Correlation of Arterial CO2 and Respiratory Impedance Values among Subjects with COPD
Abstract
1. Introduction
2. Methods
2.1. Participating COPD Subjects
2.2. Arterial Blood Gas Analysis
2.3. Pulmonary Function Test and FOT
2.4. Statistical Analysis
3. Results
3.1. Characteristics of COPD Subjects
3.2. Pulmonary Function Test and FOT Values of Enrolled Subjects with COPD
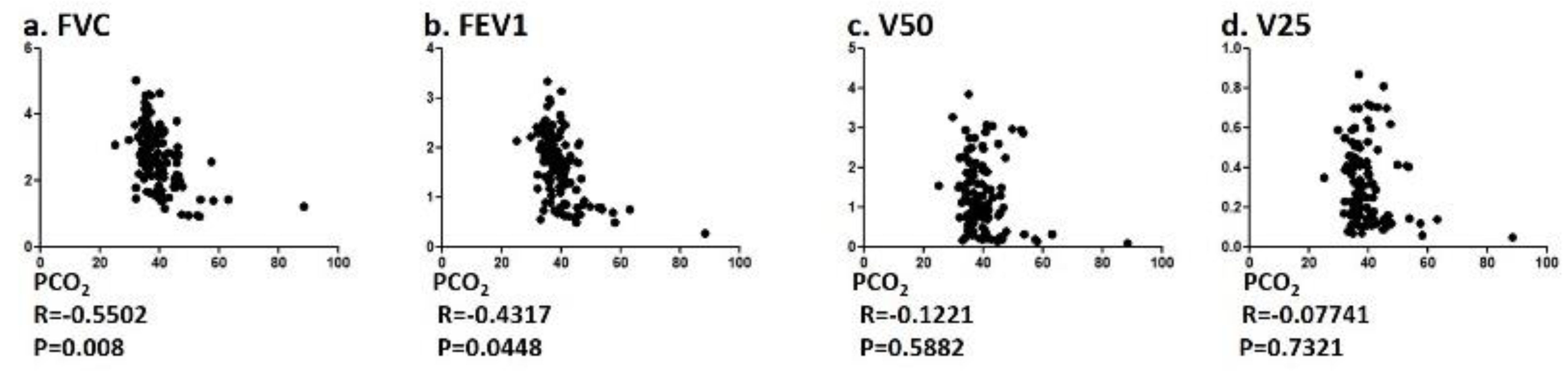
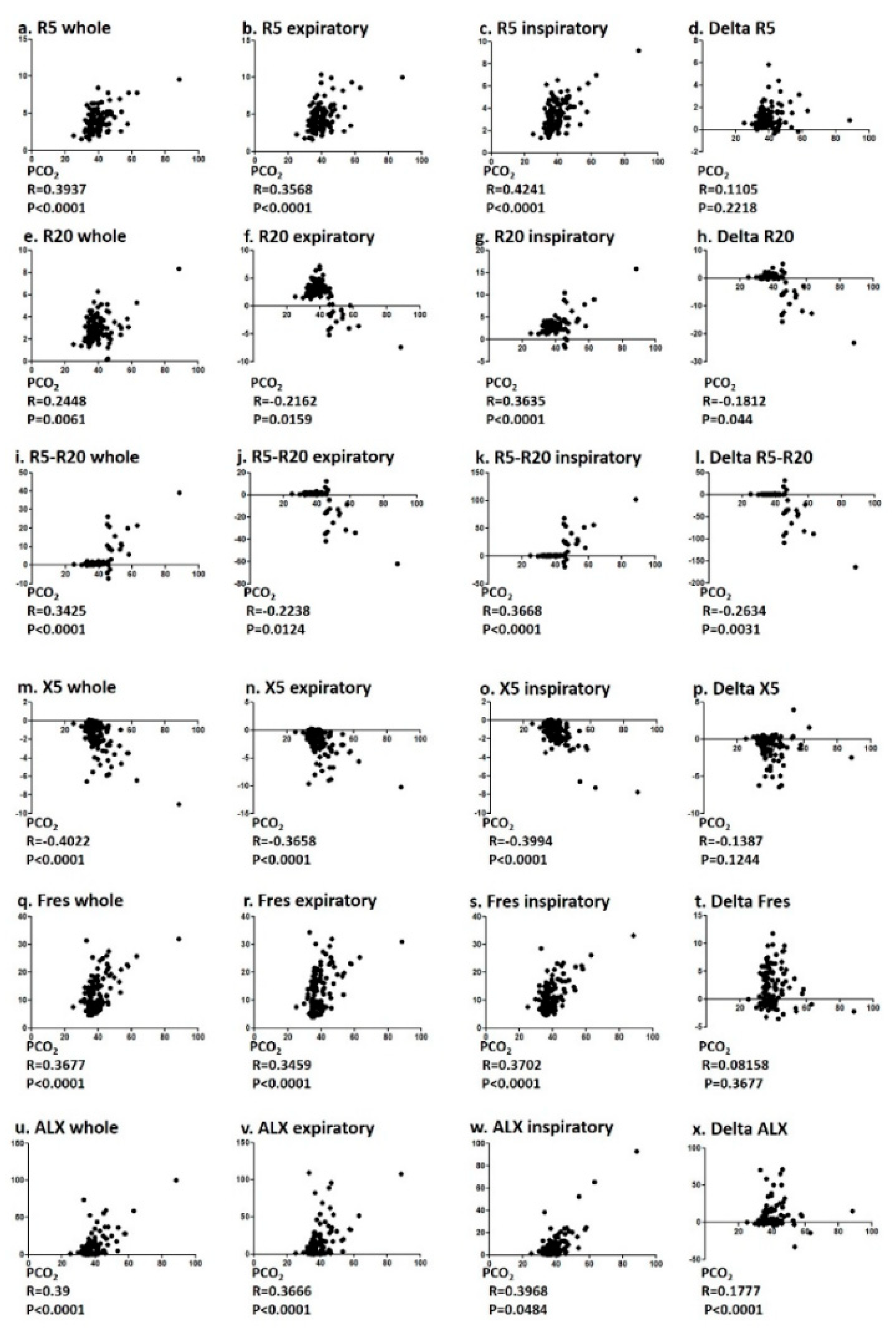
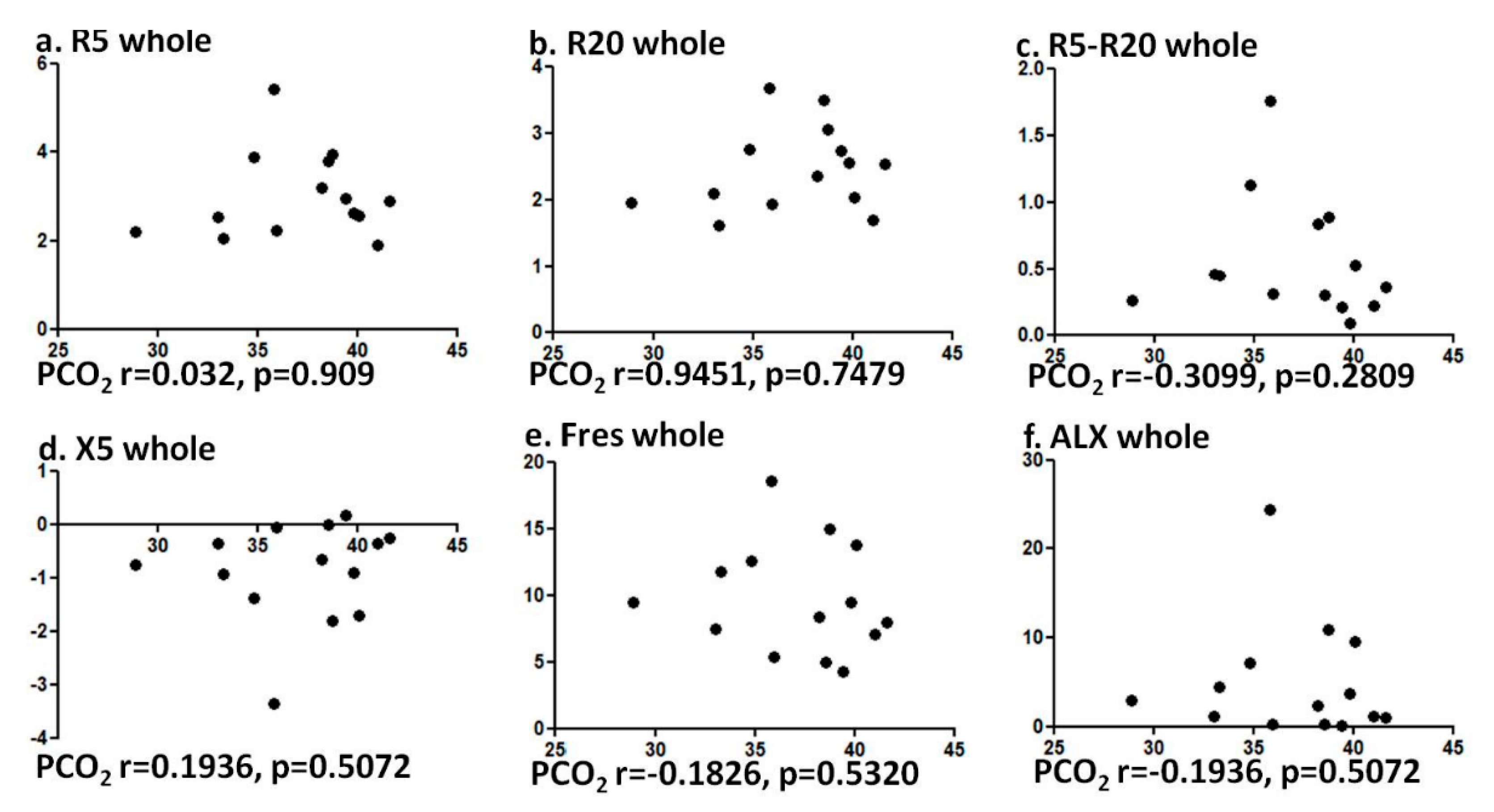
3.3. Correlation of PCO2 and Pulmonary Function Test and FOT Values
3.4. Multiple Linear Regression Analysis of Clinical Variables Associated with Hypercapnia
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calverley, P.M.; Celli, B.R.; Coxson, H.; Edwards, L.; Lomas, D.A.; MacNee, W.; Miller, B.E.; Rennard, S.I.; Silverman, E.C.; Agustí, A. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir. Res. 2010, 11, 122. [Google Scholar] [CrossRef]
- Terry, P.D.; Dhand, R. Inhalation therapy for stable COPD: 20 Years of GOLD reports. Adv. Ther. 2020, 37, 1812–1828. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Gea, J.; Faner, R. Biomarkers, the control panel and personalized COPD medicine. Respirology 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Tanaka, A.; Homma, T.; Kaneko, K.; Uno, T.; Sato, H.; Manabe, R.; Ohta, S.; Kusumoto, S.; Yamaguchi, F.; et al. Comparison of three frailty models and a sarcopenia model in elderly patients with chronic obstructive pulmonary disease. Geriatr. Gerontol. Int. 2019, 19, 896–901. [Google Scholar] [CrossRef]
- Singh, D.; Criner, G.J.; Naya, I.; Jones, P.W.; Tombs, L.; Lipson, D.A.; Han, M.K. Measuring disease activity in COPD: Is clinically important deterioration the answer? Respir. Res. 2020, 21, 134. [Google Scholar] [CrossRef]
- Shigemura, M.; Lecuona, E.; Sznajder, J.I. Effects of hypercapnia on the lung. J. Physiol. 2017, 595, 2431–2437. [Google Scholar] [CrossRef]
- Vadász, I.; Hubmayr, R.D.; Nin, N.; Sporn, P.H.S.; Sznajder, J.I. Hypercapnia: A nonpermissive environment for the lung. Am. J. Respir. Cell Mol. Boil. 2012, 46, 417–421. [Google Scholar] [CrossRef]
