1. Introduction
Craniofacial reconstructions are often time consuming and present a significant challenge for the surgeon. A typical application is a cranioplasty, which is a standard neurosurgical procedure performed to reconstruct cranial defects. The critical clinical challenges in the reconstruction of craniofacial bone defects are the ability to carry out complex reconstructions with precise implant fit and esthetic appearance. The cumulative understanding of the surgeon to overcome these clinical challenges have led to the utilization of prefabricated patient-specific implants (PSIs) for cranioplasty procedures [
1,
2,
3].
Over the past several years, polyetheretherketone (PEEK), a high-performance biopolymer, has gained substantial popularity in craniofacial reconstructions [
4,
5]. PEEK is an aromatic polymer with ether and ketone bond linkages. It is a high-temperature, semicrystalline thermoplastic material, which is chemically inert with high thermal stability and mechanical properties. Combining the characteristics intrinsic properties of PEEK, such as no artifact in medical imaging along with cortical bone-like modulus, it is an excellent alternative for metallic biomaterials in craniomaxillofacial reconstructive surgeries [
6,
7,
8].
To date, the fabrication of PEEK implants is well matched to computer-aided design and computer-aided manufacturing (CAD/CAM) machining technologies like injection molding and, specifically, milling [
9]. However, the recent advent of additive manufacturing (AM), popularly known as three-dimensional (3D) printing, is providing a replacement frontier for the design and production of prefabricated PEEK implants [
9]. Schmidt et al. [
10] first proposed AM of PEEK parts using the selective laser sintering (SLS) printing technology. Later, based on SLS technology, the EOS P800 (EOS, Electro-Optical Systems GmbH, Krailling, Germany) system was launched, which concentrated on AM of parts from PEEK powders at high temperatures [
11]. However, the EOS P800 requires expensive PEEK powders, and the concentrated laser beam restricts the sintering process in extensive areas [
12].
Compared to SLS technology, material extrusion-based fused filament fabrication (FFF) technology is already integrated into the hospitals for the fabrication of anatomical biomodels, customized surgical tools, and prosthetic aids [
9]. Besides, recent technological advancements in FFF 3D printers have made it possible to process high-temperature PEEK thermoplastic biomaterial [
13]. In the FFF process, a filament is continuously extruded from a heated nozzle in a viscous state and deposited in a layer-by-layer manner to form the desired shape of an object. FFF allows the fabrication of specific complex geometries that is not feasible by other manufacturing techniques such as milling or injection molding [
