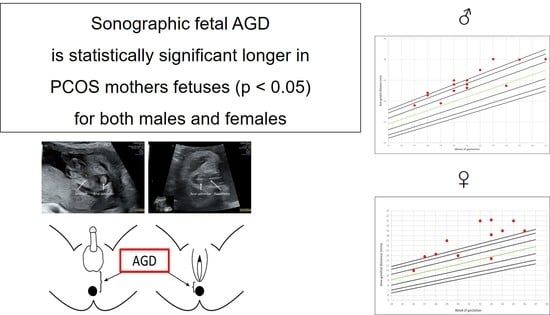
Foetal Sonographic Anogenital Distance Is Longer in Polycystic Ovary Syndrome Mothers
Abstract
1. Introduction
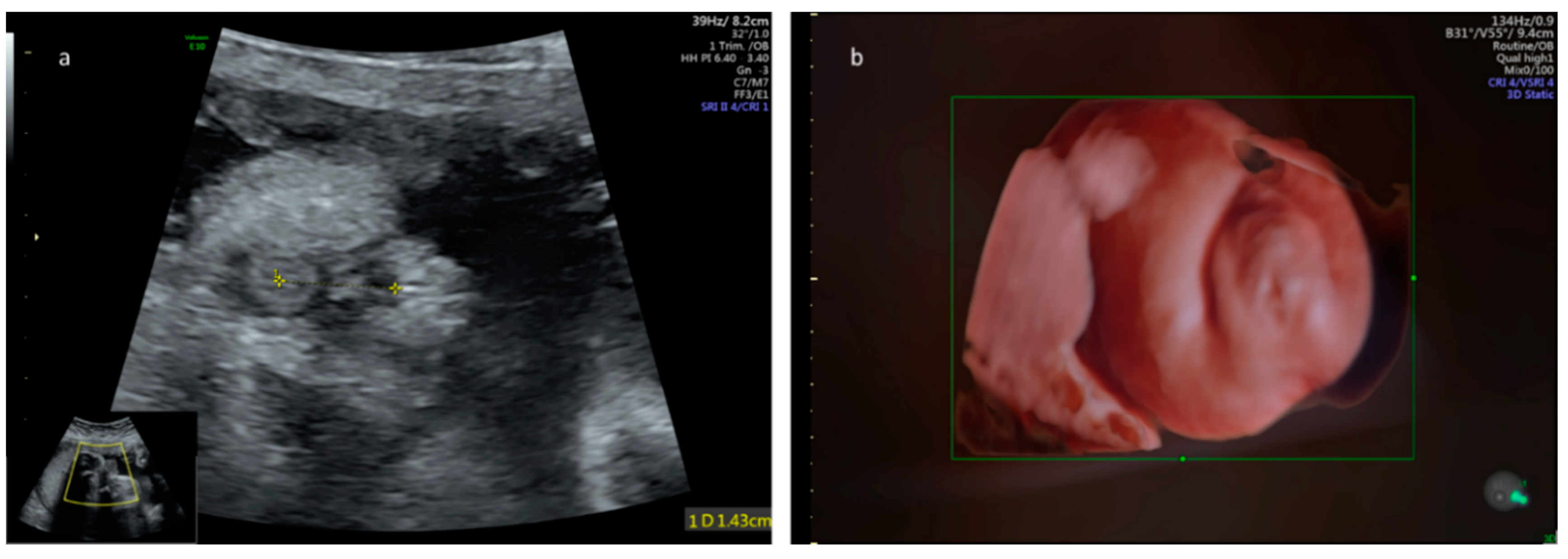
2. Methods
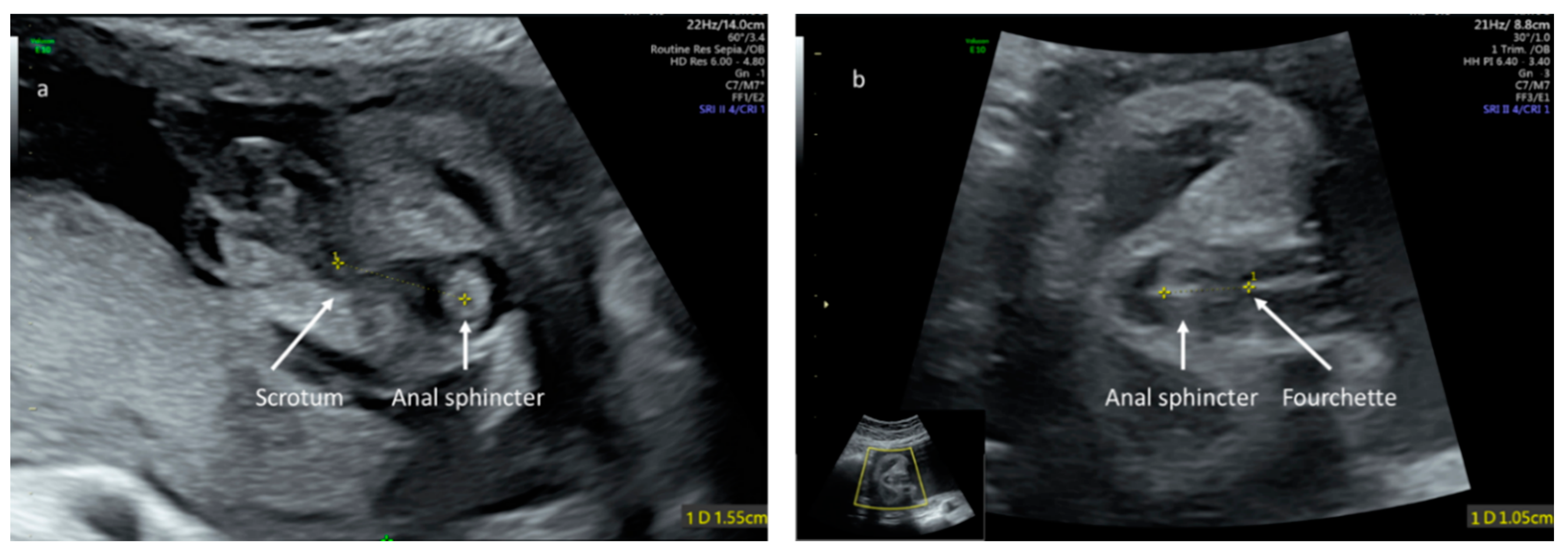
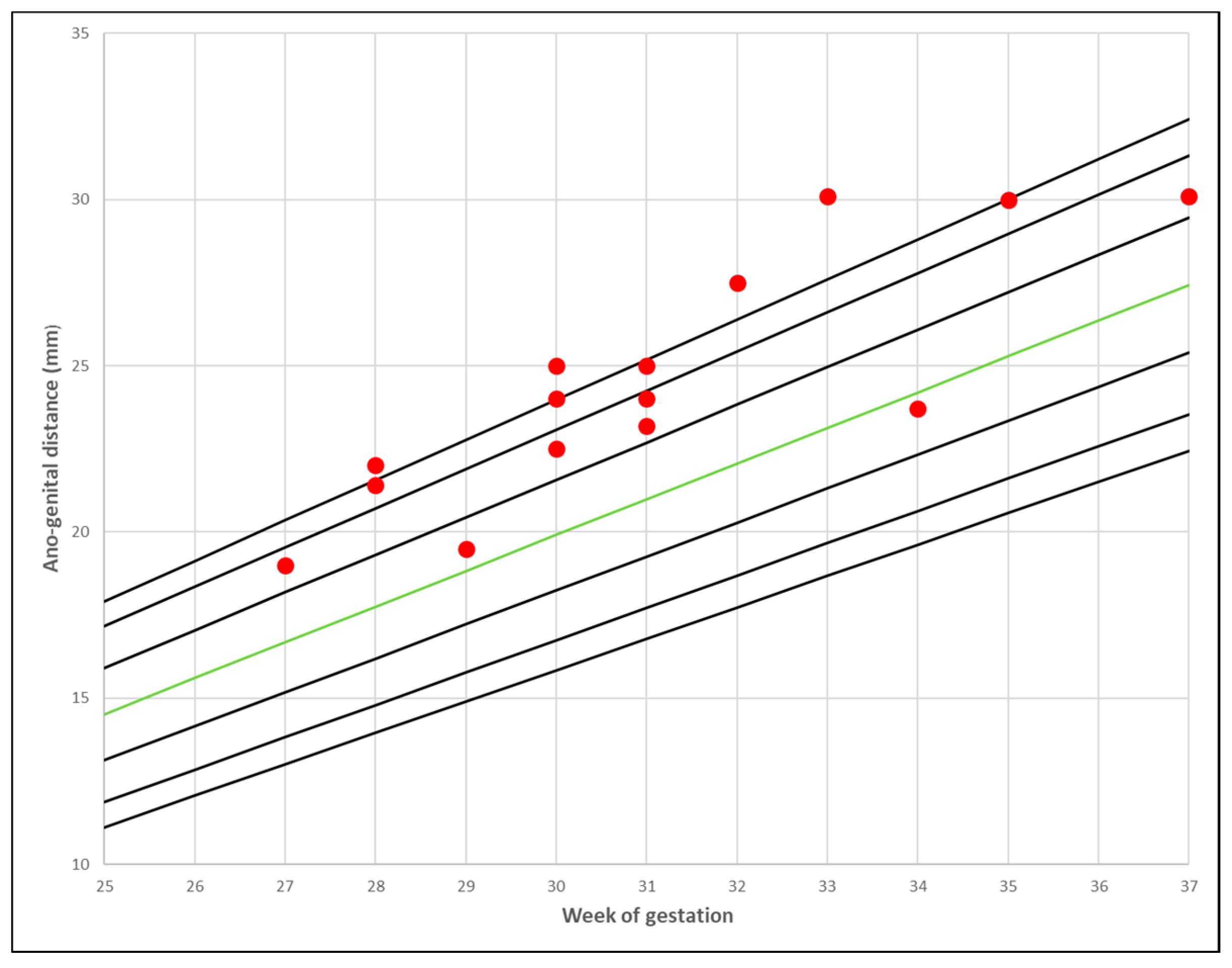
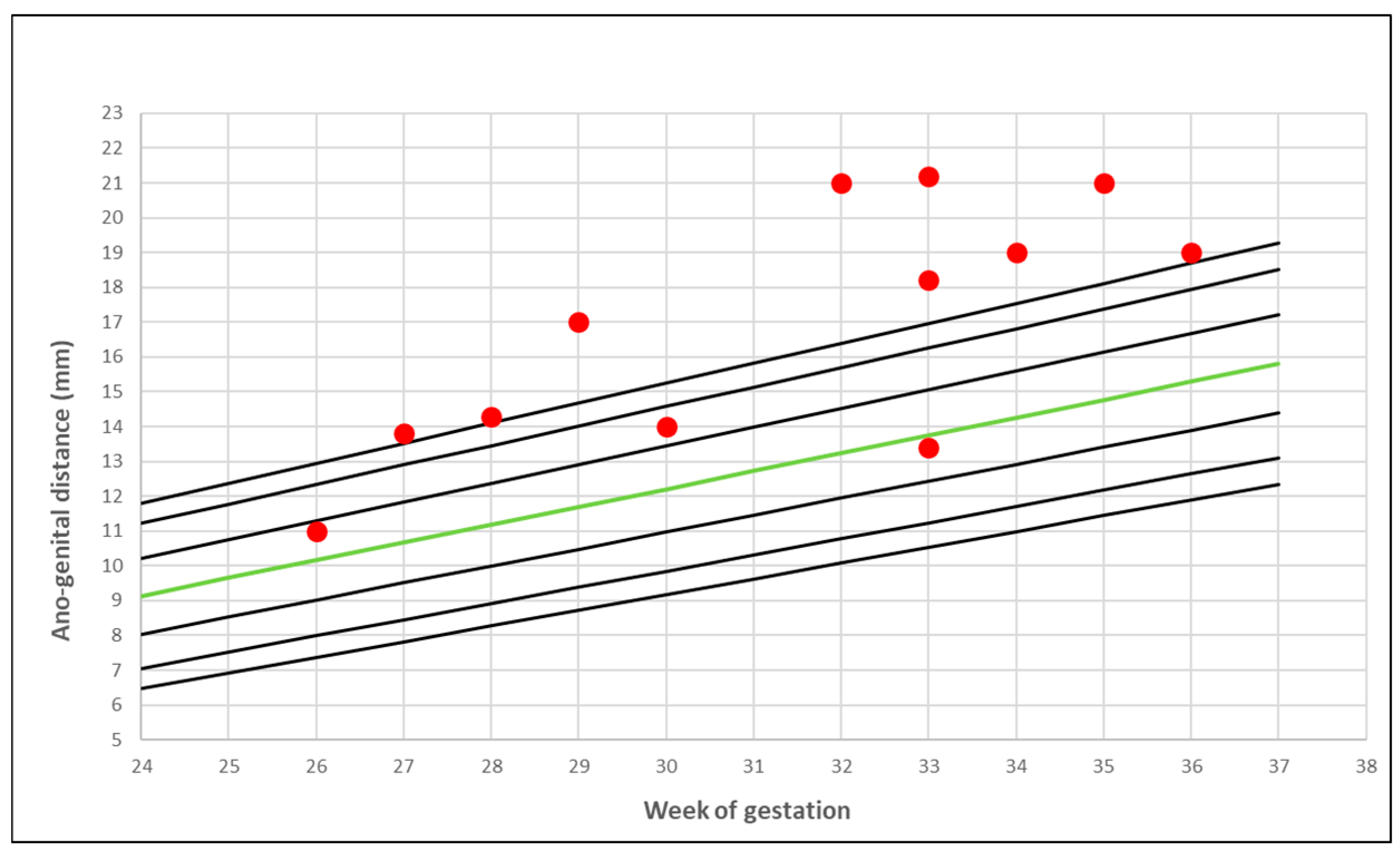
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Thankamony, A.; Ong, K.K.; Dunger, D.B.; Acerini, C.L.; Hughes, I.A. Anogenital distance from birth to 2 years: A population study. Environ. Health Perspect. 2009, 117, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Martinez, E.; Romano-Riquer, P.; Yanez-Marquez, E.; Longnecker, M.P.; Hernandez-Avila, M. Anogenital distance in human male and female newborns: A descriptive, cross-sectional study. Environ. Health 2004, 3. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Beard, L.; Zhou, C.; Grady, R. Measurement and correlates of ano-genital distance in healthy, newborn infants. Int. J. Androl. 2010, 33, 317–323. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, B.S.; Barlow, N.J.; Foster, P.M. Androgen-mediated development in male rat offspring exposed to flutamide in utero: Permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol. Sci. 2001, 62, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, A.K.; Parks-Saldutti, L.G.; Ostby, J.S.; Lambright, C.; Furr, J.; Vandenbergh, J.G.; Gray, L.E., Jr. A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol. Reprod. 2004, 71, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Macleod, D.J.; Sharpe, R.M.; Welsh, M.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Drake, A.J.; van den Driesche, S. Androgen action in the masculinization programming window and development of male reproductive organs. Int. J. Androl. 2010, 33, 279–287. [Google Scholar] [CrossRef]
