Long-Term Outcome of Epiretinal Membrane Surgery in Patients with Internal Limiting Membrane Dehiscence
Abstract
1. Introduction
2. Methods
2.1. Patients
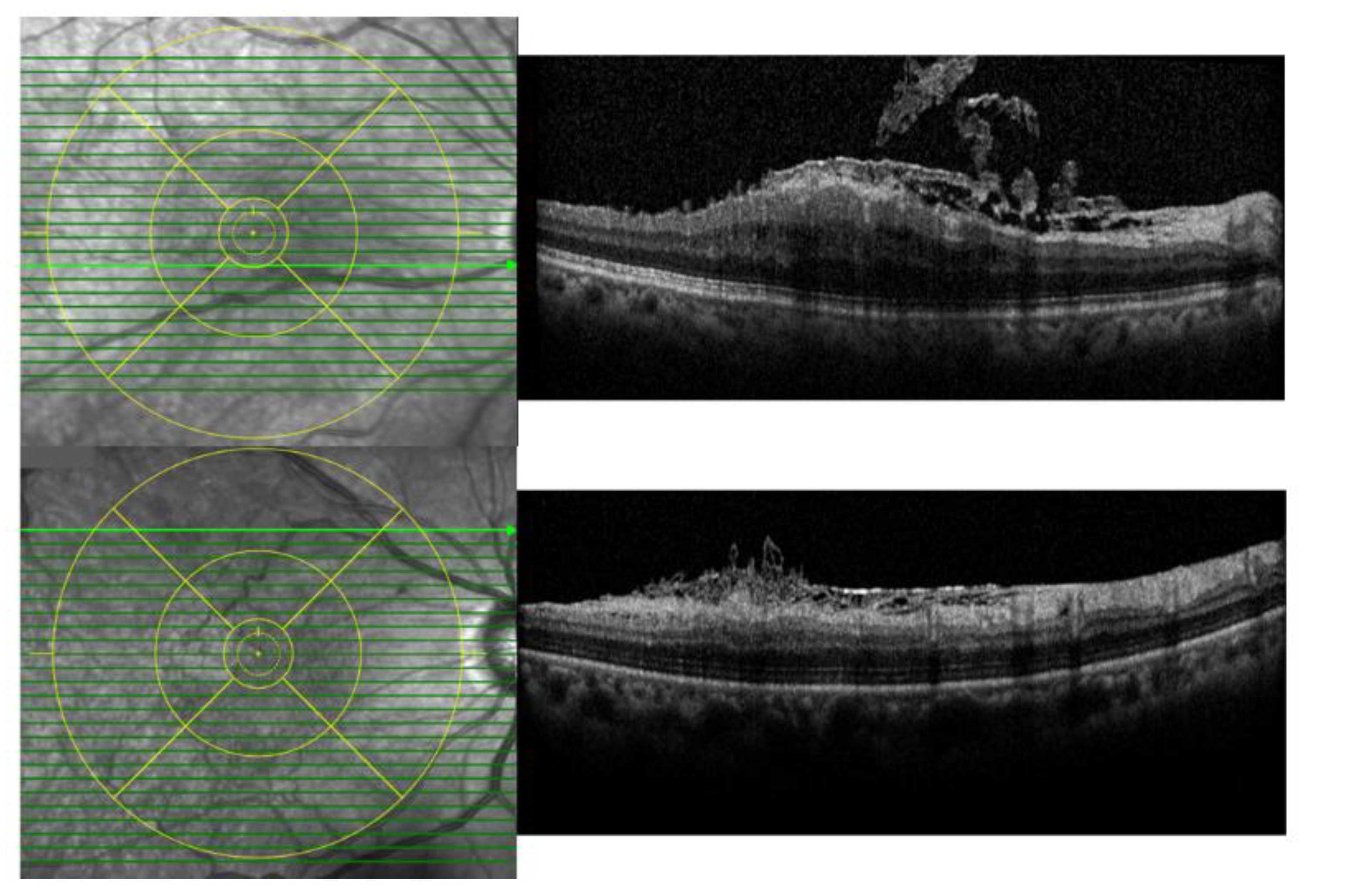
2.2. Analyses of ILM Dehiscence and Optical Coherence Tomography (OCT) Measurements
2.3. Statistical Analyses
3. Results
3.1. Demographics
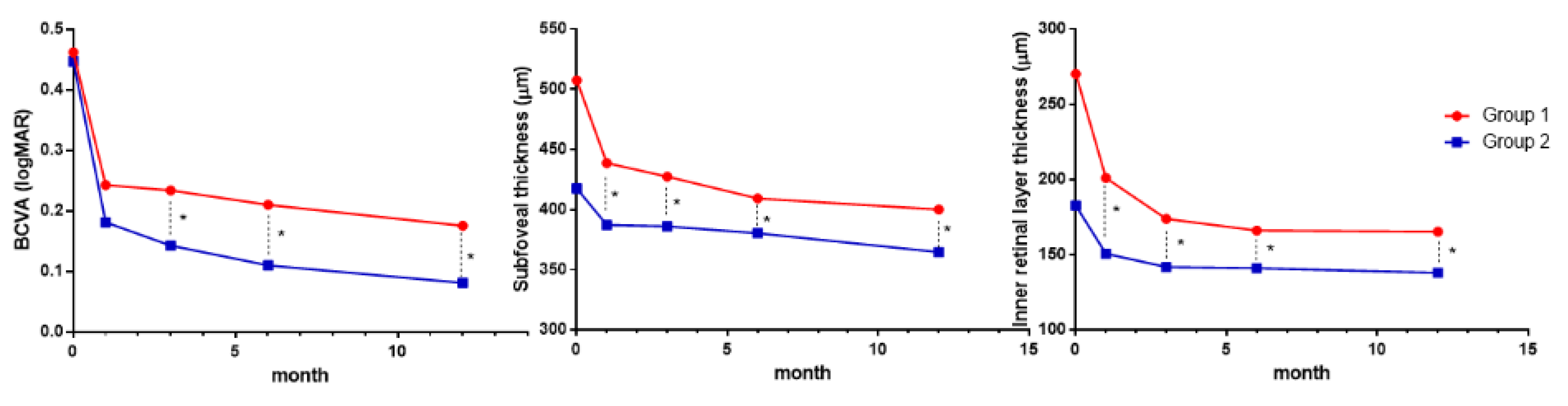
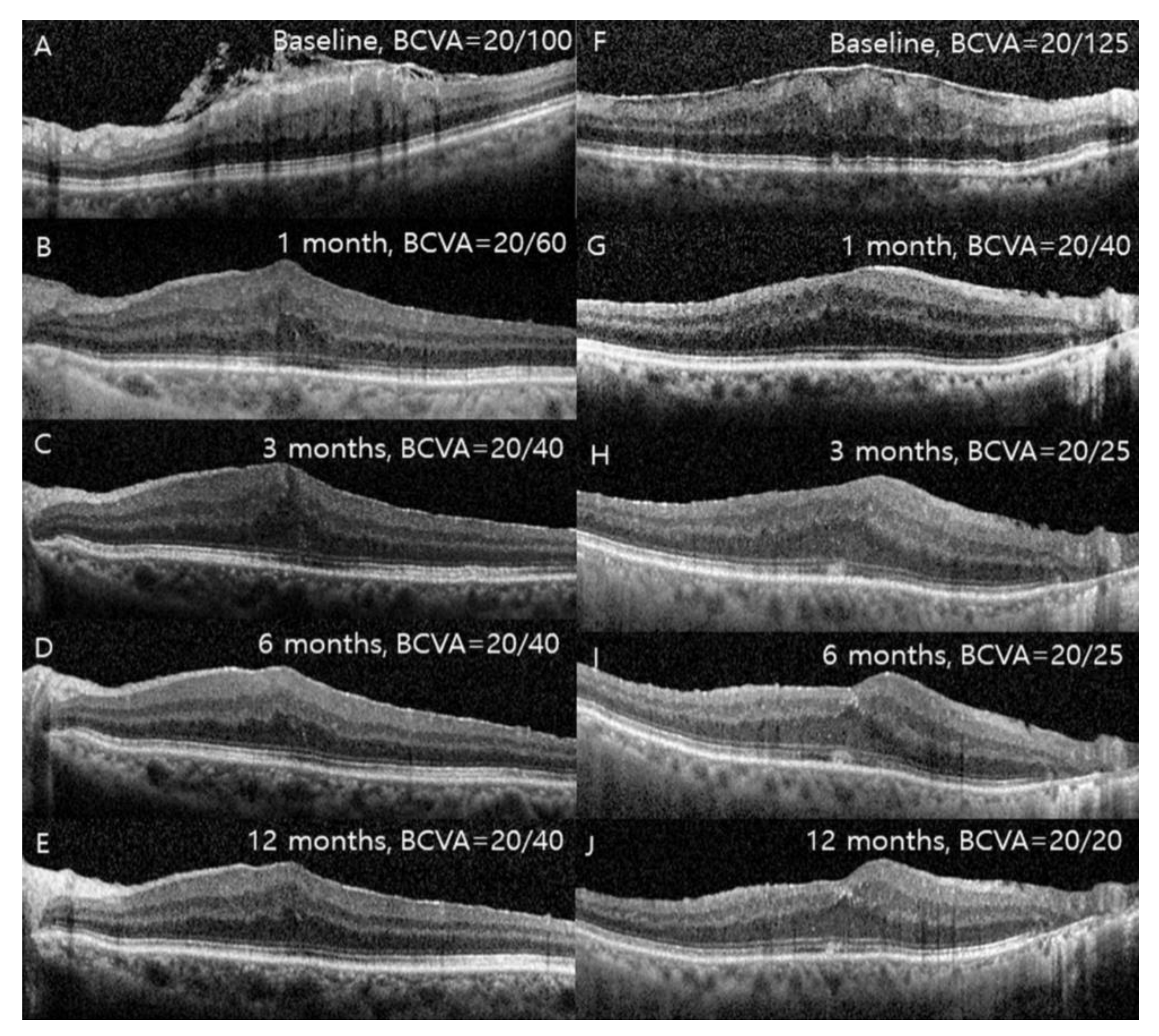
3.2. Changes in Visual Acuity, SFT, and IRLT after Surgery
3.3. Thickness of Each Retinal Layer at 12 Months after Surgery
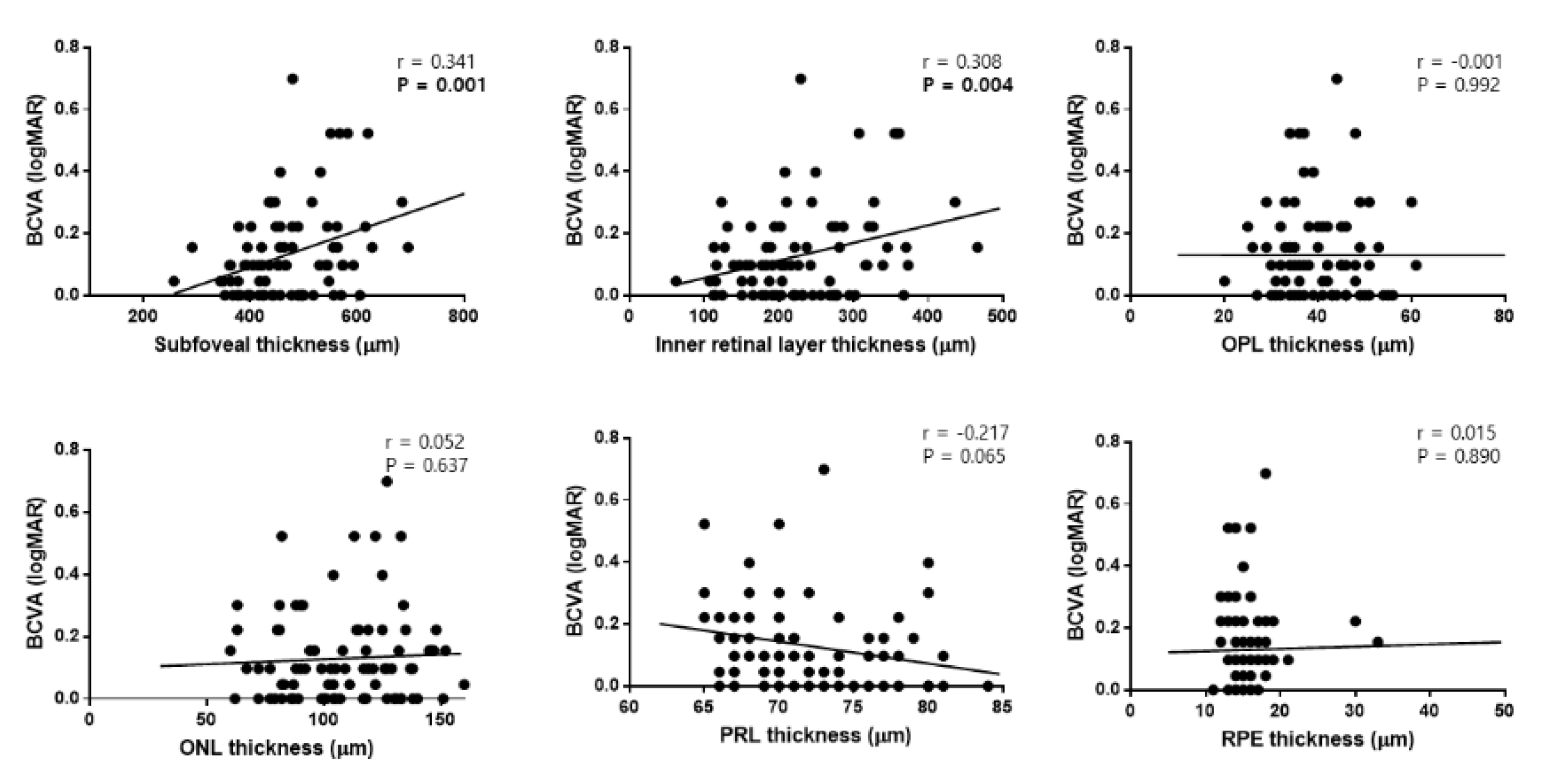
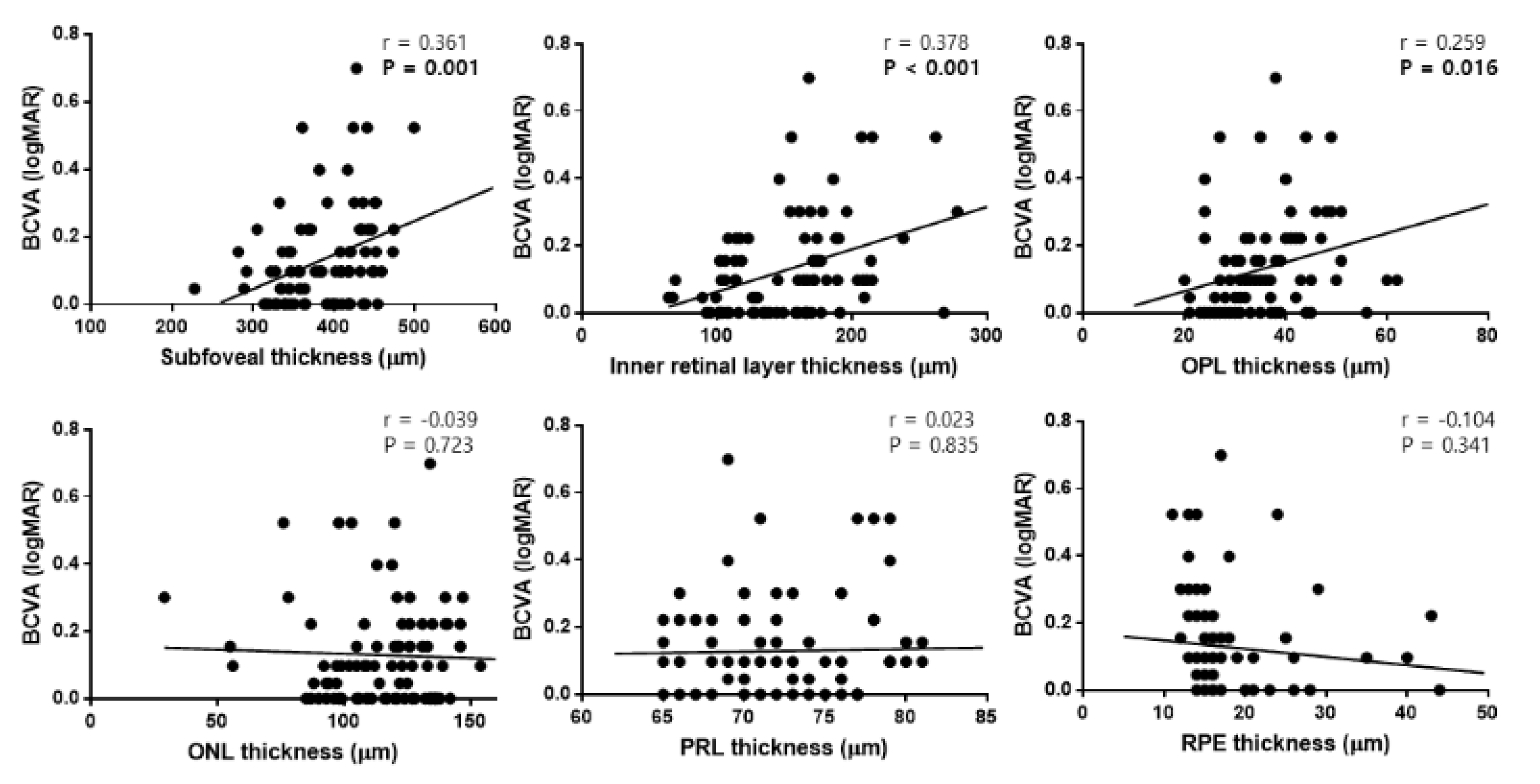
3.4. Univariate and Multivariate Linear Regression Analyses in Patients with ILM Dehiscence
3.5. Univariate and Multivariate Logistic Regression Analyses for Determining the Factors Associated with ILM Dehiscence
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mitchell, P.; Smith, W.; Chey, T.; Wang, J.J.; Chang, A. Prevalence and associations of epiretinal membranes: The Blue Mountains Eye Study, Australia. Ophthalmology 1997, 104, 1033–1040. [Google Scholar] [CrossRef]
- Wise, G.N. Clinical features of idiopathic preretinal macular fibrosis: Schoenberg Lecture. Am. J. Ophthalmol. 1975, 79, 349–357. [Google Scholar] [CrossRef]