- Shigemura, M.; Lecuona, E.; Angulo, M.; Dada, L.A.; Edwards, M.B.; Welch, L.C.; Casalino-Matsuda, S.M.; Sporn, P.H.S.; Vadász, I.; Helenius, I.T.; et al. Elevated CO2 regulates the Wnt signaling pathway in mammals, Drosophila melanogaster and Caenorhabditis elegans. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Shigemura, M.; Lecuona, E.; Angulo, M.; Homma, T.; Rodríguez, D.A.; Gonzalez-Gonzalez, F.J.; Welch, L.C.; Amarelle, L.; Kim, S.-J.; Kaminski, N.; et al. Hypercapnia increases airway smooth muscle contractility via caspase-7-mediated miR-133a-RhoA signaling. Sci. Transl. Med. 2018, 10, eaat1662. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Boil. 2009, 11, 50–61. [Google Scholar] [CrossRef]
- Kamenetsky, M.; Middelhaufe, S.; Bank, E.M.; Levin, L.R.; Buck, J.; Steegborn, C. Molecular details of cAMP generation in mammalian cells: A tale of two systems. J. Mol. Boil. 2006, 362, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Tochino, Y.; Chibana, K.; Trudeau, J.B.; Holguin, F.; Wenzel, S. Nitric oxide and related enzymes in asthma: Relation to severity, enzyme function and inflammation. Clin. Exp. Allergy 2011, 42, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; López-Barneo, J.; Buckler, K.J.; Archer, S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2005, 353, 2042–2055. [Google Scholar] [CrossRef]
- Holmes, A.P.; Nunes, A.R.; Cann, M.; Kumar, P. Ecto-5′-nucleotidase, adenosine and transmembrane adenylyl cyclase signalling regulate basal carotid body chemoafferent outflow and establish the sensitivity to hypercapnia. Adv. Exp. Med. Biol. 2015, 860, 279–289. [Google Scholar] [CrossRef]
- Vohwinkel, C.U.; Lecuona, E.; Sun, H.; Sommer, N.; Vadász, I.; Chandel, N.S.; Sznajder, J.I. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J. Boil. Chem. 2011, 286, 37067–37076. [Google Scholar] [CrossRef] [PubMed]
- Van Vu, G.; Ha, G.H.; Nguyen, C.T.; Vu, G.T.; Pham, H.Q.; Latkin, C.A.; Tran, B.X.; Ho, R.; Ho, C.S.H. Interventions to improve the quality of life of patients with chronic obstructive pulmonary disease: A global mapping during 1990–2018. Int. J. Environ. Res. Public Heal. 2020, 17, 3089. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Strassmann, S.; Schwarz, S.B.; Merten, M.; Fan, E.; Beck, J.; Sinderby, C.; Windisch, W. Control of respiratory drive by extracorporeal CO2 removal in acute exacerbation of COPD breathing on non-invasive NAVA. Crit. Care 2019, 23, 135. [Google Scholar] [CrossRef]
