Impact of Serum Lipid on Breast Cancer Recurrence
Abstract
:1. Introduction
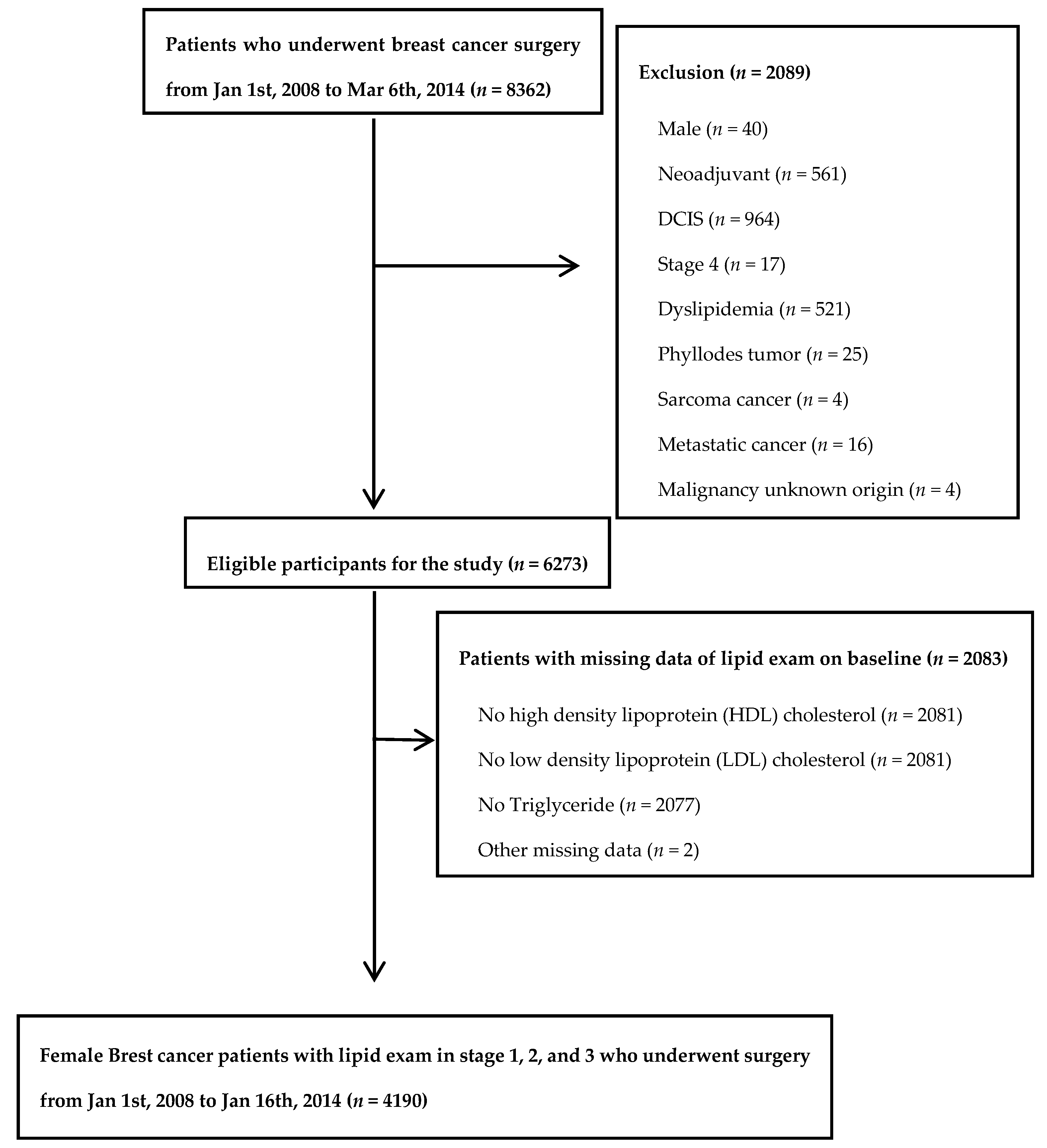
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Statistical Analysis
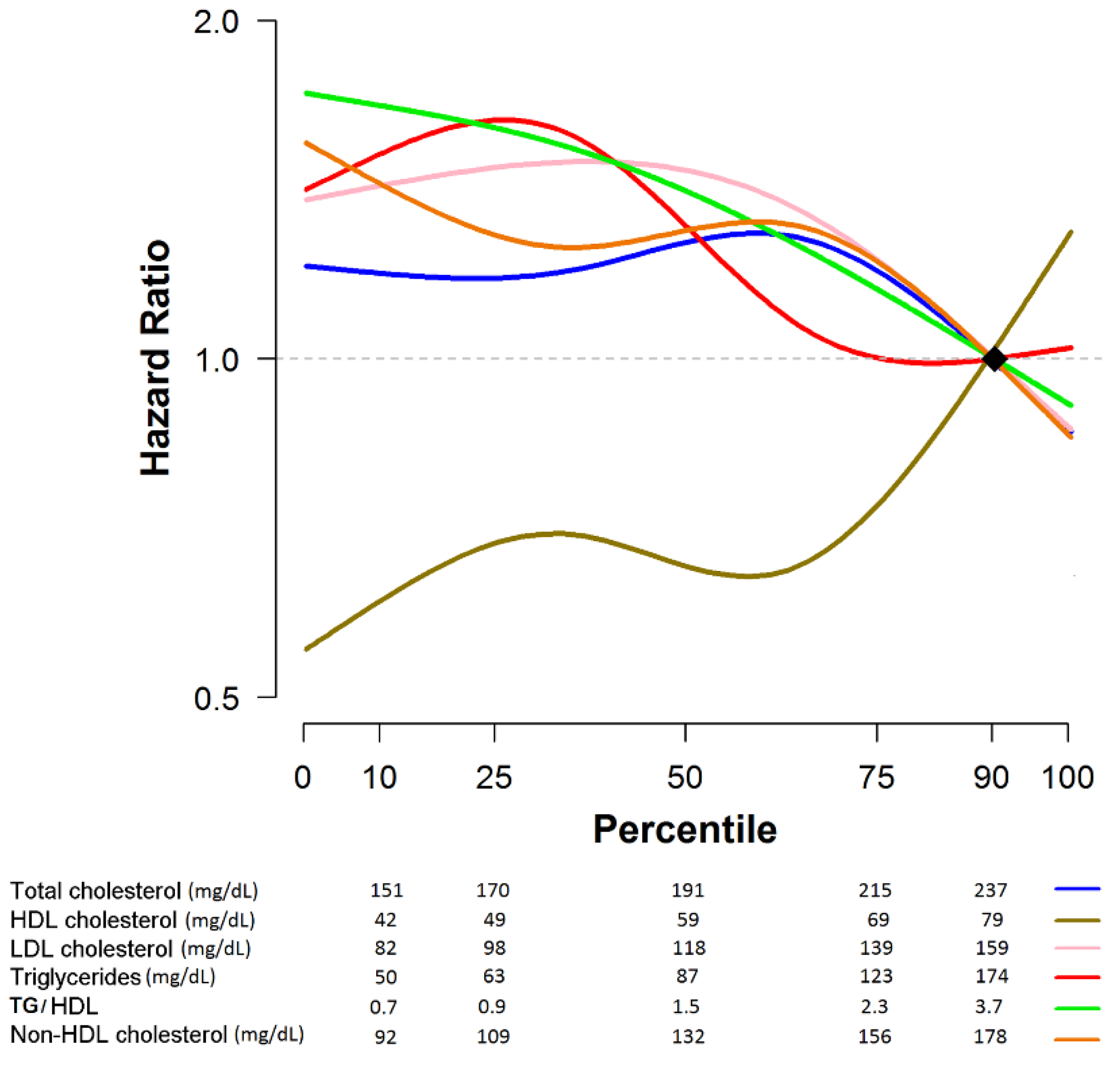
3. Results
3.1. Patients Characteristics
3.2. Prognostic Role of Lipid Profile with Regard to Disease Recurrence in Breast Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.K.; Noone, A.M.; Mariotto, A.B.; Simard, E.P.; Boscoe, F.P.; Henley, S.J.; Jemal, A.; Cho, H.; Anderson, R.N.; Kohler, B.A.; et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014, 120, 1290–1314. [Google Scholar] [CrossRef] [PubMed]
- Vuong, D.; Simpson, P.T.; Green, B.; Cummings, M.C.; Lakhani, S.R. Molecular classification of breast cancer. Virchows Arch. 2014, 465, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Smith-Warner, S.A.; Spiegelman, D.; Yaun, S.S.; van den Brandt, P.A.; Folsom, A.R.; Goldbohm, R.A.; Graham, S.; Holmberg, L.; Howe, G.R.; Marshall, J.R.; et al. Alcohol and breast cancer in women: A pooled analysis of cohort studies. JAMA 1998, 279, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Caan, B.J.; Natarajan, L.; Parker, B.; Gold, E.B.; Thomson, C.; Newman, V.; Rock, C.L.; Pu, M.; Al-Delaimy, W.; Pierce, J.P. Soy food consumption and breast cancer prognosis. Cancer Epidemiol. Prev. Biomark. 2011, 20, 854–858. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.K.; Levy, R.M.; Elliott, J.C.; Burnett, B.P. The effect of genistein aglycone on cancer and cancer risk: A review of in vitro, preclinical, and clinical studies. Nutr. Rev. 2009, 67, 398–415. [Google Scholar] [CrossRef]