- Watanabe, A.; Arimoto, S.; Nishi, O. Correlation between metamorphopsia and epiretinal membrane optical coherence tomography findings. Ophthalmology 2009, 116, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Smiddy, W.E.; Maguire, A.M.; Green, W.R.; Michels, R.G.; de la Cruz, Z.; Enger, C.; Jaeger, M.; Rice, T.A. Idiopathic epiretinal membranes: Ultrastructural characteristics and clinicopathologic correlation. Ophthalmology 1989, 96, 811–821. [Google Scholar] [CrossRef]
- Pesin, S.R.; Olk, R.J.; Grand, M.G.; Boniuk, I.; Arribas, N.P.; Thomas, M.A.; Williams, D.F.; Burgess, D. Vitrectomy for premacular fibroplasia: Prognostic factors, long-term follow-up, and time course of visual improvement. Ophthalmology 1991, 98, 1109–1114. [Google Scholar] [CrossRef]
- Rice, T.A.; De Bustros, S.; Michels, R.G.; Thompson, J.T.; Debanne, S.M.; Rowland, D.Y. Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology 1986, 93, 602–610. [Google Scholar] [CrossRef]
- Shimozono, M.; Oishi, A.; Hata, M.; Matsuki, T.; Ito, S.; Ishida, K.; Kurimoto, Y. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am. J. Ophthalmol. 2012, 153, 698–704.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.M.; Chung, E.J.; Lee, S.Y.; Koh, H.J. Structural and functional predictors of visual outcome of epiretinal membrane surgery. Am. J. Ophthalmol. 