Abstract
Sedentary behavior and cognitive impairment have a direct impact on patients’ outcomes. An energy metabolic disorder may be involved in the overlap of these comorbid conditions (motoric cognitive risk (MCR)) in patients with chronic obstructive pulmonary disease (COPD). We aimed to explore the linkage between a proapoptotic protein, growth differentiation factor (GDF)-15, and MCR. Physical activity (PA), cognitive function (Japanese version of the Montreal Cognitive Assessment: MOCA-J), and the serum GDF-15 levels were assessed in healthy subjects (n = 14), asthmatics (n = 22), and COPD patients (n = 28). In the entire cohort, serum GDF-15 had negative correlations with exercise (Ex) (ρ = −0.43, p < 0.001) and MoCA-J (ρ = −0.44, p < 0.001), and Ex and MOCA-J showed a positive correlation (ρ = 0.52, p < 0.0001). Compared to healthy subjects and asthmatics, COPD patients showed the highest serum GDF-15 levels and had a significantly higher proportion of subjects with MCR (both sedentary lifestyle (EX < 1.5) and cognitive risk (MoCA-J ≤ 25)). Also, we found that serum GDF-15 has a screening potential (100% sensitivity) greater than aging (67% sensitivity) for detecting MCR in COPD patients. In conclusion, higher serum GDF-15 had interrelationships with a sedentary lifestyle and cognitive risk. This protein was not disease-specific but could be a screening biomarker to detect MCR related to poor health outcomes of COPD patients.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of death and loss of social activities [1,2,3]. In fact, it is well known that representative comorbid conditions, sedentary behavior and cognitive impairment, have a direct impact on patients’ health outcomes [4,5,6,7]. Importantly, these two comorbid conditions commonly co-occur in the same patient, and their overlap is now recognized as motoric cognitive risk (MCR) syndrome [8]. Handschin et al. reported that a sedentary lifestyle may be a trigger for the progression of chronic disease through systemic inflammation accompanied by skeletal muscle inactivity [9]. Physical inactivity may be associated with MCR via molecular biological responses between the brain and skeletal muscle.
Growth differentiation factor-15 (GDF-15), which is a member of the transforming growth factor-β superfamily [10], is suggested to be a promising biomarker for the prognosis [11]. This protein, expressed in multiple tissues including brain, lung, and muscle [12,13,14], is induced during tissue injury, inflammation, and oxidative stress [15]. Previous reports have shown that GDF-15 is also associated with cell apoptosis, senescence, and energy metabolic disorder due to mitochondrial dysfunction, which are hallmarks of aging [13,16,17,18,19]. Actually, GDF-15 has been shown to be a promising biomarker of mortality in aging-associated disease [20]. If systemic inflammation and energy metabolic disorder due to muscle inactivity are involved in the mechanism of MCR, this proapoptotic protein may be a useful biomarker to predict the risk of poor health outcomes in COPD patients.
This study aimed to explore the linkage of GDF-15 with a sedentary lifestyle and cognitive risk, and to verify the diagnostic ability of GDF-15 to detect MCR in COPD patients.
2. Materials and Methods
2.1. Study Subjects
Subjects older than 40 years were recruited from Yamaguchi Medical University Hospital. The study protocol and its amendments were approved by the local ethics committee at Yamaguchi Medical University (institutional review board no. H28-031). The study was registered in the UMIN Clinical Trials Registry: UMIN000024645.
The subjects consisted of healthy subjects, asthmatics and patients with COPD. Asthma and COPD were diagnosed by a pulmonologist. Asthmatic patients had documented reversible airflow limitation and were treated based on the Global Initiative for Asthma (GINA) guidelines [21]. Asthmatics had adequate inhalation and good adherence to asthma therapy. The COPD patients were treated based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [22]. Asthmatics and patients with COPD were stable and had no exacerbations for at least three months prior to the study. Patients with other pulmonary diseases such as interstitial lung disease or with disorders that would prevent them from completing the study assessments were excluded. Patients received an explanation of the study protocol and gave written informed consent.