- Wolf, C.J.; Hotchkiss, A.; Ostby, J.S.; LeBlanc, G.A.; Gray, L.E., Jr. Effects of prenatal testosterone propionate on the sexual development of male and female rats: A dose-response study. Toxicol. Sci. 2002, 65, 71–86. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Sabaté, J.P.; Gallissot, F. Effects of in utero exposure to di-n-hexyl phthalate on the reproductive development of the male rat. Reprod. Toxicol. 2009, 28, 468–476. [Google Scholar] [CrossRef]
- Dean, A.; Smith, L.B.; Macpherson, S.; Sharpe, R.M. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int. J. Androl. 2012, 35, 330–339. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Hsieh, M.H.; Walters, R.C.; Krasnow, R.; Lipshultz, L.I. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS ONE 2011, 6, e18973. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Shy, M.; Walters, R.C.; Lipshultz, L.I. The relationship between anogenital distance and azoospermia in adult men. Int. J. Androl. 2012, 35, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.; Roca, M.; Mínguez-Alarcón, L.; Mira-Escolano, M.P.; López-Espín, J.J.; Barrett, E.S.; Swan, S.H.; Torres-Cantero, A.M. Anogenital distance is related to ovarian follicular number in young Spanish women: A cross-sectional study. Environ. Health 2012, 11. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Breyer, B.N.; Eisenberg, M.L.; Baskin, L.S. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr. Urol. Rep. 2008, 9, 137–142. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Eisenberg, M.L.; Hittelman, A.B.; Wilson, J.M.; Tasian, G.E.; Baskin, L.S. Caucasian male infants and boys with hypospadias exhibit reduced anogenital distance. Hum. Reprod. 2012, 27, 1577–1580. [Google Scholar] [CrossRef]
