The Relevance of Fetal Abdominal Subcutaneous Tissue Recording in Predicting Perinatal Outcome of GDM Pregnancies: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Ultrasound Parameters
2.3. Statistical Analysis
2.4. Outcome Parameters
3. Results
3.1. Patient Characteristics
3.2. Perinatal Outcome
3.3. FAST
3.4. Discrimination Ability of US Parameters for Perinatal Outcome
3.5. Subgroup Analysis
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.M.; Gale, C.; Hyde, M.J.; Santhakumaran, S.; Modi, N. Diabetes in pregnancy and infant adiposity: Systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F65–F72. [Google Scholar] [CrossRef] [PubMed]
- Muche, A.A.; Olayemi, O.O.; Gete, Y.K. Effects of gestational diabetes mellitus on risk of adverse maternal outcomes: A prospective cohort study in Northwest Ethiopia. BMC Pregnancy Childbirth 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Fadl, H.E.; Ostlund, I.K.; Magnuson, A.F.; Hanson, U.S. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet. Med. 2010, 27, 436–441. [Google Scholar] [CrossRef]
- Waters, T.P.; Dyer, A.R.; Scholtens, D.M.; Dooley, S.L.; Herer, E.; Lowe, L.P.; Oats, J.J.; Persson, B.; Sacks, D.A.; Metzger, B.E.; et al. Maternal and Neonatal Morbidity for Women Who Would Be Added to the Diagnosis of GDM Using IADPSG Criteria: A Secondary Analysis of the Hyperglycemia and Adverse Pregnancy Outcome Study. Diabetes Care 2016, 39, 2204–2210. [Google Scholar] [CrossRef]
- Martin, K.E.; Grivell, R.M.; Yelland, L.N.; Dodd, J.M. The influence of maternal BMI and gestational diabetes on pregnancy outcome. Diabetes Res. Clin. Pract. 2015, 108, 508–513. [Google Scholar] [CrossRef]
- Schäfer-Graf, U.M.; Gembruch, U.; Kainer, F.; Groten, T.; Hummel, S.; Hösli, I.; Grieshop, M.; Kaltheuner, M.; Bührer, C.; Kautzky-Willer, A.; et al. Gestationsdiabetes mellitus (GDM)–Diagnostik, Therapie und Nachsorge. Leitlinie der DDG und DGGG (S3-Niveau, AWMF-Registernummer 057/008, Februar 2018). GebFra -DGGG-Gesellschaftsausgaben 2018, 78, 1219–1231. [Google Scholar] [CrossRef]
- Bhat, R.G.; Nathan, A.; Amar, R.; Vasudeva, A.; Adiga, P.; Bhat, P.V.; Kumar, N.P. Correlation of fetal abdominal subcutaneous tissue thickness by ultrasound to predict birth weight. J. Clin. Diagn. Res. 2014, 8, OC09–OC11. [Google Scholar] [CrossRef]
- Higgins, M.F.; Russell, N.M.; Mulcahy, C.H.; Coffey, M.; Foley, M.E.; McAuliffe, F.M. Fetal anterior abdominal wall thickness in diabetic pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 43–47. [Google Scholar] [CrossRef]
- Khalifa, E.A.; Hassanein, S.A.; Eid, H.H. Ultrasound measurement of fetal abdominal subcutaneous tissue thickness as a predictor of large versus small fetuses for gestational age. Egypt. J. Radiol. Nucl. Med. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Madendag, Y.; Aksoy, U.; Col Madendag, I.; Aksoy, H. Fetal front-abdominal wall thickness in the second trimester as a predictor of abnormal fetal growth. J. Matern. Fetal Neonatal Med. 2020, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; de Vries, B.; Ross, G.P.; Gordon, A. Fetal biometry for guiding the medical management of women with gestational diabetes mellitus for improving maternal and perinatal health. Cochrane Database Syst. Rev. 2019, 9, CD012544. [Google Scholar] [CrossRef] [PubMed]
- IADPSG. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Available online: https://apps.who.int/iris/bitstream/handle/10665/85975/WHO_NMH_MND_13.2_eng.pdf?sequence=1 (accessed on 27 February 2020).