- Reynolds, P.; Hurley, S.; Goldberg, D.E.; Anton-Culver, H.; Bernstein, L.; Deapen, D.; Horn-Ross, P.L.; Peel, D.; Pinder, R.; Ross, R.K.; et al. Active smoking, household passive smoking, and breast cancer: Evidence from the California Teachers Study. J. Natl. Cancer Inst. 2004, 96, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.S.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Dignam, J.J.; Wieand, K.; Johnson, K.A.; Fisher, B.; Xu, L.; Mamounas, E.P. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J. Natl. Cancer Inst. 2003, 95, 1467–1476. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Murai, T. The role of lipid rafts in cancer cell adhesion and migration. Int. J. Cell Biol. 2012, 2012, 763283. [Google Scholar] [CrossRef] [Green Version]
- Incardona, J.P.; Eaton, S. Cholesterol in signal transduction. Curr. Opin. Cell Biol. 2000, 12, 193–203. [Google Scholar] [CrossRef]
- Yadav, N.K.; Poudel, B.; Thanpari, C.; Chandra Koner, B. Assessment of biochemical profiles in premenopausal and postmenopausal women with breast cancer. Asian Pac. J. Cancer Prev. 2012, 13, 3385–3388. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, C.C.; Stuller, I.; Rausch, P.; Muller, C. Increased serum concentrations of cholesterol and triglycerides in the progression of breast cancer. J. Cancer Res. Clin. Oncol. 1988, 114, 514–518. [Google Scholar] [CrossRef]
- Owiredu, W.K.; Donkor, S.; Addai, B.W.; Amidu, N. Serum lipid profile of breast cancer patients. Pak. J. Biol. Sci. 2009, 12, 332–338. [Google Scholar] [CrossRef]
