Left Ventricular Contraction Duration Is the Most Powerful Predictor of Cardiac Events in LQTS: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Outcome Variables
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Search Results and Trial Flow
3.2. Characteristics of Included Studies
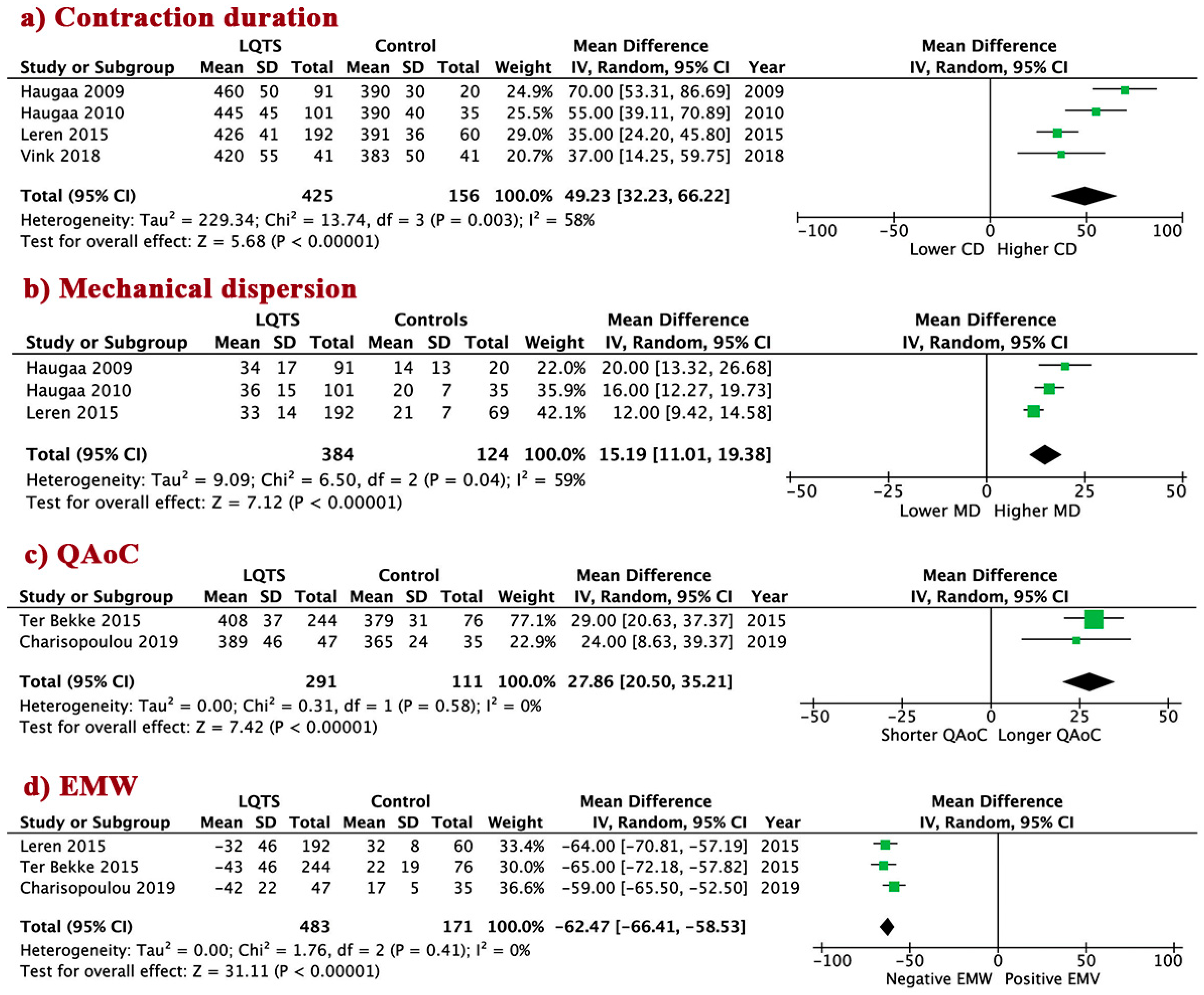
3.3. Electrical Abnormalities in LQTS vs. Controls
3.4. LV Mechanical Abnormalities in LQTS Patients vs. Controls
3.5. LV Mechanical Abnormalities in Asymptomatic vs. Symptomatic LQTS Patients
3.6. LV Mechanical Abnormalities in Asymptomatic LQTS Patients vs. Controls
3.7. EM Abnormalities in LQT1 vs. LQT2
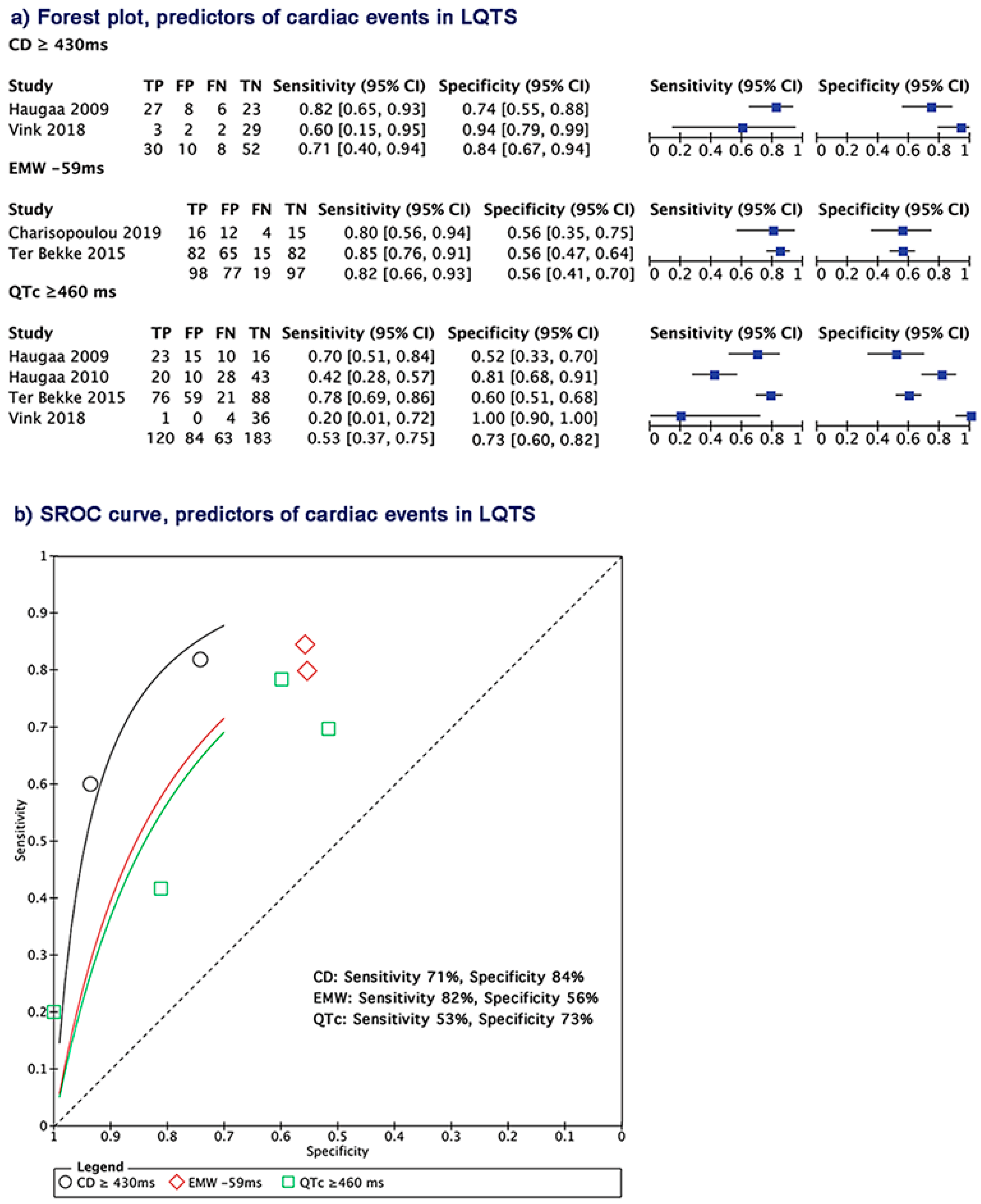
3.8. Electrical and Mechanical Predictors of Cardiac Events in LQTS Patients
3.9. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nador, F.; Beria, G.; De-Ferrari, G.M.; Stramba-Badiale, M.; Locati, E.H.; Lotto, A.; Schwartz, P. Unsuspected echocardiographic abnormality in the long QT syndrome. Diagnostic, prognostic, and pathogenetic implications. Circulation 1991, 84, 1530–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leren, I.S.; Hasselberg, N.E.; Saberniak, J.; Håland, T.F.; Kongsgård, E.; Smiseth, O.A.; Edvardsen, T.; Haugaa, K.H. Cardiac Mechanical Alterations and Genotype Specific Differences in Subjects With Long QT Syndrome. JACC Cardiovasc. Imaging 2015, 8, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, G.-X.; Antzelevitch, C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 1998, 98, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Yamanari, H.; Otsuka, F.; Fukushima, K.; Saito, H.; Fujimoto, Y.; Emori, T.; Matsubara, H.; Uchida, S.; Ohe, T. Dispersion of regional wall motion abnormality in patients with long QT syndrome. Heart 1998, 80, 245–250. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Edvardsen, T.; Leren, T.P.; Gran, J.M.; Smiseth, O.A.; Amlie, J.P. Left ventricular mechanical dispersion by tissue Doppler imaging: A novel approach for identifying high-risk individuals with long QT syndrome. Eur. Heart J. 2008, 30, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Vyas, H.; O’Leary, P.W.; Earing, M.G.; Cetta, F.; Ackerman, M.J. Mechanical Dysfunction in Extreme QT Prolongation. J. Am. Soc. Echocardiogr. 2008, 21, 511.e15–511.e17. [Google Scholar] [CrossRef]
- Savoye, C.; Klug, D.; Denjoy, I.; Ennezat, P.V.; Le Tourneau, T.; Guicheney, P.; Kacet, S. Tissue Doppler echocardiography in patients with long QT syndrome. Eur. J. Echocardiogr. 2003, 4, 209–213. [Google Scholar] [CrossRef]
- Priori, S.G.; Napolitano, C.; Diehl, L.; Schwartz, P. Dispersion of the QT interval. A marker of therapeutic efficacy in the idiopathic long QT syndrome. Circulation 1994, 89, 1681–1689. [Google Scholar] [CrossRef] [Green Version]