- Kleinwechter, H.; Schäfer-Graf, U.; Bührer, C.; Hoesli, I.; Kainer, F.; Kautzky-Willer, A.; Pawlowski, B.; Schunck, K.; Somville, T.; Sorger, M. Gestationsdiabetes mellitus (GDM). Diabetol. Stoffwechs. 2011, 6, 290–328. [Google Scholar] [CrossRef]
- Voigt, M.; Fusch, C.; Olbertz, D.; Hartmann, K.; Rochow, N.; Renken, C.; Schneider, K. Analysis of the neonatal collective in the Federal Republic of Germany 12th report. Presentation of detailed percentiles for the body measurement of newborns. Geburtshilfe Frauenheilkd. 2006, 66, 956–970. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Berghella, V.; Bilardo, C.; Hernandez-Andrade, E.; Johnsen, S.L.; Kalache, K.; Leung, K.Y.; Malinger, G.; Munoz, H.; et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet. Gynecol. 2011, 37, 116–126. [Google Scholar] [CrossRef]
- Bethune, M.; Bell, R. Evaluation of the measurement of the fetal fat layer, interventricular septum and abdominal circumference percentile in the prediction of macrosomia in pregnancies affected by gestational diabetes. Ultrasound Obstet. Gynecol. 2003, 22, 586–590. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Dubinsky, T.J.; O’Regan, J.; Sonneborn, R.; Hippe, D.S.; Dighe, M.; Moshiri, M. A Nomogram of Lateral Abdominal Wall Fat Thickness in Normal Third Trimester Fetuses. Ultrasound Q. 2019, 35, 30–34. [Google Scholar] [CrossRef]
- O’Connor, C.; Farah, N.; O’Higgins, A.; Segurado, R.; Fitzpatrick, C.; Turner, M.J.; Stuart, B.; Kennelly, M.M. Longitudinal measurement of fetal thigh soft tissue parameters and its role in the prediction of birth weight. Prenat. Diagn. 2013, 33, 945–951. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Medicine, I.o.; Council, N.R. Weight Gain During Pregnancy: Reexamining the Guidelines; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.J.; Chen, X.H.; Cao, L.; Wu, Y.; Zhu, L.J.; Lv, K.T.; Ji, C.B.; Guo, X.R. Measurement of fetal abdominal and subscapular subcutaneous tissue thickness during pregnancy to predict macrosomia: A pilot study. PLoS ONE 2014, 9, e93077. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Rigano, S.; Ferrazzi, E.; Radaelli, T.; Cetin, E.T.; Pardi, G. Sonographic measurements of subcutaneous fetal fat in pregnancies complicated by gestational diabetes and in normal pregnancies. Croat. Med. J. 2000, 41, 240–244. [Google Scholar]
- Greco, P.; Vimercati, A.; Hyett, J.; Rossi, A.C.; Scioscia, M.; Giorgino, F.; Loverro, G.; Selvaggi, L. The ultrasound assessment of adipose tissue deposition in fetuses of ‘well controlled’ insulin-dependent diabetic pregnancies. Diabet. Med. 2003, 20, 858–862. [Google Scholar] [CrossRef]
- Aksoy, H.; Aksoy, Ü.; Yücel, B.; Saygi Özyurt, S.; Aydın, T.; Alparslan Babayiğit, M. Fetal anterior abdominal wall thickness may be an early ultrasonographic sign of gestational diabetes mellitus. J. Matern. -Fetal Neonatal Med. 2016, 29, 2028–2032. [Google Scholar] [CrossRef]
- Ulrich, D.; Desoye, G.; Wadsack, C.; Haas, J.; Csapo, B.; Holzapfel-Bauer, M.; Lang, U.; Schlembach, D. Fetal anterior wall thickness and amniotic fluid insulin levels: An interdependence? Ultraschall Med. 2012, 33, E108–E113. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Kjos, S.L.; Fauzan, O.H.; Bühling, K.J.; Siebert, G.; Bührer, C.; Ladendorf, B.; Dudenhausen, J.W.; Vetter, K. A randomized trial evaluating a predominantly fetal growth-based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care 2004, 27, 297–302. [Google Scholar] [CrossRef]
- Kjos, S.L.; Schaefer-Graf, U.; Sardesi, S.; Peters, R.K.; Buley, A.; Xiang, A.H.; Bryne, J.D.; Sutherland, C.; Montoro, M.N.; Buchanan, T.A. A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care 2001, 24, 1904–1910. [Google Scholar] [CrossRef]
- GestDiab. Gestdiab 2017: Auswertung für Gestationsdiabetes; GestDiab: Neuss, Germany, 2017; Available online: https://www.windiab.de/wp-content/uploads/2019/01/190127-GDM-2017-alle-Praxen.pdf (accessed on 21 August 2020).