9,
14]. Unlike SLS, FFF offers numerous advantages, including low initial machine purchase costs, ease of use, less waste generation, and reduced risk of material contamination [
15,
16]. Considering these aspects, some FFF 3D printers are being explicitly developed for medical PEEK applications [
17]. This technology currently contributes to a new point-of-care (POC) workflow, implementing how PEEK medical implants need to be designed, developed, and manufactured for low-volume and on-demand production. Previous studies have demonstrated the feasibility of using FFF for medical PEEK printing [
13,
17]. As this technology is recognized as a prospective tool in the medical sector for in-hospital manufacturing of customized PEEK implants, the extent to which it affects the geometry and manufacturing quality remain under investigated. Therefore, to implement POC manufacturing, an understanding of the relationship between the process parameters and the quality characteristics of the printed parts is crucial.
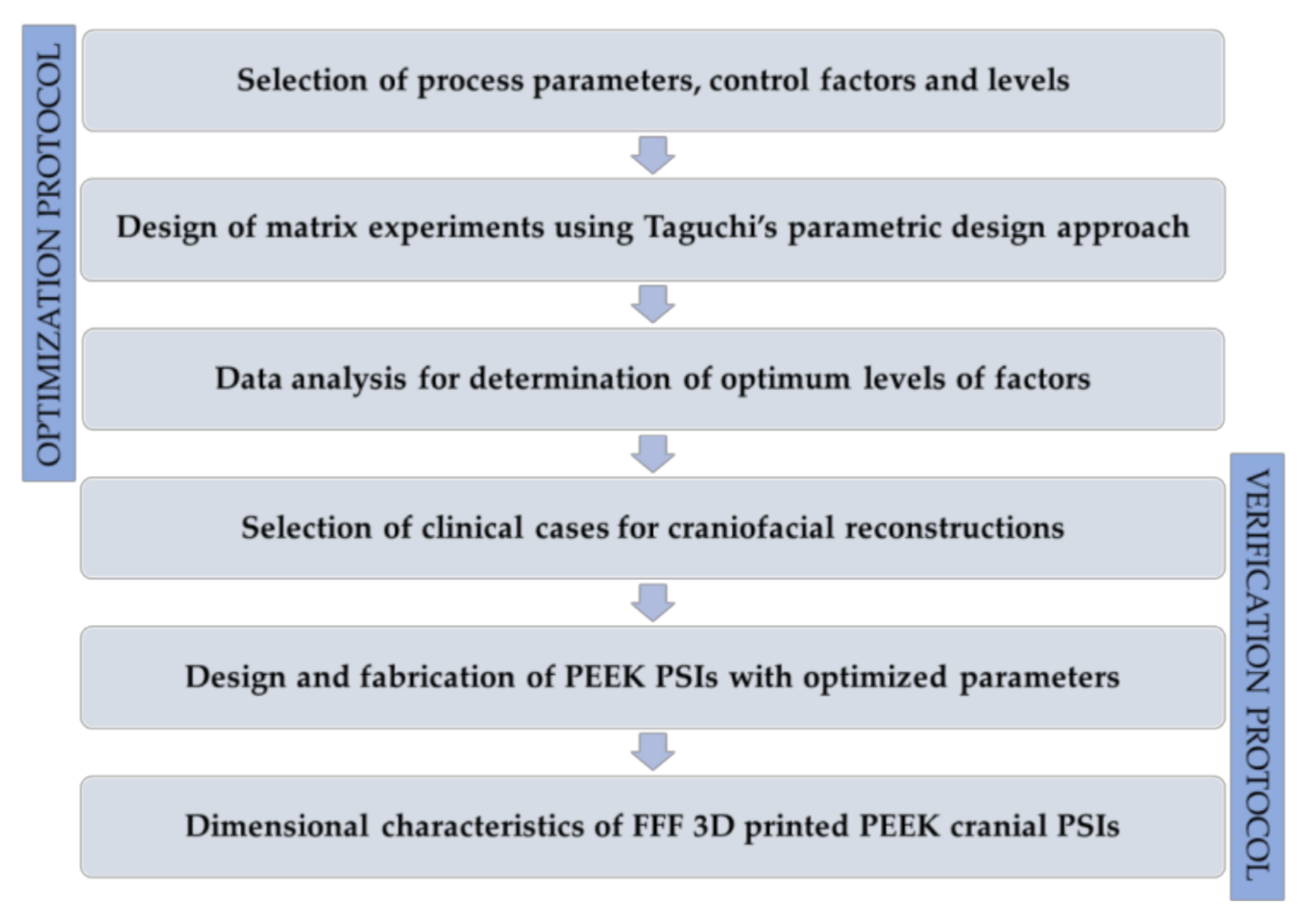
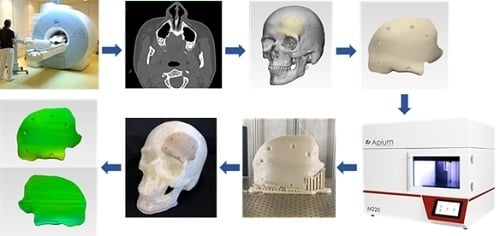
This paper aimed to analyze PEEK customized implants fabricated at the POC for craniofacial reconstructions, discussing the numerous challenges during the extrusion AM process. Furthermore, dimensional characteristics of the extrusion-based anatomically shaped PEEK cranial plates are reported for the first time in this paper.