- Brado, J.; De Chant, M.J.; Menza, M.; Komancsek, A.; Lang, C.N.; Bugger, H.; Foell, D.; Jung, B.; Stiller, B.; Bode, C.; et al. Phase-contrast magnet resonance imaging reveals regional, transmural, and base-to-apex dispersion of mechanical dysfunction in patients with long QT syndrome. Heart Rhythm. 2017, 14, 1388–1397. [Google Scholar] [CrossRef]
- De-Ferrari, G.M.; Nador, F.; Beria, G.; Sala, S.; Lotto, A.; Schwartz, P. Effect of calcium channel block on the wall motion abnormality of the idiopathic long QT syndrome. Circulation 1994, 89, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.0.2; updated September 2009; The Cochrane Collaboration: London, UK, 2009. [Google Scholar]
- Zeng, X.-T.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Amlie, J.P.; Berge, K.E.; Leren, T.P.; Smiseth, O.A.; Edvardsen, T. Transmural Differences in Myocardial Contraction in Long-QT Syndrome. Circulation 2010, 122, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Haugaa, K.H.; Johnson, J.N.; Bos, J.M.; Phillips, B.L.; Eidem, B.W.; Ackerman, M.J. Subclinical Cardiomyopathy and Long QT Syndrome: An Echocardiographic Observation: Echocardiography in LQTS. Congenit Heart Dis. 2013, 8, 352–359. [Google Scholar] [CrossRef]
- Hummel, Y.M.; Wilde, A.A.; Voors, A.A.; Bugatti, S.; Hillege, H.L.; van den Berg, M.P. Ventricular dysfunction in a family with long QT syndrome type 3. Europace 2013, 15, 1516–1521. [Google Scholar] [CrossRef]
- Ter Bekke, R.M.; Haugaa, K.H.; Wijngaard, A.V.D.; Bos, J.M.; Ackerman, M.J.; Edvardsen, T.; Volders, P.G. Electromechanical window negativity in genotyped long-QT syndrome patients: Relation to arrhythmia risk. Eur. Heart J. 2014, 36, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Robyns, T.; Willems, R.; Vandenberk, B.; Ector, J.; Garweg, C.; Kuiperi, C.; Breckpot, J.; Corveleyn, A.; Janssens, S.; Heidbuchel, H.; et al. Individualized corrected QT interval is superior to QT interval corrected using the Bazett formula in predicting mutation carriage in families with long QT syndrome. Heart Rhythm. 2017, 14, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Vink, A.S.; Kuipers, I.M.; De Bruin-Bon, R.H.A.C.M.; Wilde, A.A.M.; Blom, N.A.; Clur, S.-A.B. A Potential Diagnostic Approach for Foetal Long-QT Syndrome, Developed and Validated in Children. Pediatr. Cardiol. 2018, 39, 1413–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charisopoulou, D.; Koulaouzidis, G.; Rydberg, A.; Henein, M.Y. Exercise worsening of electromechanical disturbances: A predictor of arrhythmia in long QT syndrome. Clin. Cardiol. 2019, 42, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jervell, A.; Lange-Nielsen, F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval, and sudden death. Am. Heart J. 1957, 54, 59–68. [Google Scholar] [CrossRef]