 ; light grey), standard clinical parameters combined with standard ultrasound parameters abdominal circumference (AC) and estimated fetal weight (EFW) (- - -; broken black line) and with additional inclusion of FAST (fetal abdominal subcutaneous tissue)measurement (
; light grey), standard clinical parameters combined with standard ultrasound parameters abdominal circumference (AC) and estimated fetal weight (EFW) (- - -; broken black line) and with additional inclusion of FAST (fetal abdominal subcutaneous tissue)measurement ( ; black) in prediction of LGA in the entire cohort (a) in prediction of hypoglycemia in the subgroup of fetuses with an AC > 75th percentile (b) and for C-section when the fetus is growing below the 10th percentile (c). † means SP is included.
; black) in prediction of LGA in the entire cohort (a) in prediction of hypoglycemia in the subgroup of fetuses with an AC > 75th percentile (b) and for C-section when the fetus is growing below the 10th percentile (c). † means SP is included.
 ; light grey), standard clinical parameters combined with standard ultrasound parameters abdominal circumference (AC) and estimated fetal weight (EFW) (- - -; broken black line) and with additional inclusion of FAST (fetal abdominal subcutaneous tissue)measurement (
; light grey), standard clinical parameters combined with standard ultrasound parameters abdominal circumference (AC) and estimated fetal weight (EFW) (- - -; broken black line) and with additional inclusion of FAST (fetal abdominal subcutaneous tissue)measurement ( ; black) in prediction of LGA in the entire cohort (a) in prediction of hypoglycemia in the subgroup of fetuses with an AC > 75th percentile (b) and for C-section when the fetus is growing below the 10th percentile (c). † means SP is included.
; black) in prediction of LGA in the entire cohort (a) in prediction of hypoglycemia in the subgroup of fetuses with an AC > 75th percentile (b) and for C-section when the fetus is growing below the 10th percentile (c). † means SP is included.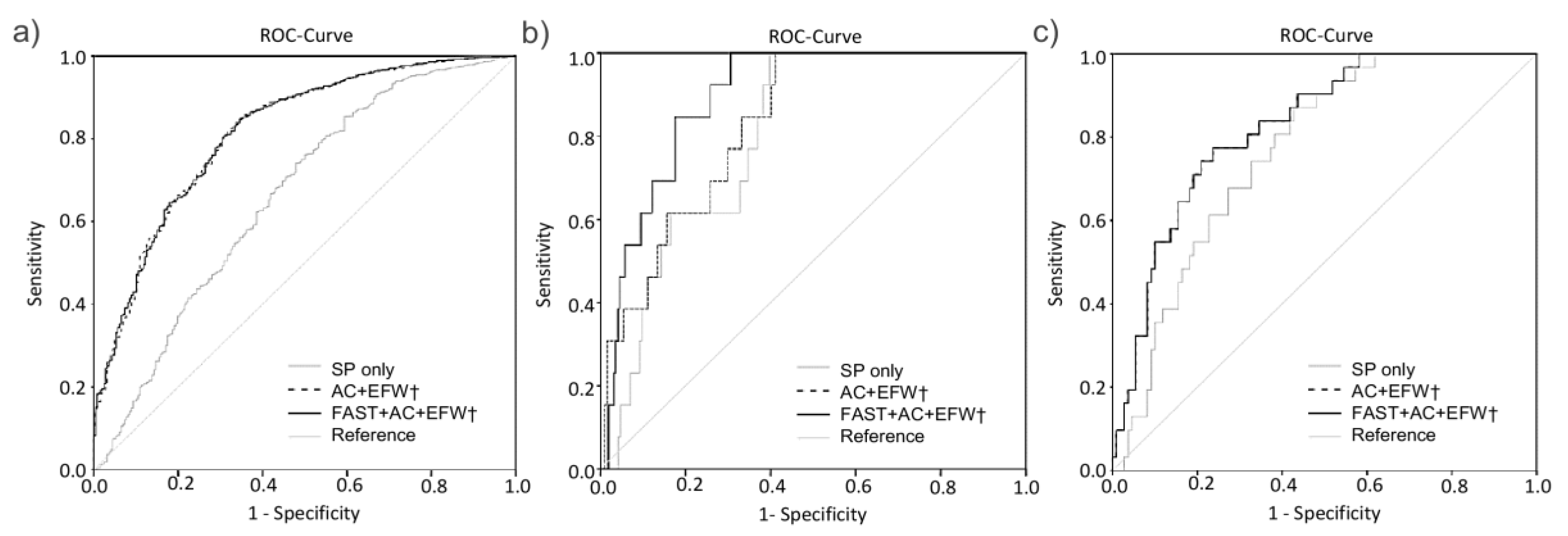
| Variable | Value |
|---|---|
| Maternal age in years (n = 800) | 31 (28–35) |
| Parity (n = 782) | 1 (0–1) |
| GA at GDM diagnosis in weeks (n = 698) | 26.3 (24.7–28.0) |