4. Discussion
Personalized medicine has revolutionized the practice of modern medicine. With advancements in CAD and AM technologies, the use of customized implants with excellent cosmetics and functional results has now become widespread [
22]. These technological improvements have led to a tremendous increase in the use of patient-specific alloplastic implants for cranioplasty applications. PEEK has been used in cranioplasty as a reliable alternative to other alloplastic materials [
23,
24]. Previous studies have shown the possibility of printing PEEK by FFF [
17,
25,
26]; however, studies on FFF 3D printed PEEK cranial implants are limited. Therefore, to investigate the outcome of quality and clinical relevance of FFF 3D printing technology at POC manufacturing, the present study was conducted. We reported quantitative assessments of dimensional accuracy characteristics of FFF 3D printed PEEK PSIs using an in-house PEEK 3D printer.
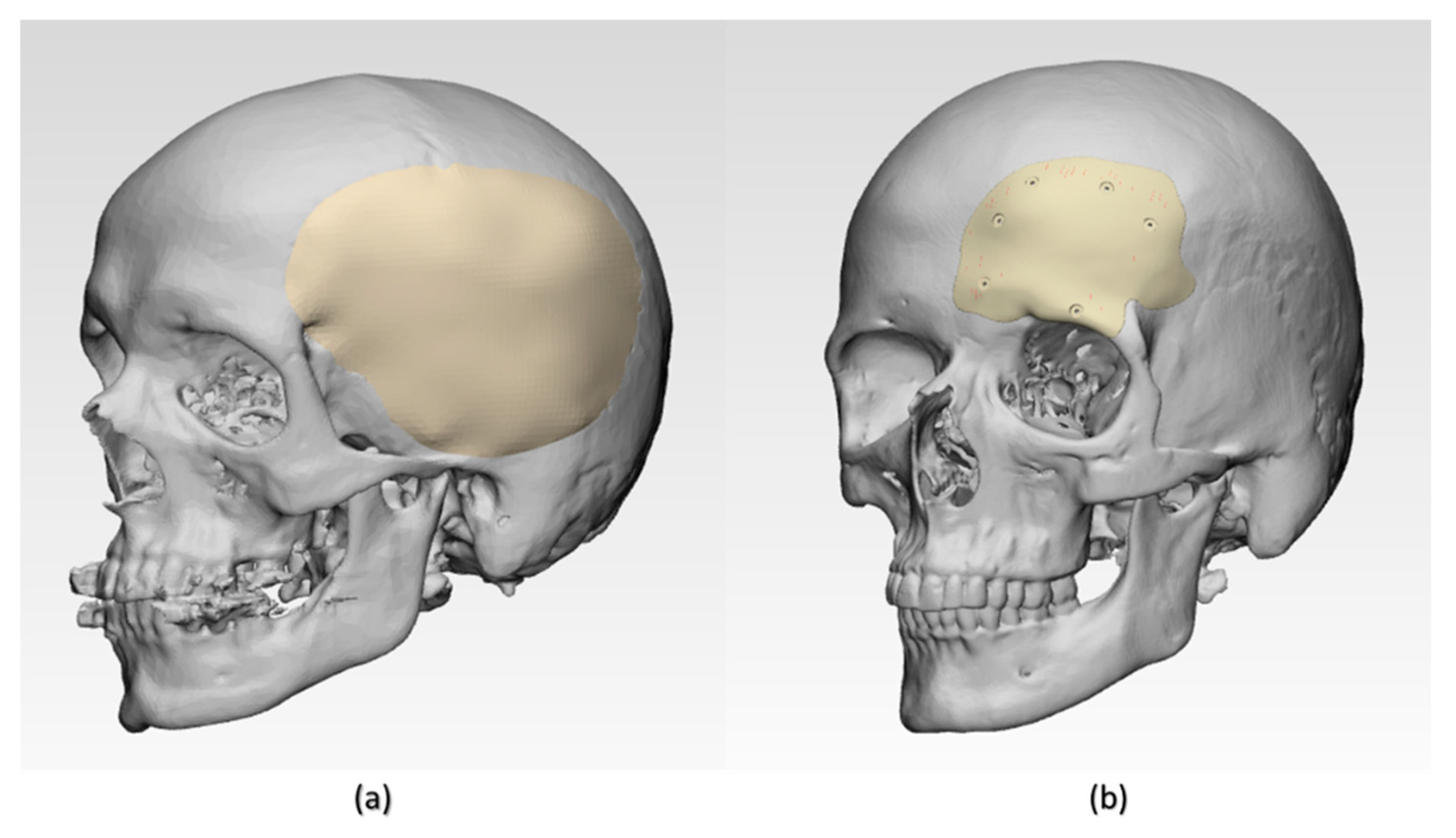
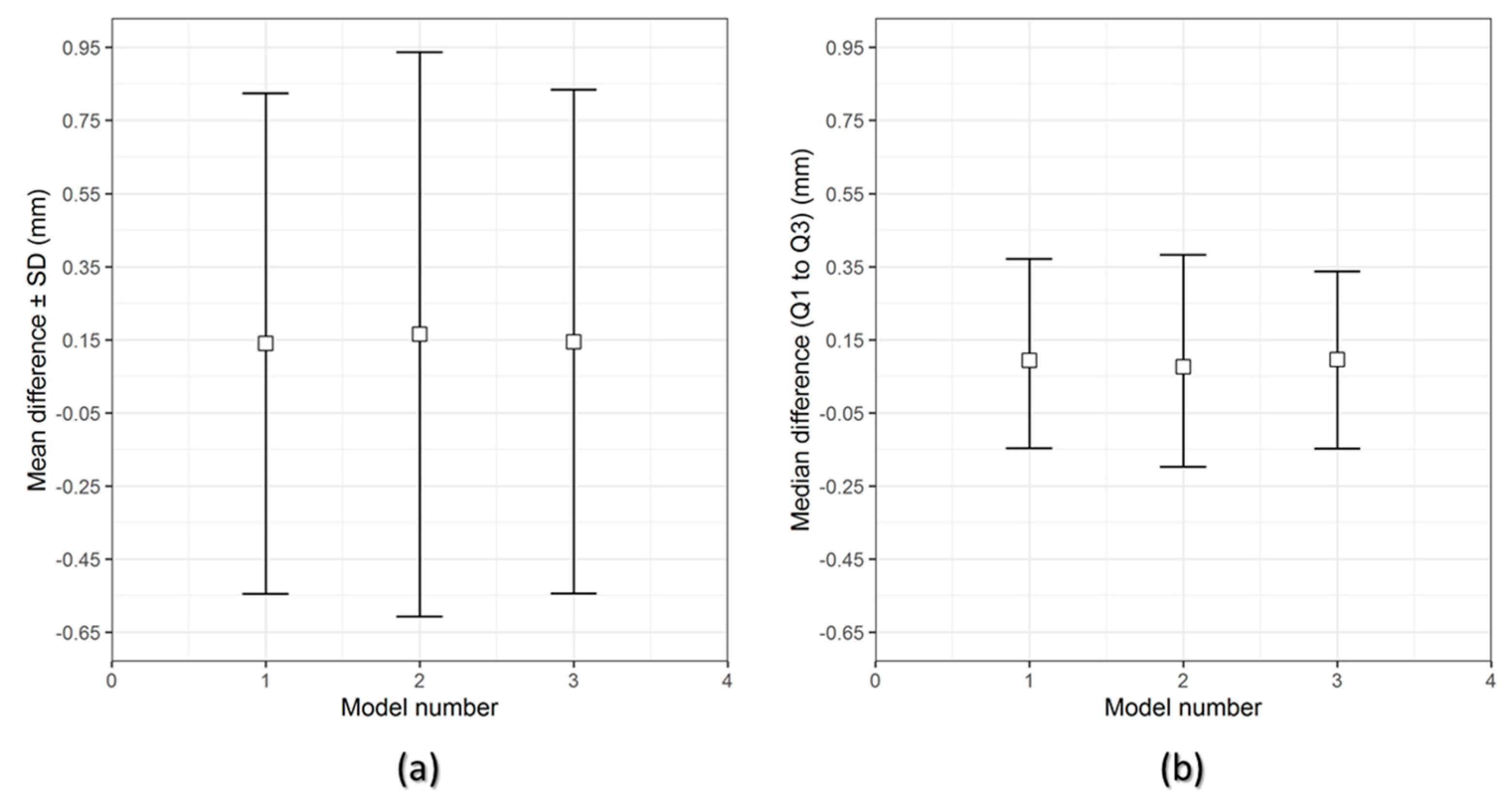
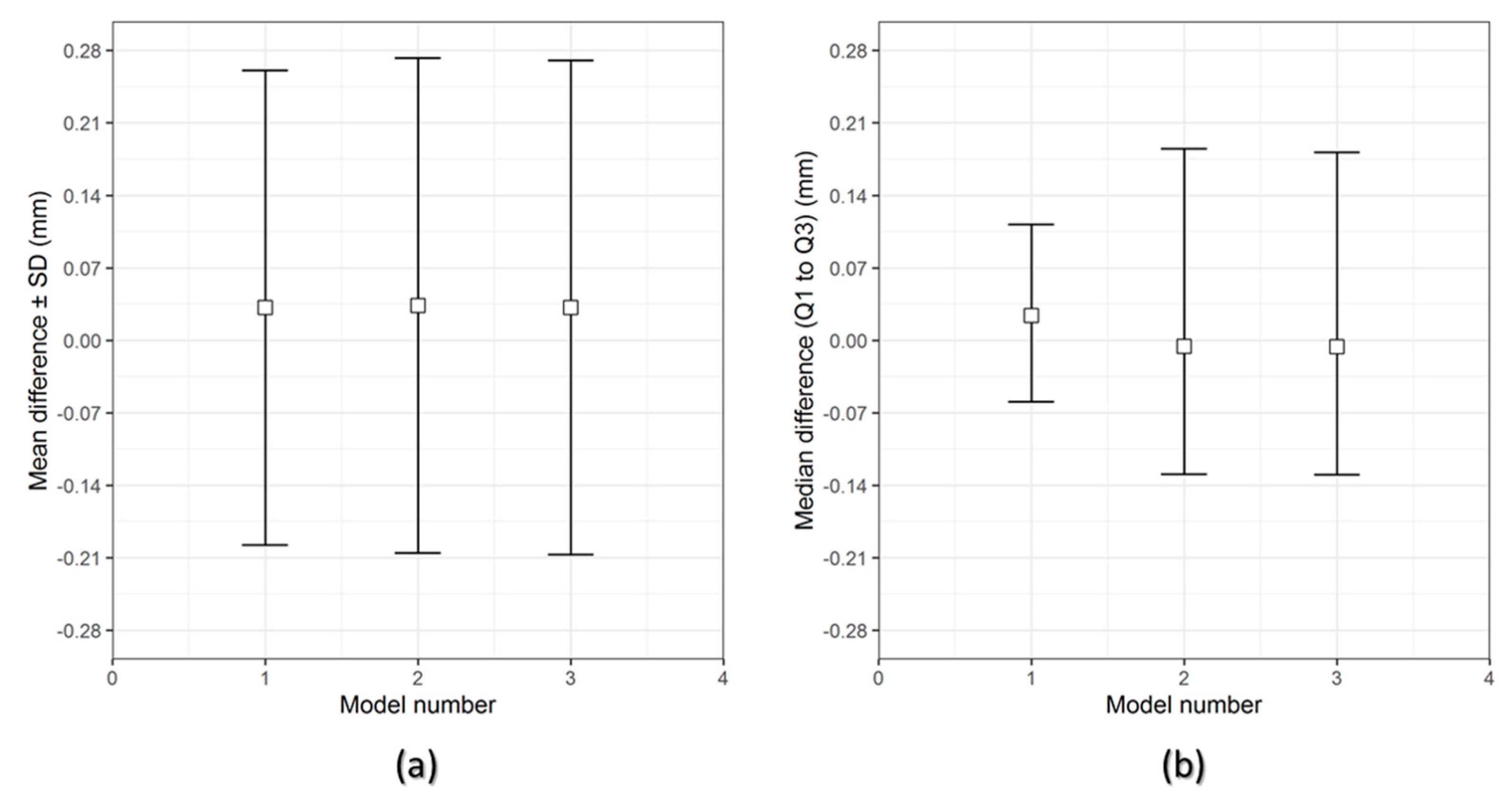
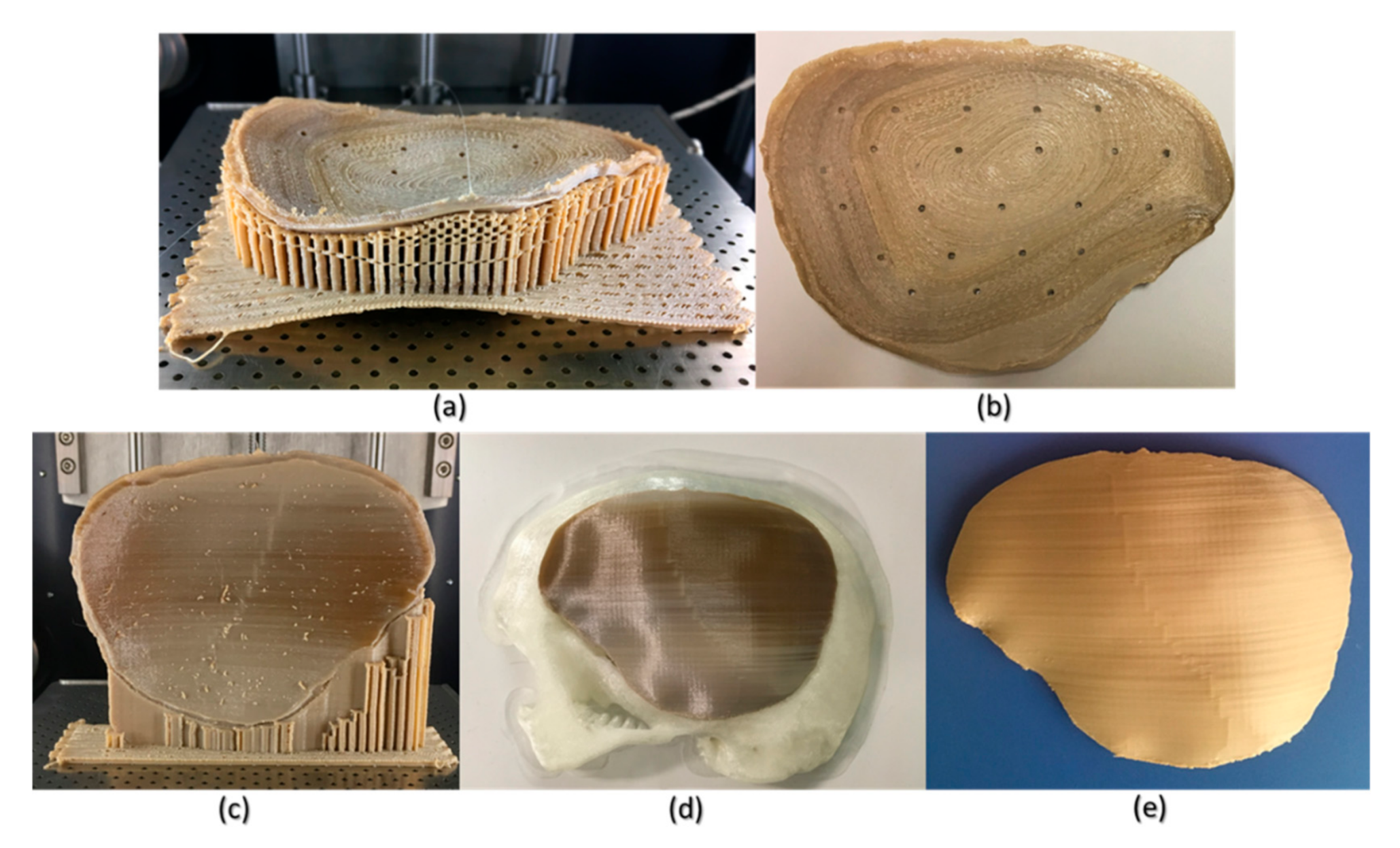