- Jain, V.G.; Singal, A.K. Shorter anogenital distance correlates with undescended testis: A detailed genital anthropometric analysis in human newborns. Hum. Reprod. 2013, 28, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Thankamony, A.; Lek, N.; Carroll, D.; Williams, M.; Dunger, D.B.; Acerini, C.L.; Ong, K.K.; Hughes, I.A. Anogenital distance and penile length in infants with hypospadias or cryptorchidism: Comparison with normative data. Environ. Health Perspect. 2014, 122, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-C.; Kuo, P.-L.; Chou, Y.-Y.; Lin, S.-J.; Lee, C.-C. Association between prenatal exposure to phthalates and the health of newborns. Environ. Int. 2009, 35, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008, 108, 177–184. [Google Scholar] [CrossRef]
- Swan, S.H.; Main, K.M.; Liu, F.; Stewart, S.L.; Kruse, R.L.; Calafat, A.M.; Mao, C.S.; Redmon, J.; Ternand, C.L.; Sullivan, S.; et al. Decrease in Anogenital Distance among Male Infants with Prenatal Phthalate Exposure. Environ. Health Perspect. 2005, 113, 1056–1061. [Google Scholar] [CrossRef]
- Fowler, P.A.; Bhattacharya, S.; Flannigan, S.; Drake, A.J.; O’Shaughnessy, P.J. Maternaligarette smoking and effects on androgen action in male offspring: Unexpected effects on second-trimester anogenital distance. J. Clin. Endocrinol. Metab. 2011, 96, E1502–E1506. [Google Scholar] [CrossRef]
- Fowler, P.A.; Filis, P.; Bhattacharya, S.; le Bizec, B.; Antignac, J.P.; Morvan, M.L.; Drake, A.J.; Soffientini, U.; O’Shaughnessy, P.J. Human anogenital distance: An update on fetal smoke-exposure and integration of the perinatal literature on sex differences. Hum. Reprod. 2016, 31, 463–472. [Google Scholar] [CrossRef]