2.2. Evaluation of Physical Activity
The Active Style Pro HJA-750C® with triaxial acceleration can be worn around the waist and continually record physical activity for two weeks. We measured the value of the metabolic equivalents (METs) and calculated the exercise (EX) that was the value of metabolic equivalents multiplied by their durations (MET × hours per day) as previously reported [23,24,25,26,27,28] According to the American College of Sports Medicine and the American Heart Association recommendation [29], we defined EX < 1.5 as a sedentary lifestyle.
2.3. Assessment of Ccognitive Function
Trained research assistants administered the Japanese version of the Montreal Cognitive Assessment (MoCA-J) according to standardized procedures for cognitive function. MoCA-J assesses short-term memory, visuospatial ability, some executive functions, attention, concentration, working memory, language, and temporal and spatial orientation. Since a MoCA-J score with a threshold is less than 25 is considered to indicate mild cognitive impairment [30,31], we defined a MoCA-J score ≤ 25 as cognitive risk.
2.4. Definition of MCR
We defined cases that fulfilled both EX < 1.5 (sedentary lifestyle) and a MoCA-J score ≤ 25 (cognitive risk) at the same time as MCR in this study.
2.5. Measurement of GDF-15
Peripheral venous blood was drawn into pyrogen-free EDTA collection tubes and centrifuged within 30 min at 2150× g for 15 min at 4 °C. The plasma was aliquoted and stored in −80 °C ultra-freezers. The plasma levels of GDF-15 were measured in the baseline samples by ELISA (Quantikine, R&D Systems, Inc., Abingdon, UK). All samples were measured in duplicate and accepted only if the intra-assay variance was <10%.
2.6. Assessment of Pulmonary Function
Under the recommendations of the American Thoracic Society/European Respiratory Society, the pulmonary function was assessed by CHESTAC-8800 DN type (Chest Ltd., Tokyo, Japan) [32].
2.7. Statistical Analysis
Data are shown as the median ± interquartile (IQR). We divided the subjects into disease groups or groups based on the EX or MoCA-J score. The Wilcoxon’s rank sum test or Kruskal–Wallis test was employed to measure the differences in each group. The Kruskal–Wallis test with post-hoc analysis was used to compare the differences between specific groups. Spearman’s rank correlation and multiple linear regression analysis using the least square method were performed to analyze the correlation between parameters. We analyzed sensitivity versus specificity by the area under the curve (AUC) and found a cutoff value for GDF-15 that could detect MCR. Statistics were performed using JMP Pro®, version 14.0.0 (SAS Institute, Inc., Cary, NC, US). A probability value less than 0.05 was considered statistically significant.
3. Results
The baseline characteristics are shown in Table 1. A total of 64 subjects were enrolled in the study. The subjects consisted of 14 healthy subjects, 22 asthmatics, and 28 patients with COPD. The subjects were 70.3% male and had a mean age of 67.4 years. The median of body mass index (BMI) was 23.1 and a total of 41(64%) subjects had a smoking history.

Table 1.
Characteristics of the study subjects.
Table 2 shows the results of physical activity (EX), cognition level (MoCA-J), and GDF-15 among subjects. The medians of EX, MoCA-J, and GDF-15 were 3.2 (MET × hours per day), 25 (scores), and 934 (pg/mL), respectively, in all subjects. The GDF-15 intra-assay variance of all samples was <10%. EX (2.0 METs × hours per day) and MoCA-J (23 scores) were lowest in COPD among the subjects (p < 0.0005, p < 0.0001, respectively). Comparing subjects by the GDF-15 levels, those in COPD (1285 pg/mL) showed trend of the highest values among the subjects (p < 0.05) and GDF-15 levels in COPD were higher than those in healthy subjects and asthmatics by post-hoc analysis of differences between the specific groups (830 pg/mL; p < 0.005, 793 pg/mL; p = 0.08, respectively).

Table 2.
Comparison of physical activity (EX), cognition level (MoCA-J), and growth differentiation factor-15 (GDF-15) among subjects.
In the entire cohort, the associations of GDF-15, EX, and MoCA-J were strong, although there was no correlation between these relationships after adjustment for age (Table 3).

Table 3.
Association of EX, MoCA-J, and GDF-15 unadjusted and adjusted by age in all subjects.
When comparing the difference in GDF-15 between categories based on physical activity or cognitive risk level, participants who belonged to the MCR category showed trend of the highest GDF-15 levels (Figure 1). Therefore, we assessed the diagnostic ability of GDF-15 and age to determine whether MCR existed or not using receiver operating characteristic (ROC) analysis. As a result, although both AUCs were almost the same, the sensitivity of GDF-15 was better than that of age (Table 4).