2012, 153, 103–110.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Kim, J.T.; Joe, S.G.; Lee, J.Y.; Yoon, Y.H. Postoperative restoration of foveal inner retinal configuration in patients with epiretinal membrane and abnormally thick inner retina. Retina 2015, 35, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Govetto, A.; Lalane, R.A., III; Sarraf, D.; Figueroa, M.S.; Hubschman, J.P. Insights into epiretinal membranes: Presence of ectopic inner foveal layers and a new optical coherence tomography staging scheme. Am. J. Ophthalmol. 2017, 175, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Govetto, A.; Virgili, G.; Rodriguez, F.J.; Figueroa, M.S.; Sarraf, D.; Hubschman, J.P. Functional and anatomical significance of the ectopic inner foveal layers in eyes with idiopathic epiretinal membranes: Surgical results at 12 months. Retina 2019, 39, 347–357. [Google Scholar] [CrossRef] [PubMed]
- González-Saldivar, G.; Berger, A.; Wong, D.; Juncal, V.; Chow, D.R. Ectopic inner foveal layer classification scheme predicts visual outcomes after epiretinal membrane surgery. Retina 2020, 40, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Hussnain, S.A.; Sharma, T.; Hood, D.C.; Chang, S. Schisis of the Retinal Nerve Fiber Layer in Epiretinal Membranes. Am. J. Ophthalmol. 