- Edler, I.; Lindström, K. The history of echocardiography. Ultrasound Med. Biol. 2004, 30, 1565–1644. [Google Scholar] [CrossRef]
- Blessberger, H.; Binder, T. NON-invasive imaging: Two dimensional speckle tracking echocardiography: Basic principles. Heart 2010, 96, 716–722. [Google Scholar] [CrossRef]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.-J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of Myocardial Mechanics Using Speckle Tracking Echocardiography: Fundamentals and Clinical Applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369. [Google Scholar] [CrossRef]
- Bshiebish, H.A.H.; Al-Musawi, A.H.; Khudeir, S.A. Role of global longitudinal strain in assessment of left ventricular systolic function in patients with heart failure with preserved ejection fraction. J. Saudi Heart Assoc. 2019, 31, 100–105. [Google Scholar] [CrossRef]
- Park, J.J.; Park, J.-B.; Park, J.-H.; Cho, G.-Y. Global Longitudinal Strain to Predict Mortality in Patients With Acute Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1947–1957. [Google Scholar] [CrossRef]
- Dabiri, Y.; Sack, K.; Shaul, S.; Sengupta, P.P.; Guccione, J.M. Relationship of Transmural Variations in Myofiber Contractility to Left Ventricular Ejection Fraction: Implications for Modeling Heart Failure Phenotype With Preserved Ejection Fraction. Front. Physiol. 2018, 9, 1003. [Google Scholar] [CrossRef] [Green Version]
- Herman, M.V.; Heinle, R.A.; Klein, M.D.; Gorlin, R. Localized disorders in myocardial contraction. Asynergy and its role in congestive heart failure. N. Engl. J. Med. 1967, 277, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Odening, K.E.; Jung, B.; Lang, C.N.; Cabrera-Lozoya, R.; Ziupa, D.; Menza, M.; Relan, J.; Franke, G.; Feliz, S.P.; Koren, G.; et al. Spatial correlation of action potential duration and diastolic dysfunction in transgenic and drug-induced LQT2 rabbits. Heart Rhythm. 2013, 10, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.J.; Priori, S.G.; Dubin, A.M.; Kowey, P.; Linker, N.J.; Slotwiner, D.; Triedman, J.K.; Van Hare, G.F.; Gold, M.R. Beta-blocker therapy for long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: Are all beta-blockers equivalent? Heart Rhythm. 2017, 14, e41–e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamim, W.; Yousufuddin, M.; Xiao, H.; Flather, M.; Henein, M.; Gibson, D. A Coats Septal q waves as an indicator of risk of mortality in elderly patients with chronic heart failure. Am. Heart J. 2002, 144, 740–744. [Google Scholar] [CrossRef]