- Wallace, R.B.; Rost, C.; Burmeister, L.F.; Pomrehn, P.R. Cancer incidence in humans: Relationship to plasma lipids and relative weight. J. Natl. Cancer Inst. 1982, 68, 915–918. [Google Scholar]
- Emaus, A.; Veierod, M.B.; Tretli, S.; Finstad, S.E.; Selmer, R.; Furberg, A.S.; Bernstein, L.; Schlichting, E.; Thune, I. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res. Treat. 2010, 121, 651–660. [Google Scholar] [CrossRef]
- Ha, M.; Sung, J.; Song, Y.M. Serum t and the risk of breast cancer in postmenopausal Korean women. Cancer Causes Control 2009, 20, 1055–1060. [Google Scholar] [CrossRef]
- Hoyer, A.P.; Engholm, G. Serum lipids and breast cancer risk: A cohort study of 5207 Danish women. Cancer Causes Control 1992, 3, 403–408. [Google Scholar] [CrossRef]
- Hiatt, R.A.; Friedman, G.D.; Bawol, R.D.; Ury, H.K. Breast cancer and serum cholesterol. J. Natl. Cancer Inst. 1982, 68, 885–889. [Google Scholar] [PubMed]
- Gaard, M.; Tretli, S.; Urdal, P. Risk of breast cancer in relation to blood lipids: A prospective study of 31,209 Norwegian women. Cancer Causes Control 1994, 5, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Vatten, L.J.; Foss, O.P. Total serum cholesterol and triglycerides and risk of breast cancer: A prospective study of 24,329 Norwegian women. Cancer Res. 1990, 50, 2341–2346. [Google Scholar]
- Furberg, A.S.; Jasienska, G.; Bjurstam, N.; Torjesen, P.A.; Emaus, A.; Lipson, S.F.; Ellison, P.T.; Thune, I. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. Cancer Epidemiol. Prev. Biomark. 2005, 14, 33–40. [Google Scholar]