The results of the dimensional accuracy in the present study revealed that Group 2 PSIs had comparatively lower deviations than Group 1 PSIs. In the illustrated Group 1 case, the highest RMS value was 0.790 mm, while in Group 2 case, the highest RMS value was 0.241 mm. The level of accuracy required in a PSI depends on the clinical application. According to our results, PSIs in Group 1 and Group 2 were within the acceptable accuracy range required in cranioplasty procedures, with overall 3D deviations under 2 mm [
27]. Further analysis of the spatial distribution of variations revealed that the deviation pattern depended on the size and shape of the cranial defect, which was reflected in the PSIs fabricated (
Figure 8). The more considerable deviations in Group 1 PSIs can be explained due to the more significant anatomical cranial defect with greater span of the curvature.
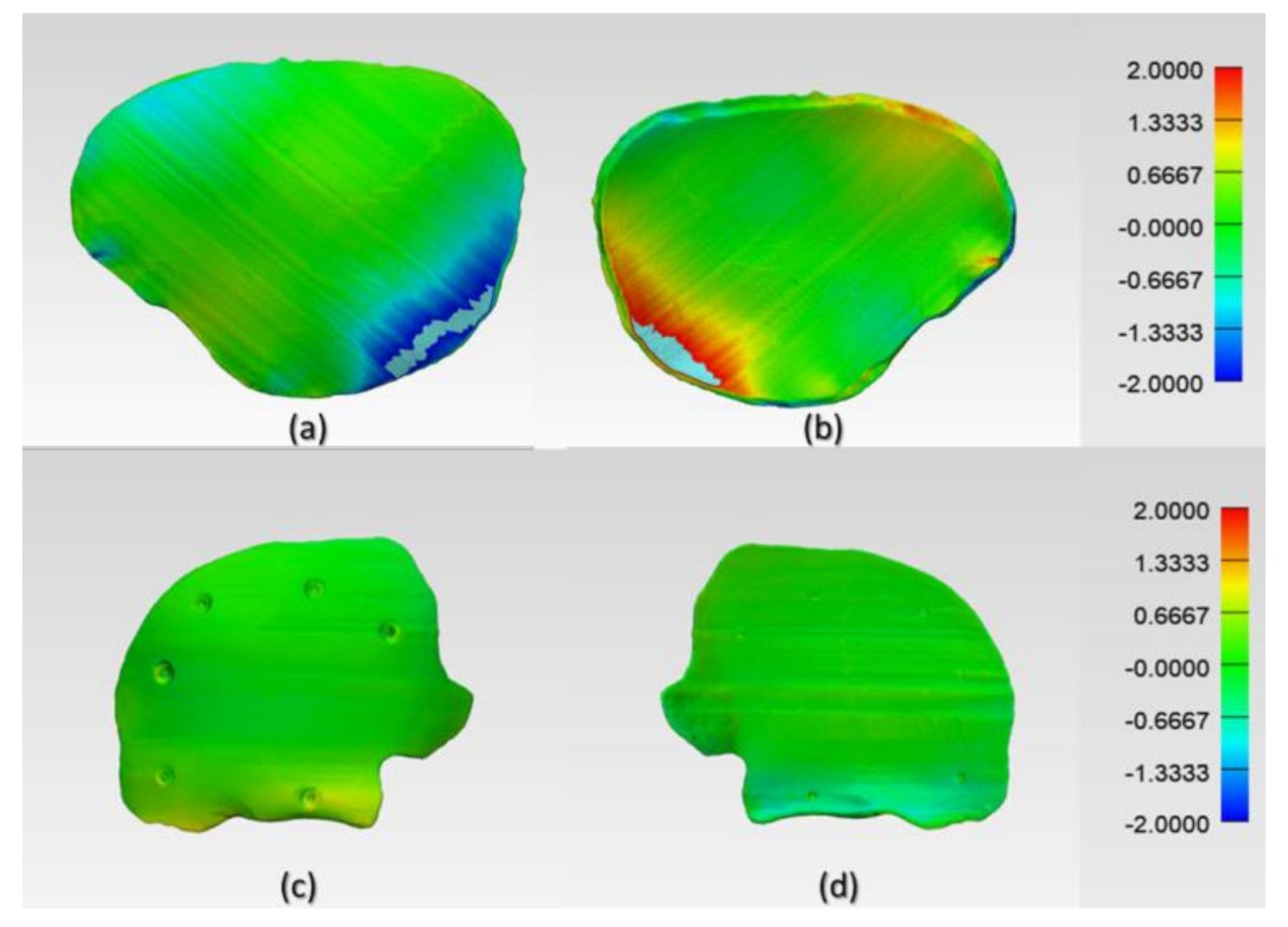
The results suggest that the dimensional characteristics of FFF 3D printed PEEK PSIs are a comprehensive consequence of a multitude of factors, including the thermal and nonthermal printing parameters of the 3D printer, the crystallinity of the parts, and the bonding interface between the printed layers. Group 1 PSIs displayed different color zones (