- Hickling, K.G.; Walsh, J.; Henderson, S.; Jackson, R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: A prospective study. Crit. Care Med. 1994, 22, 1568–1578. [Google Scholar] [CrossRef]
- Amato, M.B.P.; Barbas, C.S.V.; Medeiros, D.M.; Magaldi, R.B.; Schettino, G.P.; Lorenzi-Filho, G.; Kairalla, R.A.; Deheinzelin, D.; Muñoz, C.; Oliveira, R.; et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998, 338, 347–354. [Google Scholar] [CrossRef]
- Hitomi, Y.; Tanaka, A.; Yasunari, K.; Mikuni, H.; Tomoko, K.; Ohta, S.; Yamamoto, M.; Suzuki, S.; Ohnishi, T.; Sagara, H.; et al. Association between respiratory impedance measured by forced oscillation technique and exacerbations in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 13, 79–89. [Google Scholar] [CrossRef]
- Oostveen, E.; Macleod, D.; Lorino, H.; Farré, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Shirai, T.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Fujii, M.; Hozumi, H.; Suda, T.; et al. Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respir. Physiol. Neurobiol. 2013, 185, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.B.; Brody, A.W.; Lewis, D.H.; Burgess, B.F. Oscillation mechanics of lungs and chest in man. J. Appl. Physiol. 1956, 8, 587–594. [Google Scholar] [CrossRef]
- Lipworth, B.J.; Jabbal, S. What can we learn about COPD from impulse oscillometry? Respir. Med. 2018, 139, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Mori, K.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Fujii, M.; Suda, T.; Chida, K. Respiratory mechanics and peripheral airway inflammation and dysfunction in asthma. Clin. Exp. Allergy 2013, 43, 521–526. [Google Scholar] [CrossRef]
- Fujii, M.; Shirai, T.; Mori, K.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Suda, T. Inspiratory resonant frequency of forced oscillation technique as a predictor of the composite physiologic index in interstitial lung disease. Respir. Physiol. Neurobiol. 2015, 207, 22–27. [Google Scholar] [CrossRef]
- Paredi, P.; Goldman, M.; Alamen, A.; Ausin, P.; Usmani, O.S.; Pride, N.B.; Barnes, P.J. Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax 2010, 65, 263–267. [Google Scholar] [CrossRef]