- Kucharska-Newton, A.M.; Rosamond, W.D.; Mink, P.J.; Alberg, A.J.; Shahar, E.; Folsom, A.R. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann. Epidemiol. 2008, 18, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Ray, G.; Husain, S.A. Role of lipids, lipoproteins and vitamins in women with breast cancer. Clin. Biochem. 2001, 34, 71–76. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.K.; Han, W.; Kim, D.H.; Hong, Y.C.; Ha, E.H.; Ahn, S.H.; Noh, D.Y.; Kang, D.; Yoo, K.Y. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol. Prev. Biomark. 2009, 18, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.J.; Hou, M.F.; Tsai, S.M.; Wu, S.H.; Hou, L.A.; Ma, H.; Shann, T.Y.; Wu, S.H.; Tsai, L.Y. The association between lipid profiles and breast cancer among Taiwanese women. Clin. Chem. Lab. Med. 2007, 45, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Bosetti, C.; Talamini, R.; Levi, F.; Montella, M.; Giacosa, A.; Negri, E.; La Vecchia, C. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann. Oncol. 2011, 22, 2687–2692. [Google Scholar] [CrossRef]
- Ferraroni, M.; Gerber, M.; Decarli, A.; Richardson, S.; Marubini, E.; Crastes de Paulet, P.; Crastes de Paulet, A.; Pujol, H. HDL-cholesterol and breast cancer: A joint study in northern Italy and southern France. Int. J. Epidemiol. 1993, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liu, H.; Gao, R. Serum Lipids and Breast Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0142669. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. Updated Breast Chapter for 8th Edition. Available online: https://cancerstaging.org/references-tools/deskreferences/Pages/Breast-Cancer-Staging.aspx (accessed on 13 March 2018).