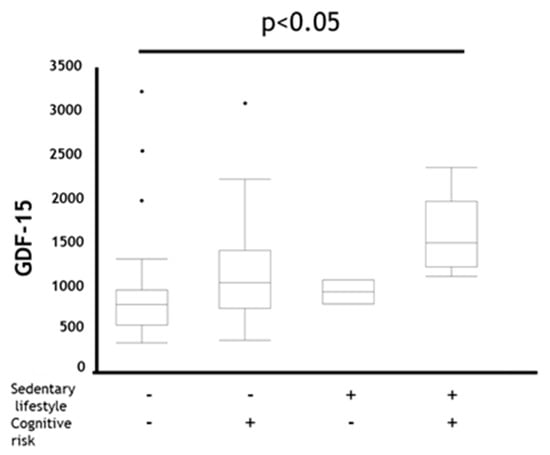
Figure 1.
Comparison of GDF-15 in coexistence status with sedentary lifestyle and cognitive risk in all subjects. Data are presented as box plot including median (IQR). P-values compared among subjects by Kruskal–Wallis test. GDF-15: Growth Differentiation Factor-15, Sedentary lifestyle + : EX (MET × hours per day) < 1.5, Sedentary lifestyle − : EX (MET × hours per day) ≥ 1.5, Cognitive risk + : MoCA-J scores ≤ 25, Cognitive risk − : MoCA-J scores > 25.

Table 4.
Diagnostic ability in age and GDF-15 to identify motoric cognitive risk (MCR) in all subjects.
As shown in Figure 2, the frequency of sedentary lifestyle (EX < 1.5) (25%) or cognitive risk (MoCA-J ≤ 25) (86%) was highest in COPD among the groups (healthy, 0%; 7% asthma, 14%; 41%, respectively). Importantly, MCR (25%) was seen most frequently in COPD compared to the healthy subjects and asthmatics (0%, 4.6%, respectively).
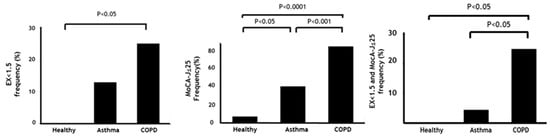
Figure 2.
Frequency of sedentary lifestyle, cognitive risk, and MCR among subjects. Data are presented as frequency. P-values show post-hoc analysis between each group. MET: Metabolic equivalent, EX: Exercise (MET × hours), MoCA-J: Japanese version of the Montreal Cognitive Assessment, MCR: Motoric cognitive risk syndrome.
Significant associations of GDF-15, EX and MoCA-J were found in COPD patients, but similar to in the result of the entire cohort, age correction weakened these relationships (Table 5).

Table 5.
Association of EX, MoCA-J, and GDF-15 adjusted by age unadjusted and adjusted by age in chronic obstructive pulmonary disease (COPD).
Additionally, trends of the GDF-15 levels in the MCR category were significantly more likely to be higher than those in other categories in patients with COPD (p < 0.05) (Figure 3).
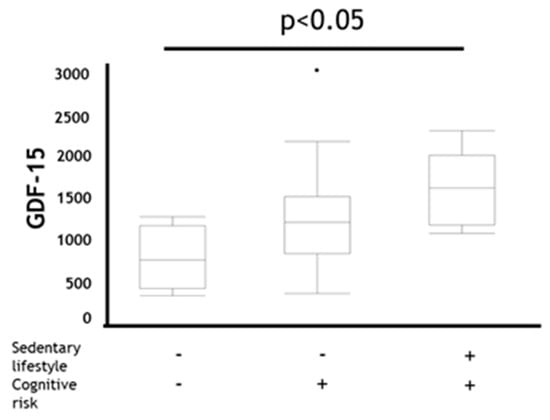
Figure 3.
Comparison of GDF-15 in coexistence status with sedentary lifestyle and cognitive risk in COPD. Data are presented as boxplot including median (IQR). P-values compared among subjects by Kruskal–Wallis test. GDF-15: Growth Differentiation Factor-15 Sedentary lifestyle +: EX (MET × hours per day) < 1.5, Sedentary lifestyle − : EX (METs × hours per day) ≥ 1.5, Cognitive risk + : MoCA-J scores ≤ 25, Cognitive risk −: MoCA-J scores > 25.