2019, 207, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Bovey, E.H.; Uffer, S. Tearing and folding of the retinal internal limiting membrane associated with macular epiretinal membrane. Retina 2008, 28, 433–440. [Google Scholar] [PubMed]
- Joe, S.G.; Lee, K.S.; Lee, J.Y.; Hwang, J.-u.; Kim, J.G.; Yoon, Y.H. Inner retinal layer thickness is the major determinant of visual acuity in patients with idiopathic epiretinal membrane. Acta Ophthalmol. 2013, 91, e242–e243. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Chhablani, J.; Chan, C.K.; Cheng, L.; Kozak, I.; Hartmann, K.; Freeman, W.R. Retinal adherence and fibrillary surface changes correlate with surgical difficulty of epiretinal membrane removal. Am. J. Ophthalmol. 2012, 153, 692–697.e2. [Google Scholar] [CrossRef] [PubMed]
- Doguizi, S.; Sekeroglu, M.A.; Ozkoyuncu, D.; Omay, A.E.; Yilmazbas, P. Clinical significance of ectopic inner foveal layers in patients with idiopathic epiretinal membranes. Eye 2018, 32, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
| Group 1 (n = 46) | Group 2 (n = 40) | p-Value | |
|---|---|---|---|
| Age | 65.5 ± 9.3 | 67.9 ± 9.1 | 0.059 |
| Sex (men, %) | 17 (37.0%) | 21 (52.5%) | 0.076 |
| Hypertension (n, %) | 17 (37.0%) | 18 (45.0%) | 0.513 |
| Diabetes (n, %) | 6 (13.0%) | 8 (20%) | 0.400 |