- Zimmermann, S.C.; Huvanandana, J.; Nguyen, C.D.; Bertolin, A.; Watts, J.C.; Gobbi, A.; Farah, C.S.; Peters, M.J.; Dellaca, R.L.; King, G.G.; et al. Day-to-day variability of forced oscillatory mechanics for early detection of acute exacerbations in COPD. Eur. Respir. J. 2020, 1901739. [Google Scholar] [CrossRef]
- Zannin, E.; Nyilas, S.; Ramsey, K.; Latzin, P.; Dellaca, R.L. Within-breath changes in respiratory system impedance in children with cystic fibrosis. Pediatr. Pulmonol. 2019, 54, 737–742. [Google Scholar] [CrossRef]
- Kregenow, D.A.; Rubenfeld, G.D.; Hudson, L.D.; Swenson, E.R. Hypercapnic acidosis and mortality in acute lung injury. Crit. Care Med. 2006, 34, 1–7. [Google Scholar] [CrossRef]
- American Thoracic Society. Standardization of spirometry, 1994. Am. J. Respir. Crit. Care Med. 1995, 152, 1107–1136. [Google Scholar] [CrossRef] [PubMed]
- Brashier, B.; Salvi, S. Measuring lung function using sound waves: Role of the forced oscillation technique and impulse oscillometry system. Breathe 2015, 11, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Galant, S.P.; Komarow, H.D.; Shin, H.-W.; Siddiqui, S.; Lipworth, B.J.; Hye-Won, S. The case for impulse oscillometry in the management of asthma in children and adults. Ann. Allergy Asthma Immunol. 2017, 118, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Ward, J.; Lipworth, B.J. Comparison of bronchodilator response in patients with asthma and healthy subjects using spirometry and oscillometry. Ann. Allergy Asthma Immunol. 2011, 107, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Rentzhog, C.H.; Janson, C.; Berglund, L.; Borres, M.P.; Nordvall, L.; Alving, K.; Malinovschi, A. Overall and peripheral lung function assessment by spirometry and forced oscillation technique in relation to asthma diagnosis and control. Clin. Exp. Allergy 2017, 47, 1546–1554. [Google Scholar] [CrossRef]
- Mori, K.; Shirai, T.; Mikamo, M.; Shishido, Y.; Akita, T.; Morita, S.; Asada, K.; Fujii, M.; Suda, T.; Chida, K. Colored 3-dimensional analyses of respiratory resistance and reactance in COPD and asthma. COPD J. Chronic Obstr. Pulm. Dis. 2011, 8, 456–463. [Google Scholar] [CrossRef]
- Matsumoto, H.; Niimi, A.; Jinnai, M.; Nakaji, H.; Takeda, T.; Oguma, T.; Otsuka, K.; Inoue, H.; Yamaguchi, M.; Matsuoka, H.; et al. Association of alveolar nitric oxide levels with pulmonary function and its reversibility in stable asthma. Respiration 2011, 81, 311–317. [Google Scholar] [CrossRef]