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Cordero, A.; Andres, E.; Ordonez, B.; Leon, M.; Laclaustra, M.; Grima, A.; Luengo, E.; Moreno, J.; Bes, M.; Pascual, I.; et al. Usefulness of triglycerides-to-high-density lipoprotein cholesterol ratio for predicting the first coronary event in men. Am. J. Cardiol. 2009, 104, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- da Luz, P.L.; Favarato, D.; Faria-Neto, J.R., Jr.; Lemos, P.; Chagas, A.C. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics 2008, 63, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar] [CrossRef] [Green Version]
- Llaverias, G.; Danilo, C.; Mercier, I.; Daumer, K.; Capozza, F.; Williams, T.M.; Sotgia, F.; Lisanti, M.P.; Frank, P.G. Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 2011, 178, 402–412. [Google Scholar] [CrossRef]
- Bahl, M.; Ennis, M.; Tannock, I.F.; Hux, J.E.; Pritchard, K.I.; Koo, J.; Goodwin, P.J. Serum lipids and outcome of early-stage breast cancer: Results of a prospective cohort study. Breast Cancer Res. Treat. 2005, 94, 135–144. [Google Scholar] [CrossRef]
- Mousa, U.; Onur, H.; Utkan, G. Is obesity always a risk factor for all breast cancer patients? c-erbB2 expression is significantly lower in obese patients with early stage breast cancer. Clin. Transl. Oncol. 2012, 14, 923–930. [Google Scholar] [CrossRef]
- Ozdemir, B.H.; Akcali, Z.; Haberal, M. Hypercholesterolemia impairs angiogenesis in patients with breast carcinoma and, therefore, lowers the risk of metastases. Am. J. Clin. Pathol. 2004, 122, 696–703. [Google Scholar] [CrossRef]
- Sherwin, R.W.; Wentworth, D.N.; Cutler, J.A.; Hulley, S.B.; Kuller, L.H.; Stamler, J. Serum cholesterol levels and cancer mortality in 361,662 men screened for the Multiple Risk Factor Intervention Trial. JAMA 1987, 257, 943–948. [Google Scholar] [CrossRef]
- Keys, A.; Aravanis, C.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Menotti, A.; Nedeljkovic, S.; Punsar, S.; et al. Serum cholesterol and cancer mortality in the Seven Countries Study. Am. J. Epidemiol. 1985, 121, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.W. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012, 21, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.J.; Sedlacek, S.M.; Paul, D.; Wolfe, P.; McGinley, J.N.; Playdon, M.C.; Daeninck, E.A.; Bartels, S.N.; Wisthoff, M.R. Effect of dietary patterns differing in carbohydrate and fat content on blood lipid and glucose profiles based on weight-loss success of breast-cancer survivors. Breast Cancer Res. 2012, 14, R1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Imai, K.; Masuda, T.; Abe, S.; Nakao, H.; Tanaka, M.; Nakamura, M. Relationship between serum total cholesterol level and nutritional status in Japanese young female. Nutr. Res. 1999, 19, 1145–1152. [Google Scholar] [CrossRef]
- Wang, J.; Hong, Z. Low Plasma Total Cholesterol Concentration: A Sensitive Evaluation Marker in Hospitalized Patients with Nutritional Deficiency Malnutrition. J. Food Nutr. Res. 2014, 2, 551–555. [Google Scholar] [CrossRef]
- Kawai, M.; Tomotaki, A.; Miyata, H.; Iwamoto, T.; Niikura, N.; Anan, K.; Hayashi, N.; Aogi, K.; Ishida, T.; Masuoka, H.; et al. Body mass index and survival after diagnosis of invasive breast cancer: A study based on the Japanese National Clinical Database-Breast Cancer Registry. Cancer Med. 2016, 5, 1328–1340. [Google Scholar] [CrossRef] [Green Version]