Figure 9) compared to Group 2 PSIs. These color changes can be explained due to varying levels of crystallinity, i.e., dark-brownish areas have a more amorphous PEEK structure, whereas the lighter areas have a higher degree of crystallinity. Studies have shown that PEEK mechanical properties are influenced by the level of crystallinity of the material. Increasing the crystallinity can improve the elastic modulus and yield strength of the fabricated PEEK part [
28]. Vaezi and Yang [
29] found that heat management during the FFF 3D printing process and optimum heat distribution around the part are essential parameters to affect the level of crystallinity in the 3D printed PEEK object. Jin et al. [
30] also showed that crystallinity in PEEK parts is influenced by the thermal processing conditions, such as the material cooling rate or thermal gradient. The amorphous regions in the printed parts can be optimized in the FFF printing process if the deposited materials are cooled down slowly or printed at a higher temperature to allow generation of crystalline PEEK structure [
31].
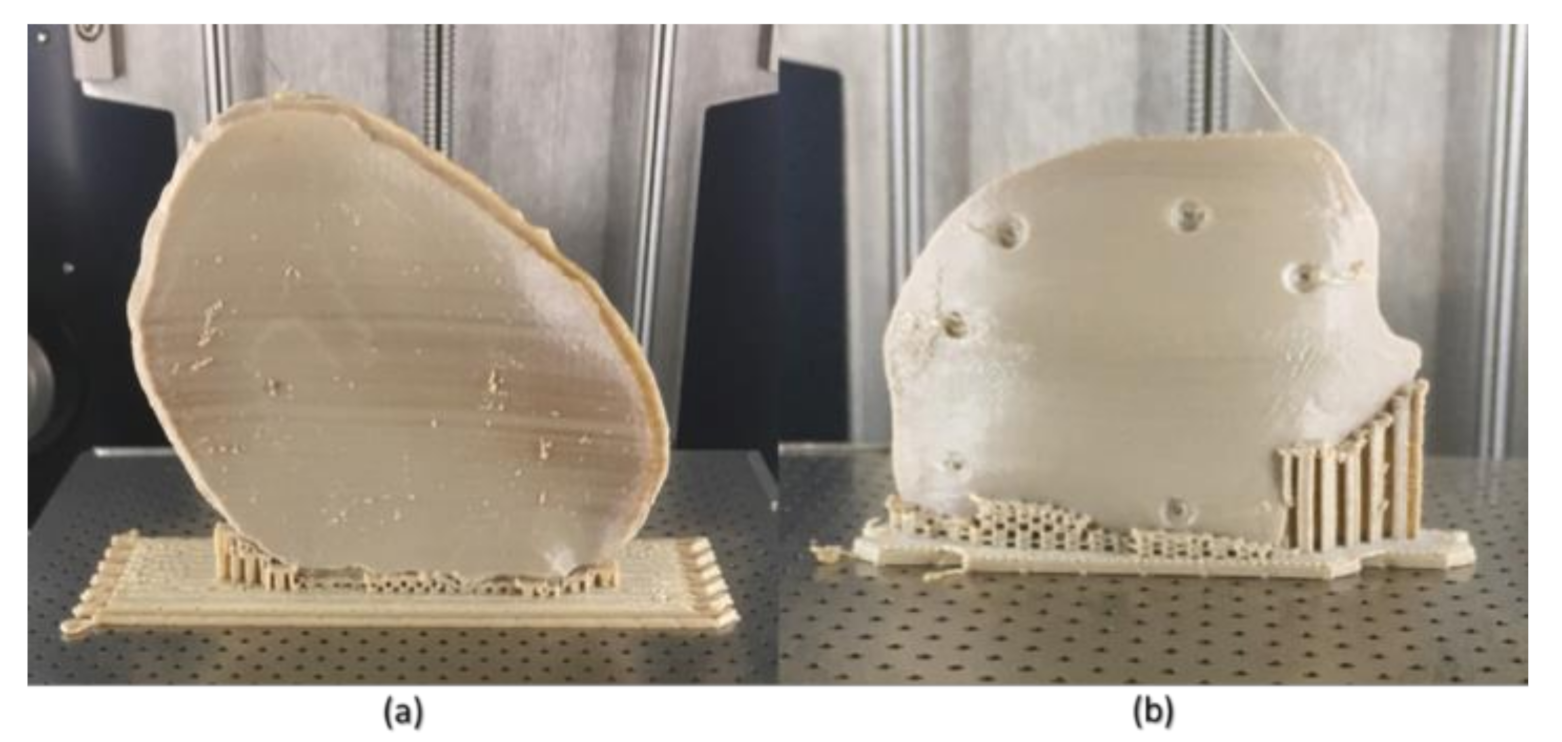
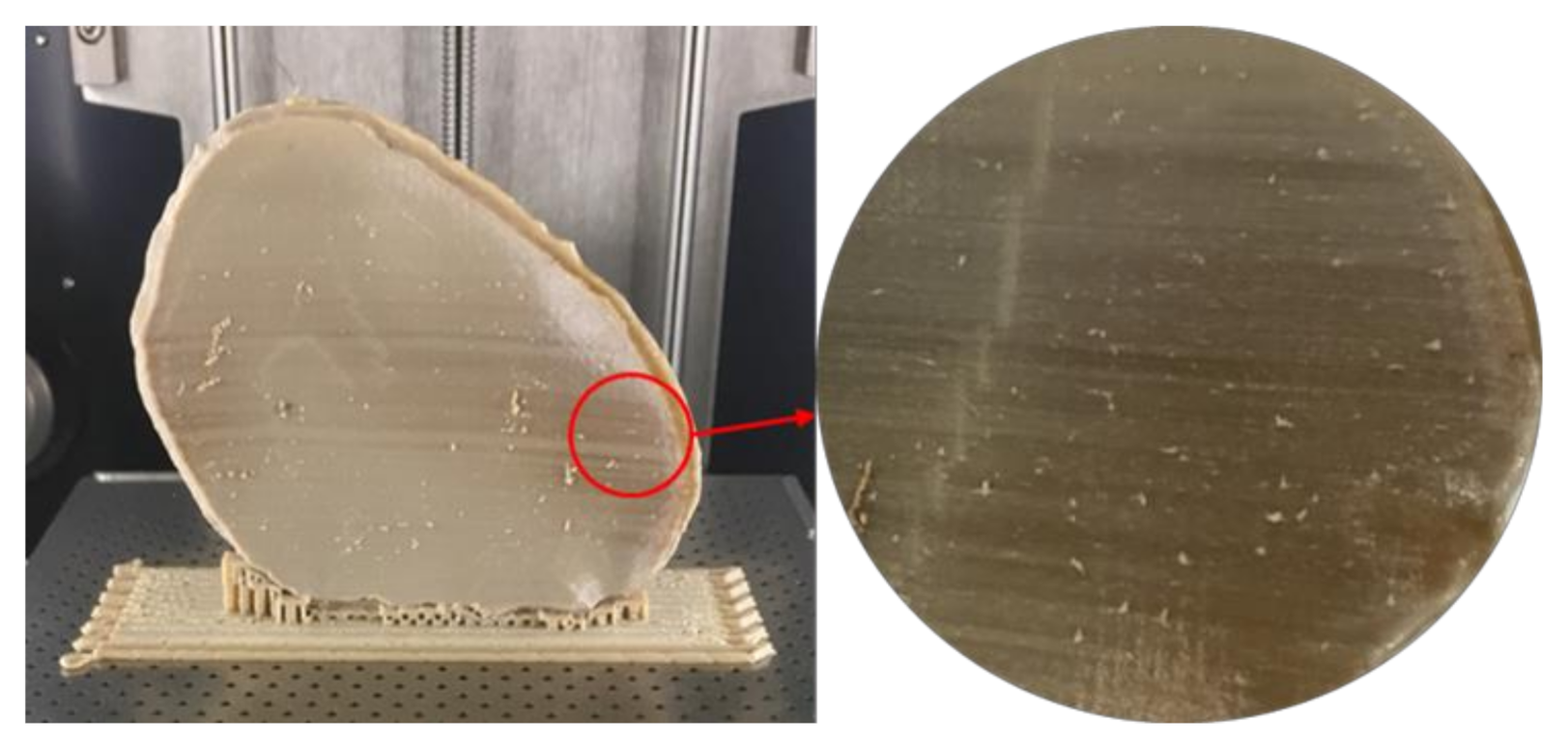
Although FFF seems like a simple process, the achievement of high efficiency and high-quality manufacturing results in PEEK printing presents significant challenges. The temperature of the illustrated FFF PEEK 3D printer used in our study was not user controlled; therefore, nonthermal printing parameters were tested to understand the dimensional characteristics. To further understand the issues with improper crystallinity, two additional cranial plates were printed; one in a horizontal build orientation and the other in a vertical build orientation positioned slightly away from the center of the build platform. We found that the test plate printed in a horizontal orientation had a very rough surface finish (
Figure 10b) but with fewer regions of amorphous PEEK. The uneven fabrication lines were caused because of the combination of the building orientation and complex geometry of the cranial implant. Moreover, by the end of the printing process, warping or detachment of the implant from the printer’s build platform was noticed (
Figure 10a). This effect can be explained because of the residual stress buildup that occurs during the printing process and the inability of the printer to maintain the required high processing temperatures consistently. In contrast, while the implant printed in a vertical orientation had a smoother surface finish, it also depicted different zones of crystallization (
Figure 10b,c). From the support structure removal perspective, the parts printed in a vertical orientation provided a much faster support removal than horizontally oriented printed counterparts. Therefore, it can be inferred that part orientation and usage of support structures affects the surface finish and dimensional accuracy in complex anatomically shaped PSIs.