- Williamson, P.A.; Clearie, K.; Menzies, D.; Vaidyanathan, S.; Lipworth, B.J. Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Lung 2010, 189, 121–129. [Google Scholar] [CrossRef]
- Wei, X.; Shi, Z.; Cui, Y.; Mi, J.; Ma, Z.; Ren, J.; Li, J.; Xu, S.; Guo, Y.-M. Impulse oscillometry system as an alternative diagnostic method for chronic obstructive pulmonary disease. Medicine 2017, 96, e8543. [Google Scholar] [CrossRef]
- Crim, C.; Celli, B.R.; Edwards, L.; Wouters, E.; Coxson, H.O.; Tal-Singer, R.; Calverley, P.M. Respiratory system impedance with impulse oscillometry in healthy and COPD subjects: ECLIPSE baseline results. Respir. Med. 2011, 105, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Haruna, A.; Oga, T.; Muro, S.; Ohara, T.; Sato, S.; Marumo, S.; Kinose, D.; Terada, K.; Nishioka, M.; Ogawa, E.; et al. Relationship between peripheral airway function and patient-reported outcomes in COPD: A cross-sectional study. BMC Pulm. Med. 2010, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Nihlen, U.; Dencker, M.; Engstrom, G.; Lofdahl, C.G.; Wollmer, P. Impulseoscillometry may be of value in detecting early manifestations of COPD. Respir. Med. 2012, 106, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann. Intern. Med. 1980, 93, 391–398. [Google Scholar] [CrossRef]
- Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Part. Lancet 1981, 317, 681–686. [CrossRef]
- Long-Term Oxygen Treatment Trial Research Group; Albert, R.K.; Au, D.H.; Blackford, A.L.; Casaburi, R.; Cooper, J.A.; Criner, G.J.; Diaz, P.; Fuhlbrigge, A.L.; Gay, S.E.; et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N. Engl. J. Med. 2016, 375, 1617–1627. [Google Scholar] [CrossRef]
- Köhnlein, T.; Windisch, W.; Köhler, D.; Drabik, A.; Geiseler, J.; Hartl, S.; Karg, O.; Laier-Groeneveld, G.; Nava, S.; Schönhofer, B.; et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respir. Med. 2014, 2, 698–705. [Google Scholar] [CrossRef]
- Suh, E.S.; Pompilio, P.; Mandal, S.; Hill, P.; Kaltsakas, G.; Murphy, P.B.; Romano, R.; Moxham, J.; Dellaca, R.; Hart, N. Auto-titrating external positive end-expiratory airway pressure to abolish expiratory flow limitation during tidal breathing in patients with severe chronic obstructive pulmonary disease: A physiological study. Eur. Respir. J. 2020. [Google Scholar] [CrossRef]