- Moon, H.G.; Han, W.; Noh, D.Y. Underweight and breast cancer recurrence and death: A report from the Korean Breast Cancer Society. J. Clin. Oncol. 2009, 27, 5899–5905. [Google Scholar] [CrossRef]
- Ahern, T.P.; Pedersen, L.; Tarp, M.; Cronin-Fenton, D.P.; Garne, J.P.; Silliman, R.A.; Sorensen, H.T.; Lash, T.L. Statin prescriptions and breast cancer recurrence risk: A Danish nationwide prospective cohort study. J. Natl. Cancer Inst. 2011, 103, 1461–1468. [Google Scholar] [CrossRef] [Green Version]
- Kwan, M.L.; Habel, L.A.; Flick, E.D.; Quesenberry, C.P.; Caan, B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res. Treat. 2008, 109, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Harputluoglu, H.; Dizdar, O.; Karaahmet, F.; Altundag, K. Post-diagnosis statin use and breast recurrence sites in early stage breast cancer survivors. Breast Cancer Res. Treat. 2008, 110, 541. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Bertoni, A.G.; Kramer, H.; Bonds, D.; Blumenthal, R.S.; Tsai, M.Y.; Psaty, B.M. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): Gender, ethnicity, and coronary artery calcium. Circulation 2006, 113, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Potter, D.; Ming, E.E. Prevalence of lipid abnormalities in the United States: The National Health and Nutrition Examination Survey 2003–2006. J. Clin. Lipidol. 2012, 6, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Vitols, S.; Gahrton, G.; Ost, A.; Peterson, C. Elevated low density lipoprotein receptor activity in leukemic cells with monocytic differentiation. Blood 1984, 63, 1186–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, C.; Vitols, S.; Rudling, M.; Blomgren, H.; Edsmyr, F.; Skoog, L. Hypocholesterolemia in cancer patients may be caused by elevated LDL receptor activities in malignant cells. Med. Oncol. Tumor Pharmacother. 1985, 2, 143. [Google Scholar] [CrossRef]


| Overall (N = 4190) | |
|---|---|
| N (%) | |
| Age, mean (SD) | 51.7 (9.8) |
| BMI, mean (SD) | 23.6 (3.2) |
| Blood lab, mean (SD) | |
| Total cholesterol (110–240) (mg/dL) | 193.3 (33.7) |
| Triglyceride (50–200) (mg/dL) | 102.8 (61.4) |
| HDL cholesterol (45–65) (mg/dL) | 59.9 (14.8) |
| LDL cholesterol (40–130) (mg/dL) | 119.4 (29.8) |
| TG/HDL | 2.0 (1.7) |
| Non-HDL cholesterol | 133.4 (33.7) |
| Menopausal status | |
| Yes | 2217 (52.9) |
| No | 1962 (46.8) |
| Unknown | 11 (0.3) |
| Comorbidity (n = 2946) * | |
| No | 2310 (78.4) |
| Yes | 636 (21.6) |
| Surgery type (n = 4188) | |
| >Mastectomy | 1166 (27.8) |
| BCS | 3022 (72.2) |
| Stage | |
| I | 2050 (48.9) |
| II | 1679 (40.1) |
| III | 461 (11.0) |
| ER | |
| Positive | 3155 (75.3) |
| Negative | 1032 (24.6) |
| Unknown | 3 (0.1) |
| PR | |
| Positive | 2894 (69.1) |
| Negative | 1293 (30.9) |
| Unknown | 3 (0.1) |
| HER2 | |
| Positive | 842 (20.1) |
| Negative | 3275 (78.2) |
| Unknown | 73 (1.7) |
| Subtype | |
| Luminal A | 2737 (65.3) |
| Luminal B | 406 (9.7) |
| HER2 type | 436 (10.4) |
| TNBC | 538 (12.8) |
| Unknown | 73 (1.7) |
| LVI | |
| No | 3018 (72.0) |
| Yes | 1149 (27.4) |
| Unknown | 23 (0.6) |
| Multiplicity | |
| No | 3274 (78.1) |
| Yes | 913 (21.8) |
| Unknown | 3 (0.1) |
| Nuclear grade | |
| Low | 847 (20.2) |