- Gilboa, Y.; Kivilevitch, Z.; Oren, M.; Cohen, Y.P.; Katorza, E.; Achiron, R. Anogenital distance in male and female fetuses at 20 to 35 weeks of gestation: Centile charts and reference ranges. Prenat. Diagn. 2014, 34, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, Y.; Perlman, S.; Kivilevitch, Z.; Messing, B.; Achiron, R. Prenatal Anogenital Distance Is Shorter in Fetuses with Hypospadias. J. Ultrasound Med. 2017, 36, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Holt, R.; Chaplin, D.; Hawkes, R.; Allison, C.; Hackett, G.; Austin, T.; Tsompanidis, A.; Gabis, L.; Ziv, S.I.; et al. Fetal anogenital distance using ultrasound. Prenat. Diagn. 2019, 39, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Sir-Petermann, T.; Maliqueo, M.; Angel, B.; Lara, H.E.; Pérez-Bravo, F.; Recabarren, S.E. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: Possible implications in prenatal androgenization. Hum. Reprod. 2002, 17, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Dollberg, S.; Haklai, Z.; Mimouni, F.B.; Gorfein, I.; Gordon, E.S. Birth weight standards in the live-born population in Israel. Isr. Med. Assoc. J. 2005, 7, 311–314. [Google Scholar]
- Ochoa, J.H.; Chiesa, M.; Vildoza, R.P.; Wong, A.E.; Sepulveda, W. Evaluation of the perianal muscular complex in the prenatal diagnosis of anorectal atresia in a high-risk population. Ultrasound Obstet. Gynecol. 2012, 39, 521–527. [Google Scholar] [CrossRef]
- Perlman, S.; Bilik, R.; Leibovitch, L.; Katorza, E.; Achiron, R.; Gilboa, Y. More than a gut feeling—Sonographic prenatal diagnosis of imperforate anus in a high-risk population. Prenat. Diagn. 2014, 34, 1307–1311. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Falbo, A.; Rocca, M.; Russo, T.; D’Ettore, A.; Tolino, A.; Zullo, F.; Orio, F.; Palomba, S. Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): Relationships with adverse outcomes. J. Ovar. Res. 2010, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Falbo, A.; Chiossi, G.; Muscogiuri, G.; Fornaciari, E.; Orio, F.; Tolino, A.; Colao, A.; La Sala, G.B.; Zullo, F. Lipid profile in nonobese pregnant women with polycystic ovary syndrome: A prospective controlled clinical study. Steroids 2014, 88, 36–43. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Musacchio, M.C.; Cappelli, V.; Massaro, M.G.; Morgante, G.; Petraglia, F. Genetic, hormonal and metabolic aspects of PCOS: An update. Reprod. Biol. Endocrinol. 2016, 14, 1–17. [Google Scholar] [CrossRef]
- Abbott, D.H.; Barnett, D.K.; Bruns, C.M.; Dumesic, D.A. Androgen excess fetal programming of female reproduction: A developmental aetiology for polycystic ovary syndrome? Hum. Reprod. Update 2005, 11, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Puttabyatappa, M.; Cardoso, R.C.; Padmanabhan, V. Effect of maternal PCOS and PCOS-like phenotype on the offspring’s health. Mol. Cell Endocrinol. 2016, 435, 29–39. [Google Scholar] [CrossRef]
- Thankamony, A.; Pasterski, V.; Ong, K.K.; Acerini, C.L.; Hughes, I.A. Anogenital distance as a marker of androgen exposure in humans. Andrology 2016, 4, 616–625. [Google Scholar] [CrossRef]
- Mira-Escolano, M.P.; Mendiola, J.; Mínguez-Alarcón, L.; Roca, M.; Cutillas-Tolín, A.; López-Espín, J.J.; Torres-Cantero, A.M. Anogenital distance of women in relation to their mother’s gynaecological characteristics before or during pregnancy. Reprod. Biomed. Online 2014, 28, 209–215. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, G.; Chen, S.; Zheng, C.; Liao, D.; Xie, M. Polycystic ovary syndrome is associated with anogenital distance, a marker of prenatal androgen exposure. Hum. Reprod. 2017, 32, 937–943. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, M.L.; Mendiola, J.; Hernández-Peñalver, A.I.; Corbalán-Biyang, S.; Carmona-Barnosi, A.; Prieto-Sánchez, M.T.; Nieto, A.; Torres-Cantero, A.M. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Hum. Reprod. 2017, 32, 2315–2323. [Google Scholar] [CrossRef]
- Barrett, E.S.; Hoeger, K.M.; Sathyanarayana, S.; Abbott, D.H.; Redmon, J.B.; Nguyen, R.H.N.; Swan, S.H. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J. Dev. Orig. Health Dis. 2018, 9, 307–314. [Google Scholar] [CrossRef]
- Mendiola, J.; Stahlhut, R.W.; Jørgensen, N.; Liu, F.; Swan, S.H. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ. Health Perspect. 2011, 119, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.D.; Mendiola, J.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Anogenital distance and reproductive parameters in young men. Andrologia 2016, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.V.; Ashwin, E.; Auyeung, B.; Chakrabarti, B.; Taylor, K.; Hackett, G.; Bullmore, E.T.; Baron-Cohen, S. Fetal Testosterone Influences Sexually Dimorphic Gray Matter in the Human Brain. J. Neurosci. 2012, 32, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Kosidou, K.; Dalman, C.; Widman, L.; Arver, S.; Lee, B.K.; Magnusson, C.; Gardner, R.M. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: A population-based nationwide study in Sweden. Mol. Psychiatry 2016, 21, 1441–1448. [Google Scholar] [CrossRef]
- Kosidou, K.; Dalman, C.; Widman, L.; Arver, S.; Lee, B.K.; Magnusson, C.; Gardner, R.M. Maternal Polycystic Ovary Syndrome and Risk for Attention-Deficit/Hyperactivity Disorder in the Offspring. Biol. Psychiatry 2017, 82, 651–659. [Google Scholar] [CrossRef]