- Shamim, W.; Yousufuddin, M.; Cicoria, M.; Gibson, D.G.; Coats, A.J.S.; Henein, M. Incremental changes in QRS duration in serial ECGs over time identify high risk elderly patients with heart failure. Heart 2002, 88, 47–51. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, C.A.; Ramzy, I.S.; Li, W.; Sutton, R.; Coats, A.J.S.; Gibson, D.G.; Henein, M.Y. The effect of the localization of Q wave myocardial infarction on ventricular electromechanics. Int. J. Cardiol. 2002, 84, 241–247. [Google Scholar] [CrossRef]
- Duncan, A.M.; O’Sullivan, C.A.; Gibson, D.G.; Henein, M.Y. Electromechanical interrelations during dobutamine stress in normal subjects and patients with coronary artery disease: Comparison of changes in activation and inotropic state. Heart 2001, 85, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Duncan, A.B.; Lim, E.; Clague, J.; Gibson, D.G.; Henein, M. Comparison of segmental and global markers of dyssynchrony in predicting clinical response to cardiac resynchronization. Eur. Heart J. 2006, 27, 2426–2432. [Google Scholar] [CrossRef]
- Henein, M. The relationship between diastolic function of the left ventricle and QT dispersion in patients with myocardial infarction. Int. J. Cardiol. 1999, 71, 195. [Google Scholar] [CrossRef]
- Foster, F.; Zhang, M.; Zhou, Y.; Liu, G.; Mehi, J.; Cherin, E.; Harasiewicz, K.; Starkoski, B.; Zan, L.; Knapik, D.; et al. A new ultrasound instrument for in vivo microimaging of mice. Ultrasound Med. Biol. 2002, 28, 1165–1172. [Google Scholar] [CrossRef]
- Moran, C.M.; Pye, S.D.; Ellis, W.; Janeczko, A.; Morris, K.D.; McNeilly, A.S.; Fraser, H.M. A Comparison of the Imaging Performance of High Resolution Ultrasound Scanners for Preclinical Imaging. Ultrasound Med. Biol. 2011, 37, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran, C.M.; Thomson, A.J.W.; Rog-Zielinska, E.; Gray, G.A. High-resolution echocardiography in the assessment of cardiac physiology and disease in preclinical models. Exp. Physiol. 2013, 98, 629–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, S. High Resolution Electrocardiography. Indian Pacing Electrophysiol. J. 2002, 2, 50–56. [Google Scholar] [PubMed]
- Smiseth, O.A.; Remme, E.W. Regional Left Ventricular Electric and Mechanical Activation and Relaxation⁎⁎Editorials published in the Journal of the American College of Cardiologyreflect the views of the authors and do not necessarily represent the views of JACCor the American College of Cardiology. J. Am. Coll. Cardiol. 2005, 47, 173–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P.P.; Korinek, J.; Belohlavek, M.; Narula, J.; Vannan, M.A.; Jahangir, A.; Khandheria, B.K. Left Ventricular Structure and Function. J. Am. Coll. Cardiol. 2006, 48, 1988–2001. [Google Scholar] [CrossRef] [Green Version]