| BMI in kg/m2 (n = 795) | 26.3 (22.9–31.6) |
| BMI < 18.5 kg/m2 (underweight) | 9 (1.1%) |
| BMI 18.5–24.9 kg/m2 (normal weight) | 326 (41%) |
| BMI 25–29.9 kg/m2 (overweight) | 208 (26.2%) |
| BMI 30–34.9 kg/m2 (obesity class I) | 131 (16.5%) |
| BMI 35–39.9 kg/m2 (obesity class II) | 84 (10.6%) |
| BMI ≥ 40 kg/m2 (obesity class III) | 37 (4.7%) |
| GWG in kg (n = 761) | 12 (8.2–16.5) |
| Excessive GWG (n = 761) | 337 (44.3%) |
| Insulin Treatment (n = 800) | 340 (42.5%) |
| Mean HbA1c levels in % (n = 767) | 5.3 (5.1–5.5) |
| HbA1c at delivery in % (n = 622) | 5.5 (5.2–5.7) |
| MBG (mmol/L) (n = 787) | 5.8 (5.6–6.1) |
| Variable | Value |
|---|---|
| GA at delivery in weeks (n = 683) | 39 (38–40) |
| Birth weight in g (n = 685) | 3440 (3127–3768) |
| Percentile at Birth (n = 667) | 56 (30–77) |
| LGA (n = 667) | 81 (12.1%) |
| SGA (n = 667) | 41 (6.1%) |
| Male Sex (n = 675) | 373 (55.3%) |
| Female Sex (n = 675) | 302 (44.7% |
| Induction of Birth (n = 656) | 259 (39.5%) |
| Spontaneous Delivery (n = 685) | 440 (64.2%) |
| Vaginal Operative Delivery (n = 685) | 28 (4.1%) |
| C-section (n = 685) | 217 (31.7%) |
| Shoulder Dystocia (n = 279) | 7 (2.5%) |
| pH (n = 644) | 7.26 (7.20–7.31) |
| APGAR 5 min (n = 670) | 9 (9–10) |
| NICU Admission (n = 624) | 70 (11.2%) |
| Pre-eclampsia (n = 566) | 39 (6.9%) |
| Jaundice (n = 560) | 110 (19.6%) |
| Hyperbilirubinemia (n = 476) | 122 (25.6%) |
| Phototherapy (n = 546) | 32 (5.9%) |
| Hypoglycemia < 2 mmol/L (n = 402) | 18 (4.5%) |
| Weeks of Gestation | n | 10th Percentile (in mm) | 50th Percentile (in mm) | 90th Percentile (in mm) |
|---|---|---|---|---|
| <24 | 70 | 1.50 | 2.00 | 2.89 |
| 24 | 57 | 1.48 | 2.10 | 2.72 |
| 25 | 95 | 1.76 | 2.30 | 3.00 |
| 26 | 136 | 1.80 | 2.30 | 3.00 |
| 27 | 196 | 1.90 | 2.50 | 3.10 |
| 28 | 189 | 1.90 | 2.50 | 3.30 |
| 29 | 244 | 2.10 | 2.70 | 3.50 |
| 30 | 247 | 2.10 | 2.90 | 3.60 |
| 31 | 262 | 2.40 | 3.10 | 3.90 |
| 32 | 261 | 2.40 | 3.20 | 4.00 |
| 33 | 252 | 2.60 | 3.40 | 4.40 |
| 34 | 267 | 2.60 | 3.40 | 4.50 |
| 35 | 217 | 2.70 | 3.60 | 4.90 |
| 36 | 269 | 2.90 | 3.70 | 4.90 |
| 37 | 184 | 3.05 | 3.90 | 5.05 |
| 38 | 176 | 3.00 | 3.90 | 5.30 |
| 39 | 64 | 3.15 | 4.20 | 5.10 |
| 40 | 15 | 1.00 | 3.80 | 5.00 |
| >40 | 4 | 2.40 | 4.75 | n.a. |