| Laterality (right, %) | 26 (56.5%) | 21 (52.5%) | 0.709 |
| Spherical equivalent | −0.34 ± 1.59 | −0.14 ± 1.69 | 0.570 |
| BCVA | 0.46 ± 0.29 | 0.45 ± 0.25 | 0.801 |
| Intraocular pressure | 14.7 ± 3.0 | 13.8 ± 3.6 | 0.210 |
| Axial length | 23.4 ± 1.1 | 23.5 ± 0.9 | 0.704 |
| Lens status (phakic, %) | 28 (60.9%) | 28 (70.0%) | 0.497 |
| Photoreceptor layer disruption (%) | 24 (52.2%) | 17 (42.5%) | 0.395 |
| Stage of ERM | 2.9 ± 0.8 | 2.6 ± 0.8 | 0.116 |
| Retinal layer thickness | |||
| SFT | 507.4 ± 80.0 | 417.6 ± 63.6 | <0.001 |
| IRLT | 270.2 ± 74.3 | 182.6 ± 60.4 | <0.001 |
| OPL | 40.2 ± 7.1 | 38.9 ± 9.0 | 0.449 |
| ONL | 110.4 ± 24.5 | 104.6 ± 24.2 | 0.274 |
| PRL | 72.0 ± 4.8 | 72.1 ± 4.2 | 0.995 |
| RPE | 15.6 ± 2.8 | 15.8 ± 3.3 | 0.778 |
| Group 1 | Group 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BCVA | SFT | IRLT | BCVA | SFT | IRLT | p-Value * | p-Value † | p-Value ‡ | |
| Baseline | 0.46 ± 0.29 | 507.4 ± 80.0 | 270.2 ± 74.3 | 0.45 ± 0.25 | 417.6 ± 63.6 | 182.6 ± 60.4 | 0.801 | <0.001 | <0.001 |
| 1 month | 0.24 ± 0.21 | 438.8 ± 62.9 | 201.1 ± 54.9 | 0.18 ± 0.16 | 387.3 ± 71.8 | 150.7 ± 46.9 | 0.137 | 0.001 | <0.001 |
| 3 months | 0.23 ± 0.21 | 427.5 ± 56.2 | 173.9 ± 42.1 | 0.14 ± 0.14 | 386.1 ± 69.7 | 141.9 ± 43.2 | 0.019 | 0.003 | 0.001 |