- Contreras, M.; Masterson, C.; Laffey, J. Permissive hypercapnia. Curr. Opin. Anaesthesiol. 2015, 28, 26–37. [Google Scholar] [CrossRef]
- Vadász, I.; Dada, L.A.; Briva, A.; Trejo, H.E.; Welch, L.C.; Chen, J.; Toth, P.T.; Lecuona, E.; Witters, L.A.; Schumacker, P.T.; et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J. Clin. Investig. 2008, 118, 752–762. [Google Scholar] [CrossRef]
- Gates, K.L.; Howell, H.A.; Nair, A.; Vohwinkel, C.U.; Welch, L.C.; Beitel, G.J.; Hauser, A.R.; Sznajder, J.I.; Sporn, P.H.S. Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am. J. Respir. Cell Mol. Boil. 2013, 49, 821–828. [Google Scholar] [CrossRef]
- Casalino-Matsuda, S.M.; Nair, A.; Beitel, G.J.; Gates, K.L.; Sporn, P.H.S. Hypercapnia inhibits autophagy and bacterial killing in human macrophages by increasing expression of Bcl-2 and Bcl-xL. J. Immunol. 2015, 194, 5388–5396. [Google Scholar] [CrossRef] [PubMed]
- Dada, L.A.; Bittar, H.E.T.; Welch, L.C.; Vagin, O.; Deiss-Yehiely, N.; Kelly, A.M.; Baker, M.R.; Capri, J.; Cohn, W.; Whitelegge, J.P.; et al. High CO2Leads to Na,K-ATPase endocytosis via c-Jun amino-terminal kinase-induced LMO7b phosphorylation. Mol. Cell. Boil. 2015, 35, 3962–3973. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bharat, A.; Graf, N.; Mullen, A.; Kanter, J.; Andrei, A.-C.; Sporn, P.H.S.; DeCamp, M.M.; Sznajder, J.I. Pleural hypercarbia after lung surgery is associated with persistent alveolopleural fistulae. Chest 2016, 149, 220–227. [Google Scholar] [CrossRef] [PubMed]
| Hypercapnia | Normocapnia | p Value | |
|---|---|---|---|
| (n = 22) | (n = 102) | ||
| Age, years-old | 76.5 (68.25–80.75) | 73 (67.25–77.75) | 0.245 |
| Gender, male/female, n | 9/13 | 74/28 | 0.303 |
| Smoking history, pack-years | 55 (32.5–105.12) | 54 (39–91.87) | 0.816 |
| Hight, cm | 157.35 (151.72–168) | 161 (154.37–167.22) | 0.786 |
| Body weight, kg | 52.85 (48.17–67.25) | 57.9 (50.15–68.8) | 0.252 |
| BMI, kg/m2 | 20.88 (18.66–25.60) | 23.36 (20.11–25.74) | 0.18 |
| Body surface area, m2/kg2 | 1.53 (1.45–1.69) | 1.58 (1.47–1.75) | 0.308 |
| Arterial blood gas | |||
| pH | 7.42 (7.39–7.42) | 7.44 (7.43–7.46) | <0.001 |
| pCO2, mmHg | 46.60 (45.7–53.2) | 36.55 (35–39.7) | <0.001 |
| pO2, mmHg | 71.2 (67.8–84.52) | 84.55 (75.62–90.45) | <0.003 |
| HCO3-, mmol/L | 30 (27.97–32.52) | 24.9 (23.32–26.37) | <0.001 |