- Haugaa, K.H.; Edvardsen, T. Myocardial Contraction and Long QT Syndrome. Trends Cardiovasc. Med. 2011, 21, 65–69. [Google Scholar] [CrossRef]
- Goldenberg, I.; Zareba, W.; Moss, A.J. Long QT Syndrome. Curr. Probl. Cardiol. 2008, 33, 629–694. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Priori, S.G.; Spazzolini, C.; Moss, A.J.; Vincent, G.M.; Napolitano, C.; Denjoy, I.; Guicheney, P.; Breithardt, G.; Keating, M.T.; et al. Genotype-phenotype correlation in the long-QT syndrome: Gene-specific triggers for life-threatening arrhythmias. Circulation 2001, 103, 89–95. [Google Scholar] [CrossRef] [Green Version]


| Study (Year) | Study Design | Long QT Mutation | Inclusion Criteria | Exclusion Criteria | Electrical Abnormalities | Echocardiographic Abnormalities | Type of Long QT |
|---|---|---|---|---|---|---|---|
| Priori et al., 1994 | Observational | Y—RWS | RWS Patients | Follow-up patients after institution of therapy of at least 1 year | QT, QTC, RR, | NR | RWS |
| relative QT dispersion, | |||||||
| relative QTC dispersion | |||||||
| Nakayama et al., 1998 | Observational—Prospective | NR | NR | NR | QT, QTC, QT | ThT | NR |
| dispersion | |||||||
| Savoye et al., 2003 | Observational | NR | Screened after identifying symptomatic LQTS patients in family | Age ≥ 16, patients with pacemaker, valvular or myocardial disease | RR, QTC | LVDd, EF, E/A | LQT1 |
| LQT2 | |||||||
| Leren et al., 2015 | Cross-sectional | Y—DM and SM | LQTS mutation-positive subjects were included from our outpatient clinic. | Concomitant cardiac disease of other origin. | QTC, HR | EF%, EDV, GLS, MD, CD, EMW, e’, E deceleration time, IVRT, LAVI, E/A, E/e’ | LQT1 |
| LQT2 | |||||||
| Patients with a diagnosis of hypertension or taking antihypertensive medication or with diabetes mellitus were excluded from diastolic measurements. | LQT3 | ||||||
| Only subjects with a pathogenic mutation were included. | |||||||
| Haugaa et al., 2009 | Observational | Y—SM and DM | Patients with previous cardiac | NR | QTC, RR | EF, CD by velocity, CD by strain, time to aortic valve closure, peak ejection velocity, PEV, Onset E’ wave, E/, E deceleration time | A and S |
| Haugaa et al., 2010 | Observational | Y—Hom and Het | Genotyped patients | Asymptomatic patients younger than 18 years old | HR, QTC, QTC dispersion | EF, Global strain, CD longitudinal, CD circumferential, MD longitudinal, MD circumferential | A and S; |
| LQT1 | |||||||
| LQT2 | |||||||