| 6 months | 0.21 ± 0.22 | 409.4 ± 53.2 | 166.2 ± 40.9 | 0.11 ± 0.12 | 380.5 ± 60.7 | 141.1 ± 44.0 | 0.014 | 0.021 | 0.010 |
| 12 months | 0.18 ± 0.17 | 400.2 ± 47.1 | 165.4 ± 41.2 | 0.08 ± 0.09 | 364.8 ± 53.7 | 138.1 ± 44.2 | 0.002 | 0.002 | 0.004 |
| Group 1 | Group 2 | p-Value | |
|---|---|---|---|
| SFT | 400.2 ± 47.1 | 364.8 ± 53.7 | 0.002 |
| IRLT | 165.4 ± 41.2 | 138.1 ± 44.2 | 0.004 |
| RNFL | 28.8 ± 23.6 | 22.7 ± 19.1 | 0.189 |
| GCL | 43.5 ± 11.5 | 35.9 ± 15.2 | 0.010 |
| IPL | 40.3 ± 10.1 | 35.6 ± 10.2 | 0.003 |
| INL | 52.9 ± 12.0 | 46.0 ± 13.9 | 0.016 |
| OPL | 36.7 ± 8.8 | 33.2 ± 8.8 | 0.070 |
| ONL | 114.9 ± 22.6 | 113.7 ± 21.3 | 0.807 |
| PRL | 73.0 ± 4.3 | 72.3 ± 4.3 | 0.498 |
| RPE | 17.6 ± 6.5 | 17.2 ± 6.2 | 0.727 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Age | 0.002 (−0.004–0.008) | 0.457 | ||
| Sex | 0.054 (−0.060–0.168) | 0.344 | ||
| Hypertension | 0.018 (−0.089–0.125) | 0.738 | ||
| Diabetes | −0.037 (−0.190–0.116) | 0.628 | ||
| Laterality | −0.015 (−0.120–0.089) | 0.767 | ||
| SE | −0.017 (−0.050–0.015) | 0.286 | ||
| IOP | −0.001 (−0.018–0.017) | 0.922 | ||
| Axial length | 0.003 (−0.045–0.050) | 0.902 | ||
| Baseline BCVA | 0.174 (0.004–0.344) | 0.045 | 0.109 (−0.082–0.300) | 0.254 |
| Baseline SFT | 0.001 (<0.001–0.001) | 0.027 | <0.001 (−0.002–0.001) | 0.353 |