| Intermediate | 1942 (46.4) |
| High | 1393 (33.3) |
| Unknown | 8 (0.2) |
| Chemotherapy | |
| No | 1532 (36.6) |
| Yes | 2586 (61.7) |
| Unknown | 72 (1.7) |
| Radiotherapy | |
| No | 882 (21.1) |
| Yes | 3205 (76.5) |
| Unknown | 103 (2.5) |
| Hormone therapy | |
| No | 781 (18.6) |
| Yes | 3409 (81.4) |
| AI ‡ | 1439 (34.3) |
| SERM § | 1887 (45.0) |
| Patients with Events | Crude | Adjusted | |||
|---|---|---|---|---|---|
| Hazard Ratio | p-Value | Hazard Ratio | p-Value | ||
| (95% CI) | (95% CI) | ||||
| Total cholesterol | |||||
| Quartile I | 34 | 1.49 (0.89, 2.48) | 0.13 | 1.34 (0.78, 2.30) | 0.28 |
| Quartile II | 29 | 1.18 (0.70, 2.01) | 0.54 | 1.14 (0.66, 1.96) | 0.63 |
| Quartile III | 36 | 1.60 (0.96, 2.65) | 0.07 | 1.47 (0.88, 2.45) | 0.14 |
| Quartile IV | 26 | Reference | Reference | ||
| LDL cholesterol | |||||
| Quartile I | 33 | 1.68 (0.97, 2.90) | 0.06 | 1.71 (0.96, 3.06) | 0.07 |
| Quartile II | 35 | 1.72 (0.99, 2.95) | 0.05 | 1.62 (0.92, 2.83) | 0.09 |
| Quartile III | 36 | 1.88 (1.09, 3.22) | 0.02 | 1.88 (1.09, 3.27) | 0.02 |
| Quartile IV | 21 | Reference | Reference | ||
| HDL cholesterol | |||||
| Quartile I | 33 | 0.58 (0.37, 0.93) | 0.02 | 0.54 (0.33, 0.88) | 0.01 |
| Quartile II | 33 | 0.65 (0.41, 1.04) | 0.07 | 0.59 (0.37, 0.96) | 0.03 |
| Quartile III | 20 | 0.43 (0.25, 0.75) | <0.001 | 0.45 (0.26, 0.77) | < 0.01 |
| Quartile IV | 39 | Reference | Reference | ||
| Triglyceride | |||||
| Quartile I | 34 | 1.75 (0.05, 2.93) | 0.03 | 1.88 (1.07, 3.29) | 0.03 |
| Quartile II | 34 | 1.55 (0.93, 2.59) | 0.09 | 1.66 (0.97, 2.82) | 0.06 |
| Quartile III | 31 | 1.36 (0.81, 2.29) | 0.25 | 1.32 (0.78, 2.25) | 0.31 |
| Quartile IV | 26 | Reference | Reference | ||
| TG/HDL | |||||
| Quartile I | 32 | 1.59 (0.95, 2.64) | 0.08 | 1.82 (1.04, 3.21) | 0.04 |
| Quartile II | 30 | 1.40 (0.83, 2.35) | 0.21 | 1.57 (0.91, 2.73) | 0.11 |
| Quartile III | 35 | 1.43 (0.87, 2.35) | 0.16 | 1.45 (0.87, 2.42) | 0.16 |
| Quartile IV | 28 | Reference | Reference | ||
| Non-HDL cholesterol | |||||
| Quartile I | 31 | 1.41 (0.85, 2.35) | 0.19 | 1.53 (0.89, 2.66) | 0.13 |
| Quartile II | 32 | 1.37 (0.83, 2.28) | 0.22 | 1.45 (0.85, 2.48) | 0.17 |
| Quartile III | 34 | 1.47 (0.89, 2.43) | 0.13 | 1.55 (0.92, 2.61) | 0.10 |
| Quartile IV | 28 | Reference | Reference | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.M.; Kang, D.; Guallar, E.; Yu, J.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Cho, J.; Lee, S.K. Impact of Serum Lipid on Breast Cancer Recurrence. J. Clin. Med. 2020, 9, 2846. https://doi.org/10.3390/jcm9092846
Jung SM, Kang D, Guallar E, Yu J, Lee JE, Kim SW, Nam SJ, Cho J, Lee SK. Impact of Serum Lipid on Breast Cancer Recurrence. Journal of Clinical Medicine. 2020; 9(9):2846. https://doi.org/10.3390/jcm9092846
Chicago/Turabian StyleJung, Sung Mi, Danbee Kang, Eliseo Guallar, Jonghan Yu, Jeong Eon Lee, Seok Won Kim, Seok Jin Nam, Juhee Cho, and Se Kyung Lee. 2020. "Impact of Serum Lipid on Breast Cancer Recurrence" Journal of Clinical Medicine 9, no. 9: 2846. https://doi.org/10.3390/jcm9092846
APA StyleJung, S. M., Kang, D., Guallar, E., Yu, J., Lee, J. E., Kim, S. W., Nam, S. J., Cho, J., & Lee, S. K. (2020). Impact of Serum Lipid on Breast Cancer Recurrence. Journal of Clinical Medicine, 9(9), 2846. https://doi.org/10.3390/jcm9092846