In order to clarify the role of age on the results, we performed univariate and multivariate analysis of age with MCR related parameters including EX, MoCA-J and GDF-15. As a result, in both all subjects and COPD, age was independently associated with GDF-15 (F = 5.6, p < 0.05, F = 4.9, p < 0.05, respectively) (Table S1). However, when comparing the relationship between age and GDF-15 for each subject group, the slope of GDF-15-Age (39.8) in COPD group was steeper than those in other groups (healthy; 2.9, asthma; 22.7, respectively) (Figure 4). Finally, a GDF-15 level > 1118 pg/mL secured 100% sensitivity and 50% specificity to detect MCR in COPD (AUC = 0.74), while AUC of age was 0.61 (57% sensitivity, 67% specificity) (Figure 5).
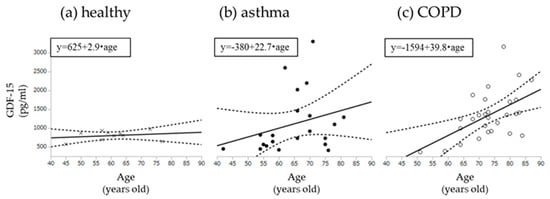
Figure 4.
Univariable models for age with GDF-15. (a) Correlation with age and GDF-15 among healthy subjects; (b) Correlation with age and GDF-15 among asthma; (c) Correlation with age and GDF-15 among COPD. GDF-15: Growth Differentiation Factor-15. Dotted lines show 95% confidence intervals.
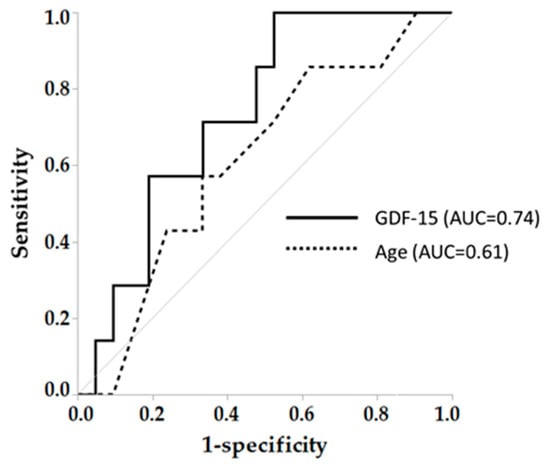
Figure 5.
Diagnostic ability in GDF-15 and age to identify MCR in COPD. AUC: Area under the curve, MCR: Motoric cognitive risk syndrome. Arrows mean cut-off point to identify coexistence of sedentary lifestyle and cognitive risk (MCR).
4. Discussion
4.1. Principal Findings
We observed that higher serum GDF-15 levels showed interrelationships with a sedentary lifestyle and cognitive risk. These data suggest that a systemic effect and an energy metabolic disorder may be involved in a pathway associated with these comorbid conditions. Compared to healthy subjects and asthmatics, the COPD patients showed the highest serum GDF-15 levels, which tended to be steeper with aging and had a significantly higher proportion of subjects with MCR. We found that this proapoptotic protein was not disease-specific but appeared to have the superior ability to detect MCR than aging in COPD patients.
4.2. Comparison with Other Studies
The mechanisms underlying the effects of GDF-15 secretion on neuron, muscle, and airway are not fully understood. The secretary mechanism of GDF-15 may be associated with skeletal muscle dysfunction by physical inactivity, because the molecule is a footprint of an energy metabolic disorder due to mitochondrial dysfunction [9]. On the other hand, Mullican et al. recently identified glial cell line-derived neurotrophic factor family receptor-a–like (GFRAL), the receptor tyrosine kinase RET, as a specific receptor in the brain for GDF-15 [33]. They described that the GDF-15/GFRAL pathway regulates appetite and could be involved with energy metabolic disorder. These data may help to explain our findings that higher serum GDF-15 levels showed interrelationships and with a sedentary lifestyle and cognitive risk.