| Outcome | Included Parameters | AUC | CI (95%) | p-Value ‡ |
|---|---|---|---|---|
| LGA | SP only | 0.661 | 0.621–0.700 | <0.01 |
| FAST † | 0.703 | 0.666–0.741 | <0.01 | |
| AC † | 0.804 | 0.773–0.835 | <0.01 | |
| EFW † | 0.804 | 0.774–0.834 | <0.01 | |
| FAST + AC † | 0.806 | 0.776–0.836 | <0.01 | |
| FAST + EFW † | 0.805 | 0.775–0.835 | <0.01 | |
| AC + EFW † | 0.815 * | 0.786–0.844 | <0.01 | |
| FAST + AC + EFW † | 0.816 * | 0.787–0.845 | <0.01 | |
| NICU | SP only | 0.631 | 0.592-0.669 | <0.01 |
| FAST † | 0.631 | 0.593–0.670 | <0.01 | |
| AC † | 0.637 | 0.598–0.676 | <0.01 | |
| EFW † | 0.640 | 0.601–0.678 | <0.01 | |
| FAST + AC † | 0.639 | 0.600–0.678 | <0.01 | |
| FAST + EFW † | 0.642 | 0.603–0.680 | <0.01 | |
| AC + EFW † | 0.639 | 0.601–0.678 | <0.01 | |
| FAST + AC + EFW † | 0.642 | 0.603–0.680 | <0.01 | |
| C-section | SP only | 0.599 | 0.574–0.625 | <0.01 |
| FAST † | 0.600 | 0.574–0.625 | <0.01 | |
| AC † | 0.609 | 0.583–0.634 | <0.01 | |
| EFW † | 0.609 | 0.584–0.635 | <0.01 | |
| FAST + AC † | 0.609 | 0.583–0.634 | <0.01 | |
| FAST + EFW † | 0.609 | 0.583–0.635 | <0.01 | |
| AC + EFW † | 0.610 | 0.585–0.635 | <0.01 | |
| FAST + AC + EFW † | 0.610 | 0.585–0.636 | <0.01 | |
| Hyperbilirubinemia | SP only | 0.553 | 0.522–0.585 | <0.01 |
| FAST † | 0.554 | 0.542–0.604 | <0.01 | |
| AC † | 0.573 | 0.542–0.604 | <0.01 | |
| EFW † | 0.566 | 0.536–0.597 | <0.01 | |
| FAST + AC † | 0.573 | 0.542–0.603 | <0.01 | |
| FAST + EFW † | 0.566 | 0.535–0.597 | <0.01 | |
| AC + EFW † | 0.573 | 0.535–0.597 | <0.01 | |
| FAST + AC + EFW † | 0.573 | 0.543–0.604 | <0.01 | |
| Hypoglycemia | SP only | 0.562 | 0.485–0.640 | 0.10 |
| FAST † | 0.567 | 0.494–0.639 | 0.08 | |
| AC † | 0.563 | 0.485–0.640 | 0.10 | |
| EFW † | 0.572 | 0.497–0.647 | 0.06 | |
| FAST + AC † | 0.567 | 0.494–0.640 | 0.08 | |
| FAST + EFW † | 0.573 | 0.502–0.643 | 0.08 | |
| AC + EFW † | 0.572 | 0.502–0.643 | 0.06 | |
| FAST + AC + EFW † | 0.575 | 0.507–0.643 | <0.05 |
| Subgroup | Outcome | Included Parameters | AUC | CI (95%) | p |
|---|---|---|---|---|---|
| AC > 75th Percentile | LGA | SP | 0.716 | 0.667–0.766 | <0.01 |
| AC + EFW † | 0.719 | 0.670–0.768 | <0.01 | ||
| FAST + AC + EFW † | 0.719 | 0.670–0.768 | <0.01 | ||
| NICU | SP | 0.799 | 0.732–0.865 | <0.01 | |
| AC + EFW † | 0.810 | 0.745–0.875 | <0.01 | ||