| Baseline IRLT | 0.001 (<0.001–0.001) | 0.036 | −0.001 (−0.002–0.000) | 0.555 |
| Lens status | 0.063 (−0.0420–0.167) | 0.231 | ||
| Tear size | 0.073 (−0.029–0.175) | 0.159 | ||
| Tear location | −0.135 (−0.231−0.039) | 0.007 | −0.105 (−0.203−0.008) | 0.034 |
| PRL disruption | 0.101 (0.002–0.200) | 0.045 | 0.069 (−0.024–0.162) | 0.144 |
| Stage of ERM | 0.051 (−0.009–0.112) | 0.094 | ||
| Final SFT | 0.001 (< 0.001–0.002) | 0.020 | <0.001 (−0.002–0.002) | 0.725 |
| Final IRLT | 0.002 (0.001–0.003) | 0.009 | 0.001 (0.000–0.002) | 0.046 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| B (OR) | p-Value | B (95% CI) | p-Value | |
| Age | −0.055 (0.947) | 0.027 | −0.051 (0.950) | 0.103 |
| Sex | 1.158 (3.184) | 0.100 | ||
| Hypertension | 0.448 (1.565) | 0.310 | ||
| Diabetes | 0.455 (1.576) | 0.441 | ||
| Laterality | 0.265 (1.303) | 0.542 | ||
| SE | −0.089 (0.915) | 0.513 | ||
| IOP | 0.091 (1.095) | 0.181 | ||
| Axial length | −0.092 (0.912) | 0.675 | ||
| Baseline BCVA | 0.240 (1.271) | 0.764 | ||
| Baseline SFT | 0.019 (1.020) | <0.001 | 0.009 (1.009) | 0.367 |
| Baseline IRLT | 0.021 (1.021) | <0.001 | 0.018 (1.018) | <0.001 |
| Lens status | 0.268 (1.308) | 0.556 | ||
| Photoreceptor layer disruption | 0.478 (1.613) | 0.272 | ||