| BE, mmol/L | 4.1 (2.95–7.5) | 0.9 (−0.3–2.2) | <0.001 |
| tCO2, mmHg | 26 (24–27.7) | 30.75 (29.2–33.82) | <0.001 |
| Hypercapnia | Normocapnia | p Value | |
|---|---|---|---|
| (n = 22) | (n = 102) | ||
| FVC, L | 1.97 (1.40–2.57) | 2.89 (2.33–3.47) | <0.001 |
| %FVC, % | 65.85 (51.40–82) | 97.3 (84.85–110.07) | <0.001 |
| FEV1, L | 0.8 (0.66–1.31) | 1.73 (1.32–2.19) | <0.001 |
| %FEV1, % | 36.82 (29.1–62.95) | 74.7 (55.12–84.45) | <0.001 |
| FEV1%, % | 53.71 (31.88–68.94) | 63.15 (50.84–71.19) | 0.228 |
| PEF, L | 2.98 (2.54–4.18) | 5.11 (3.76–6.69) | 0.781 |
| %PEF, % | 39.20 (29.55–39.20) | 69.35 (48.02–81.15) | <0.001 |
| V50, L | 0.58 (0.21–1.43) | 1.15 (0.65–1.89) | 0.044 |
| %V50, % | 18.35 (7.15–67.75) | 35.05 (19.27–61.4) | 0.101 |
| V25, L | 0.14 (0.12–0.34) | 0.26 (0.16–0.42) | 0.006 |
| %V25, % | 18.1 (10.72–29.27) | 27.2 (16.22–39.57) | 0.015 |
| Hypercapnia | Normocapnia | p Value | |
|---|---|---|---|
| (n = 22) | (n = 102) | ||
| R5, cmH2O/L/s | |||
| Whole breath | 5.05 (3.58–6.43) | 3.50 (2.72–4.32) | 0.001 |
| Inspiratory | 5.56 (3.62–7.75) | 3.91 (3.00–5.02) | 0.004 |
| Exspiratory | 4.32 (3.28–5.07) | 3.09 (2.34–3.83) | 0.005 |
| delta R5 | 1.17 (0.25–2.07) | 0.77 (0.47–1.29) | 0.001 |
| R20, cmH2O/L/s | |||
| Whole breath | 2.78 (2.01–3.77) | 2.56 (2.21–3.31) | 0.891 |
| Inspiratory | −1.47 (−3.45–0.22) | 2.87 (2.33–3.62) | <0.001 |
| Exspiratory | 3.94 (1.87–7.51) | 2.34 (1.90–3.06) | 0.009 |
| delta R20 | −5.46 (−11.25–−0.21) | 0.41 (0.22–0.75) | <0.001 |
| R5–R20, cmH2O/L/s | |||
| Whole breath | 9.29 (2.54–18.76) | 0.80 (0.53–1.19) | <0.001 |
| Inspiratory | −14.75 (−30.01–−1.01) | 1.00 (0.66–1.47) | <0.001 |
| Exspiratory | 24.04 (3.71–48.77) | 0.57 (0.34–0.89) | <0.001 |
| delta (R5-R20) | −38.78 (−78.77–−2.98) | 0.38 (0.18–0.58) | <0.001 |
| X5, cmH2O/L/s | |||
| Whole breath | −2.66 (−4.51–−1.12) | −0.74 (−1.90–−0.37) | <0.001 |
| Inspiratory | −3.34 (−5.26–−1.09) | −0.78 (−2.41–−0.35) | <0.001 |
| Exspiratory | −1.96 (−3.01–−1.16) | −0.74 (−1.34–−0.39) | <0.001 |
| delta X5 | −0.79 (−2.29–0.09) | −0.09 (−0.96–0.18) | 0.108 |
| Fres, Hz | |||
| Whole breath | 19.03 (11.99–22.57) | 9.90 (7.44–15.05) | <0.001 |
| Inspiratory | 20.87 (12.16–23.89) | 10.47 (7.46–16.73) | <0.001 |
| Exspiratory | 16.76 (11.66–22.18) | 9.81 (7.34–13.04) | <0.001 |