The detailed biological mechanisms by which the GDF-15 pathway links with the coexistence of physical inactivity and cognitive risk in COPD remain unclear. However, it is suggested that there is a pathophysiological basis for the comorbidity through systemic inflammation accompanied by physical inactivity in chronic disease including COPD [9,34,35]. In addition, mobility and cognition tasks share common brain regions, such as the cortex in order to execute each function [36], and this area may be susceptible to systemic inflammation [37,38]. Actually, systemic inflammation is suggested to have a pathogenic role in accelerating pathologic processes underlying cognitive decline [35]. Mitochondrial dysfunction is also reported to contribute to neural injury via neuronal apoptosis [39,40,41], which may be closely linked with the biological responses between muscle and neuron. Therefore, our findings could support the hypothesis that there is inter-organ crosstalk between the brain and pulmonary and skeletal muscle [9,34,35].
4.3. Strength and Limitations
To the best of our knowledge, this is the first study that investigated the relationship between MCR and GDF-15 in COPD. An overlap of these comorbid conditions may have greater predictive value for worsening QOL and prognosis than a single comorbidity in patients with COPD because multiple treatments may be necessary to deal with various diseases, which could complicate the management of such patients [42]. In addition, such conditions are more likely to share pathways of accelerated aging [43]. Therefore, in this study, we expanded the evidence that GDF-15 could be a novel biomarker for detecting MCR and elucidating the mechanism of accelerated aging in COPD. The detection of MCR using GDF-15 may lead to novel precautions and interventions that target common pathways of accelerated aging.
Some previous reports have shown that elevated serum GDF-15 levels contribute to subclinical coronary atherosclerosis [44] and are correlated with adverse outcomes such as exacerbation and lung function decline in COPD [45]. Our results are in accord with these findings in that GDF-15 adds a novel measurable value to a predictor model based on a conventional method. Since a serum GDF-15 cutoff value to detect MCR showed high sensitivity, it is suggested that GDF-15 could be a useful screening tool for predementia in COPD.
The current study has some limitations. The first is the small number of subjects. Next, this cross-sectional study could not clarify a causal relationship between GDF-15 and MCR. More large-scaled longitudinal studies are needed. The GDF-15 was not a specific biomarker to identify MCR in the study subjects. In order to overcome this point, we need to explore whether GDF-15 combined with the results from brain imaging, for example, could be useful to specifically identify MCR. Finally, it is well known that aging is strongly associated with most outcomes in our study. In fact, when adjusted for age, the relationship between GDF-15 and comorbid conditions was greatly diminished. However, these data do not preclude the significance of GDF-15 as a screening biomarker to detect MCR. The sensitivity of GDF-15 to detect MCR was superior to that of age not only in entire cohort, but also in COPD. In addition, the AUC of GDF-15 in COPD was higher than that of age, and the increasing rate of GDF-15 with aging in COPD accelerated than that in other groups. This indicates that higher GDF-15 is originated from combination of aging and specific disease effect in COPD. Our study revealed the relationship between GDF-15 and comorbid conditions that are associated with heavy burdens on the lives of COPD patients.
5. Conclusions
Our study demonstrated that higher GDF-15 levels had interrelationships with a sedentary lifestyle and cognitive risk. We provide the novel insight that this protein is not disease-specific but could be a screening biomarker to detect MCR, which is related to poor health outcomes in COPD patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/9/2737/s1, Table S1. Association of age and EX, MoCA-J and GDF-15.
Author Contributions
Conceptualization, T.H., S.T., T.D., and K.M. (Kazuto Matsunaga); methodology, T.H. and K.D., S.T. and K.M. (Kazuto Matsunaga); formal analysis, T.H. and S.T.; investigation, T.H. and K.D.; resources, T.H.; data curation, T.H., K.D., and K.S.; writing—original draft preparation, T.H. and K.M. (Kazuto Matsunaga); writing—review and editing, T.H., S.T., T.D., K.S., K.O., K.Y., Y.M. (Yusuke Mimura), K.D., M.H., S.S., K.M. (Kazuto Matsunaga), A.C., Y.O., K.M. (Keita Murakawa), S.U., K.H., S.O., Y.M. (Yoriyuki Murata), Y.Y., M.A.-N., N.E., T.K., and K.M. (Kazuki Matsuda); project administration, T.H. and K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Brent Bell for reading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kochanek, K.D.; Murphy, S.; Xu, J.; Arias, E. Mortality in the United States, 2016; NCHS data brief; Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2017; pp. 1–8.