- Cherskov, A.; Pohl, A.; Allison, C.; Zhang, H.; Payne, R.A.; Baron-Cohen, S. Polycystic ovary syndrome and autism: A test of the prenatal sex steroid theory. Transl. Psychiatry 2018, 136. [Google Scholar] [CrossRef]
| Gestational Age at Measurement (Mean) | 31.2 Weeks ± 3 Days SD |
|---|---|
| Maternal BMI (mean) | 29.36 ± 8.89 SD |
| Fetal estimated weight at examination (g; percentile [27]) | 1852 g ± 592 g SD; 52.1% ± 19.3 |
| Birthweight (g, percentile [27]) | 3110 g ± 483 g SD; 52.9% ± 26.5 |
| Diabetes (n, %) | 14 (51.9%) |
| Assisted reproduction technology (n, %) | 14 (51.9%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perlman, S.; Toledano, Y.; Kivilevitch, Z.; Halevy, N.; Rubin, E.; Gilboa, Y. Foetal Sonographic Anogenital Distance Is Longer in Polycystic Ovary Syndrome Mothers. J. Clin. Med. 2020, 9, 2863. https://doi.org/10.3390/jcm9092863
Perlman S, Toledano Y, Kivilevitch Z, Halevy N, Rubin E, Gilboa Y. Foetal Sonographic Anogenital Distance Is Longer in Polycystic Ovary Syndrome Mothers. Journal of Clinical Medicine. 2020; 9(9):2863. https://doi.org/10.3390/jcm9092863
Chicago/Turabian StylePerlman, Sharon, Yoel Toledano, Zvi Kivilevitch, Nufar Halevy, Elena Rubin, and Yinon Gilboa. 2020. "Foetal Sonographic Anogenital Distance Is Longer in Polycystic Ovary Syndrome Mothers" Journal of Clinical Medicine 9, no. 9: 2863. https://doi.org/10.3390/jcm9092863
APA StylePerlman, S., Toledano, Y., Kivilevitch, Z., Halevy, N., Rubin, E., & Gilboa, Y. (2020). Foetal Sonographic Anogenital Distance Is Longer in Polycystic Ovary Syndrome Mothers. Journal of Clinical Medicine, 9(9), 2863. https://doi.org/10.3390/jcm9092863