| delta Fres | 2.34 (0.89–4.35) | 1.18 (−0.62–4.14) | 0.431 |
| ALX, cmH2O/L/s Hz | |||
| Whole breath | 22.70 (5.17–36.94) | 2.91 (1.24–12.16) | <0.001 |
| Inspiratory | 29.53 (5.20–47.61) | 2.98 (1.12–16.00) | <0.001 |
| Exspiratory | 14.31 (5.75–23.49) | 2.92 (1.28–7.25) | <0.001 |
| delta ALX | 7.76 (0.81–18.79) | 0.74 (−0.47–7.24) | 0.059 |
| Coefficient | SE | 95%CI | p Value | |
|---|---|---|---|---|
| Age | −0.101 | 0.064 | −0.229 to 0.026 | 0.118 |
| Gender | 2.127 | 1.292 | −0.431 to 4.687 | 0.102 |
| BMI | −0.214 | 0.269 | −0.474 to 0.046 | 0.105 |
| R5 whole breath | 1.46 | 0.679 | 0.114 to 2.805 | 0.033 |
| R20 whole breath | −1.138 | 0.693 | −2.5116 to 0.234 | 0.103 |
| Fres whole breath | −0.414 | 0.269 | −0.948 to 0.118 | 0.126 |
| X5 whole breath | −1.482 | 2.231 | −5.903 to 2.938 | 0.507 |
| ALX whole breath | 0.21 | 0.179 | −0.145 to 0.566 | 0.244 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uno, T.; Homma, T.; Shigemura, M.; Fukuda, Y.; Kimura, T.; Onitsuka, C.; Kawahara, T.; Sato, H.; Akimoto, K.; Suganuma, H.; et al. Correlation of Arterial CO2 and Respiratory Impedance Values among Subjects with COPD. J. Clin. Med. 2020, 9, 2819. https://doi.org/10.3390/jcm9092819
Uno T, Homma T, Shigemura M, Fukuda Y, Kimura T, Onitsuka C, Kawahara T, Sato H, Akimoto K, Suganuma H, et al. Correlation of Arterial CO2 and Respiratory Impedance Values among Subjects with COPD. Journal of Clinical Medicine. 2020; 9(9):2819. https://doi.org/10.3390/jcm9092819
Chicago/Turabian StyleUno, Tomoki, Tetsuya Homma, Masahiko Shigemura, Yosuke Fukuda, Tomoyuki Kimura, Chihiro Onitsuka, Tomoko Kawahara, Hiroki Sato, Kaho Akimoto, Hiromitsu Suganuma, and et al. 2020. "Correlation of Arterial CO2 and Respiratory Impedance Values among Subjects with COPD" Journal of Clinical Medicine 9, no. 9: 2819. https://doi.org/10.3390/jcm9092819
APA StyleUno, T., Homma, T., Shigemura, M., Fukuda, Y., Kimura, T., Onitsuka, C., Kawahara, T., Sato, H., Akimoto, K., Suganuma, H., Kashima, A., Yamamoto, S., Ebato, T., Matsunaga, T., Kaneko, K., Mikuni, H., Sato, H., Uchida, Y., Fujiwara, A., ... Sagara, H. (2020). Correlation of Arterial CO2 and Respiratory Impedance Values among Subjects with COPD. Journal of Clinical Medicine, 9(9), 2819. https://doi.org/10.3390/jcm9092819