| LQT3 | |||||||
| LQT5 | |||||||
| Haugaa et al., 2013 | Observational—Retrospective Study | Y | Patients evaluated in Mayo’s LQT Clinic between August 1998 till December 2008 | 24 patients who did not accept to participate | HR, QTC, | EF, Left atrial volume indexed, E, e/, E/e’, E deceleration time | A and S; |
| LQT1 | |||||||
| LQT2 | |||||||
| LQT3 | |||||||
| LQT4 | |||||||
| LQT5 | |||||||
| LQT7 | |||||||
| Hummel et al., 2013 | Observational | Y—SCN5a mutation 1795insD | Genotyped asymptomatic patients | NR | NR | LV end-diastolic dimension, LV mass, LA volume, EF, E, A-wave, E/A, DT, IVRT, e’ | A |
| LQT3 | |||||||
| ter Bekke et al., 2015 | Observational | Y—SM and DM—Hom and Het | Genotyped patients | NR | RR, QT, QTC | EF, EMW, QAoC | A and S |
| LQT1 | |||||||
| LQT2 | |||||||
| LQT3 | |||||||
| LQT6 | |||||||
| Robyns et al., 2017 | Cross-sectional | Y | Patients with LQTS mutation and their genotype-negative family members who had both resting ECG and Holter recordings available were included | One patient with LQTS was excluded due to QT-RR correlation below 0.5 | ECG RR, ECG QTC, ECG QT, Holter T-wave amplitude, Holter QT-RR slope, Holter mean QT, Holter number of templates, Holter RR | NR | NR |
| Children younger than 8 years were excluded because of high heart rates | |||||||
| Vink et al., 2018 | Cross-sectional | Y | Children under 18 years old | Children above 18 years old | RR, QTC, QT | CD | NR |
| Charisopoulou et al., 2019 | Observational—Retrospective Study | Y | RR, HR, QTC | EF, e’, a’, E/A, FT, ET, IVRT | LQT1 | ||
| LQT2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsayed, M.; Bytyçi, I.; Rydberg, A.; Henein, M.Y. Left Ventricular Contraction Duration Is the Most Powerful Predictor of Cardiac Events in LQTS: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2820. https://doi.org/10.3390/jcm9092820
Abdelsayed M, Bytyçi I, Rydberg A, Henein MY. Left Ventricular Contraction Duration Is the Most Powerful Predictor of Cardiac Events in LQTS: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(9):2820. https://doi.org/10.3390/jcm9092820
Chicago/Turabian StyleAbdelsayed, Mena, Ibadete Bytyçi, Annika Rydberg, and Michael Y. Henein. 2020. "Left Ventricular Contraction Duration Is the Most Powerful Predictor of Cardiac Events in LQTS: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 9: 2820. https://doi.org/10.3390/jcm9092820
APA StyleAbdelsayed, M., Bytyçi, I., Rydberg, A., & Henein, M. Y. (2020). Left Ventricular Contraction Duration Is the Most Powerful Predictor of Cardiac Events in LQTS: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(9), 2820. https://doi.org/10.3390/jcm9092820