- Murray, C.J.; Barber, R.M.; Foreman, K.J.; Abbasoglu Ozgoren, A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Abu-Raddad, L.J.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- Juncos-Rabadan, O.; Pereiro, A.X.; Facal, D.; Rodriguez, N.; Lojo, C.; Caamaño, J.A.; Sueiro, J.; Boveda, J.; Eiroa, P. Prevalence and correlates of cognitive impairment in adults with subjective memory complaints in primary care centres. Dement. Geriatr. Cogn. Disord. 2012, 33, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Waschki, B.; Kirsten, A.; Holz, O.; Muller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Pitta, F.; Troosters, T.; Spruit, M.A.; Probst, V.S.; Decramer, M.; Gosselink, R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Minakata, Y.; Sugino, A.; Kanda, M.; Ichikawa, T.; Akamatsu, K.; Koarai, A.; Hirano, T.; Nakanishi, M.; Sugiura, H.; Matsunaga, K.; et al. Reduced level of physical activity in Japanese patients with chronic obstructive pulmonary disease. Respir. Investig. 2014, 52, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.W.; Getov, S.V.; Jones, P.W. Cognitive function in COPD. Eur. Respir. J. 2010, 35, 913–922. [Google Scholar] [CrossRef]
- Verghese, J.; Wang, C.; Lipton, R.B.; Holtzer, R. Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef]
- Luan, H.H.; Wang, A.; Hilliard, B.K.; Carvalho, F.; Rosen, C.E.; Ahasic, A.M.; Herzog, E.L.; Kang, I.; Pisani, M.A.; Yu, S.; et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell 2019, 178, 1231–1244. [Google Scholar] [CrossRef]
- Corre, J.; Hébraud, B.; Bourin, P. Concise review: Growth differentiation factor 15 in pathology: A clinical role? Stem Cells Transl. Med. 2013, 2, 946–952. [Google Scholar] [CrossRef]
- Mullican, S.E.; Rangwala, S.M. Uniting GDF15 and GFRAL: Therapeutic opportunities in obesity and beyond. Trends Endocrinol. Metab. 2018, 29, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Bottner, M.; Suter-Crazzolara, C.; Schober, A.; Unsicker, K. Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res. 1999, 297, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.K.; Ryu, D.; Kim, K.S.; Chang, J.Y.; Kim, Y.K.; Yi, H.S.; Kang, S.G.; Choi, M.J.; Lee, S.E.; Jung, S.B.; et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 2017, 216, 149–165. [Google Scholar] [CrossRef]
- Unsicker, K.; Spittau, B.; Krieglstein, K. The multiple facets of the TGF-beta family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev. 2013, 24, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Fujita, Y.; Taniguchi, Y.; Shinkai, S.; Tanaka, M.; Ito, M. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr. Gerontol. Int. 2016, 16 (Suppl. S1), 17–29. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Fernandes, A.; Aguilar-Pimentel, J.A.; de Angelis, M.H.; Guedes, J.R.; Brito, M.A.; Ortolano, S.; Pani, G.; Athanasopoulou, S.; Gonos, E.S.; et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res. Rev. 2018, 47, 214–277. [Google Scholar] [CrossRef]
- Jang, J.Y.; Blum, A.; Liu, J.; Finkel, T. The role of mitochondria in aging. J. Clin. Investig. 2018, 128, 3662–3670. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. 2020. Available online: www.ginasthma.com (accessed on 21 July 2020).
- Global Initiative for Chronic Obstructive Lung Disease. 2020. Available online: www.goldcopd.com (accessed on 21 July 2020).