| FAST + AC + EFW † | 0.810 | 0.745–0.875 | <0.01 | ||
| C-section | SP | 0.639 | 0.594–0.685 | <0.01 | |
| AC + EFW † | 0.652 | 0.607–069.7 | <0.01 | ||
| FAST + AC + EFW † | 0.651 | 0.606–0.697 | <0.01 | ||
| Hyperbilirubinemia | SP | 0.561 | 0.488–0.634 | 0.11 | |
| AC + EFW † | 0.612 | 0.539–0.686 | <0.01 | ||
| FAST + AC + EFW † | 0.613 | 0.539–0.686 | <0.05 | ||
| Hypoglycemia | SP | 0.800 | 0.720–0.881 | <0.01 | |
| AC + EFW † | 0.830 | 0.746–0.915 | <0.01 | ||
| FAST + AC + EFW † | 0.894 * | 0.838–0.949 | <0.01 | ||
| EFW < 10th Percentile | NICU | SP | 0.695 | 0.609–0.782 | <0.01 |
| AC + EFW † | 0.698 | 0.610–0.785 | <0.01 | ||
| FAST + AC + EFW † | 0.703 | 0.614–0.792 | <0.01 | ||
| C-section | SP | 0.768 | 0.687–0.849 | <0.01 | |
| AC + EFW † | 0.827 | 0.753–0.901 | <0.01 | ||
| FAST + AC + EFW † | 0.827 | 0.753–0.901 | <0.01 | ||
| Hyperbilirubinemia | SP | 0.691 | 0.556–0.826 | <0.01 | |
| AC + EFW † | 0.705 | 0.576–0.835 | <0.01 | ||
| FAST + AC + EFW † | 0.715 | 0.588–0.842 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weschenfelder, F.; Baum, N.; Lehmann, T.; Schleußner, E.; Groten, T. The Relevance of Fetal Abdominal Subcutaneous Tissue Recording in Predicting Perinatal Outcome of GDM Pregnancies: A Retrospective Study. J. Clin. Med. 2020, 9, 3375. https://doi.org/10.3390/jcm9103375
Weschenfelder F, Baum N, Lehmann T, Schleußner E, Groten T. The Relevance of Fetal Abdominal Subcutaneous Tissue Recording in Predicting Perinatal Outcome of GDM Pregnancies: A Retrospective Study. Journal of Clinical Medicine. 2020; 9(10):3375. https://doi.org/10.3390/jcm9103375
Chicago/Turabian StyleWeschenfelder, Friederike, Nadin Baum, Thomas Lehmann, Ekkehard Schleußner, and Tanja Groten. 2020. "The Relevance of Fetal Abdominal Subcutaneous Tissue Recording in Predicting Perinatal Outcome of GDM Pregnancies: A Retrospective Study" Journal of Clinical Medicine 9, no. 10: 3375. https://doi.org/10.3390/jcm9103375
APA StyleWeschenfelder, F., Baum, N., Lehmann, T., Schleußner, E., & Groten, T. (2020). The Relevance of Fetal Abdominal Subcutaneous Tissue Recording in Predicting Perinatal Outcome of GDM Pregnancies: A Retrospective Study. Journal of Clinical Medicine, 9(10), 3375. https://doi.org/10.3390/jcm9103375