| Stage of ERM | 0.472 (1.603) | 0.080 | ||
| Final BCVA | 6.310 (55.190) | 0.004 | 4.431 (83.987) | 0.039 |
| Final SFT | 0.015 (1.015) | 0.002 | −0.004 (0.996) | 0.364 |
| Final IRLT | 0.018 (1.018) | 0.003 | 0.009 (1.009) | 0.438 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-W.; Jung, I.; Song, Y.-Y.; Baek, S.-K.; Lee, Y.-H. Long-Term Outcome of Epiretinal Membrane Surgery in Patients with Internal Limiting Membrane Dehiscence. J. Clin. Med. 2020, 9, 2470. https://doi.org/10.3390/jcm9082470
Lee M-W, Jung I, Song Y-Y, Baek S-K, Lee Y-H. Long-Term Outcome of Epiretinal Membrane Surgery in Patients with Internal Limiting Membrane Dehiscence. Journal of Clinical Medicine. 2020; 9(8):2470. https://doi.org/10.3390/jcm9082470
Chicago/Turabian StyleLee, Min-Woo, Il Jung, Yong-Yeon Song, Seung-Kook Baek, and Young-Hoon Lee. 2020. "Long-Term Outcome of Epiretinal Membrane Surgery in Patients with Internal Limiting Membrane Dehiscence" Journal of Clinical Medicine 9, no. 8: 2470. https://doi.org/10.3390/jcm9082470
APA StyleLee, M.-W., Jung, I., Song, Y.-Y., Baek, S.-K., & Lee, Y.-H. (2020). Long-Term Outcome of Epiretinal Membrane Surgery in Patients with Internal Limiting Membrane Dehiscence. Journal of Clinical Medicine, 9(8), 2470. https://doi.org/10.3390/jcm9082470