- Miyamoto, S.; Minakata, Y.; Azuma, Y.; Kawabe, K.; Ono, H.; Yanagimoto, R.; Suruda, T. Verification of a motion sensor for evaluating physical activity in COPD patients. Can. Respir. J. 2018, 2018, 8343705. [Google Scholar] [CrossRef]
- Sugino, A.; Minakata, Y.; Kanda, M.; Akamatsu, K.; Koarai, A.; Hirano, T.; Sugiura, H.; Matsunaga, K.; Ichinose, M. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration 2012, 83, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.E.; Ainsworth, B.E.; Thompson, R.W.; Bassett, D.R., Jr. Sources of variance in daily physical activity levels as measured by an accelerometer. Med. Sci. Sports Exerc. 2002, 34, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Burkett, L.; Reis, J.P.; Ainsworth, B.E.; Macera, C.A.; Wilson, D.K. How many days of pedometer monitoring predict weekly physical activity in adults? Prev. Med. 2005, 40, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Matsumura, Y.; Yamamoto, M.; Kitado, T.; Nakao, Y.; Nakao, Y.; Suzuki, T.; Yoshikawa, T.; Fujimoto, S. The relationship between body weight reduction and intensity of daily physical activities assessed with 3-dimension accelerometer. Jpn. J. Phys. Fit. Sports Med. 2006, 55, 385–391. [Google Scholar] [CrossRef][Green Version]
- Matsumura, Y.; Yamamoto, M.; Kitado, T.; Nakamura, H.; Kidera, K.; Fujimoto, S. High-accuracy physical activity monitor utilizing three-axis accelerometer. Natl. Tech. Rep. 2008, 56, 60–66. [Google Scholar]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Suzuki, H.; Yasunaga, M.; Sugiyama, M.; Ijuin, M.; Sakuma, N.; Inagaki, H.; Iwasa, H.; Ura, C.; Yatomi, N.; et al. Brief screening tool for mild cognitive impairment in older Japanese: Validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr. Gerontol. Int. 2010, 10, 225–232. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Al-shair, K.; Kolsum, U.; Dockry, R.; Morris, J.; Singh, D.; Vestbo, J. Biomarkers of systemic inflammation and depression and fatigue in moderate clinically stable COPD. Respir. Res. 2011, 12, 3. [Google Scholar] [CrossRef]
- Walker, K.A.; Gottesman, R.F.; Wu, A.; Knopman, D.S.; Gross, A.L.; Mosley, T.H., Jr.; Selvin, E.; Windham, B.G. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology 2019, 92, e1256–e1267. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Verghese, J.; Zwerling, J.L. Cognition and gait in older people. Maturitas 2016, 93, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, F.; Ren, J.; Driscoll, M.J.; Culver, B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp. Neurol. 2005, 191, 318–325. [Google Scholar] [CrossRef] [PubMed]
- D’Avila, J.C.; Siqueira, L.D.; Mazeraud, A.; Azevedo, E.P.; Foguel, D.; Castro-Faria-Neto, H.C.; Sharshar, T.; Chretien, F.; Bozza, F.A. Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation. J. Neuroinflammation 2018, 15, 28. [Google Scholar] [CrossRef]
- Pollack, M.; Phaneuf, S.; Dirks, A.; Leeuwenburgh, C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann. N. Y. Acad. Sci. 2002, 959, 93–107. [Google Scholar] [CrossRef]
- Siu, P.M.; Alway, S.E. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J. Physiol. 2005, 565, 309–323. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lu, M.H.; Yuan, D.J.; Xu, D.E.; Yao, P.P.; Ji, W.L.; Chen, H.; Liu, W.L.; Yan, C.X.; Xia, Y.Y.; et al. Mitochondrial dysfunction in neural injury. Front. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef]
- Barnes, P.J. Senescence in COPD and its comorbidities. Ann. Rev. Physiol. 2017, 79, 517–539. [Google Scholar] [CrossRef]
- Barnes, P.J. Mechanisms of development of multimorbidity in the elderly. Eur. Respir. J. 2015, 45, 790–806. [Google Scholar] [CrossRef]
- Martinez, C.H.; Freeman, C.M.; Nelson, J.D.; Murray, S.; Wang, X.; Budoff, M.J.; Dransfield, M.T.; Hokanson, J.E.; Kazerooni, E.A.; Kinney, G.L.; et al. GDF-15 plasma levels in chronic obstructive pulmonary disease are associated with subclinical coronary artery disease. Respir. Res. 2017, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Husebo, G.R.; Gronseth, R.; Lerner, L.; Gyuris, J.; Hardie, J.A.; Bakke, P.S.; Eagan, T.M. Growth differentiation factor-15 is a predictor of important disease outcomes in patients with COPD. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).