Several studies have reported that further postprocessing procedures such as annealing [
28,
32] could eliminate the dark amorphous regions caused due to irregular crystallization in PEEK parts.
Figure 10d,e illustrates a PSI, before and after annealing postprocessing procedure. We noticed that the annealed cranial implant had an incomprehensible shrinkage and was, therefore, considered unfit for its clinical application. We, therefore, believe that although annealing helps to eliminate the amorphous regions and increases the mechanical strength of a part [
33], it also results in marked dimensional deviations, especially in complex anatomically shaped cranial implants. However, to further comprehend whether the variations with annealing postprocessing procedure are influenced by the dimensions and contours of a cranial prosthesis, further studies are required. Nonetheless, the issues with recrystallization of amorphous regions and an additional requirement of high-temperature postprocessing procedures can limit the use of FFF 3D printed PEEK cranial implants for in-hospital manufacturing.
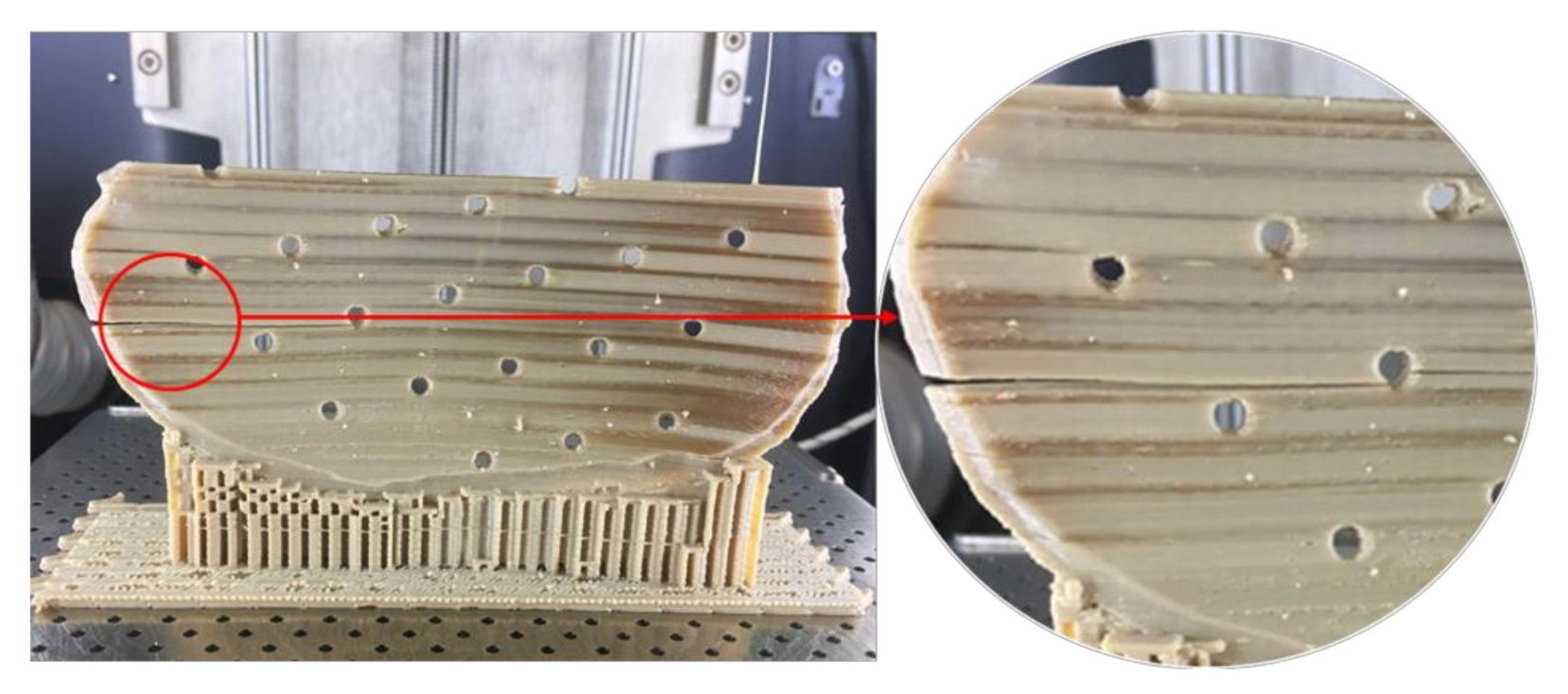
Unlike Group 2, we printed the PSIs in Group 1 with no drainage holes. We resorted to this fabrication method, seeing the best-fit option for the illustrated PSI in our study. We learned that the orientation of the cranial implant profoundly influenced the fabrication feasibility of cranial implants with holes. As Group 1 PSIs were more extensive in dimensions than Group 2 PSIs, the fabrication of implant with drainage holes was possible only in a horizontal orientation, which resulted in a very rough surface finish. Moreover, printing the implant with holes in a vertical orientation resulted in plate breakage during the fabrication process (
Figure 11). In FFF PEEK printing, the printed object attempts to accommodate these structural design effects, consequently leading to the initiation of internal stresses within the object. This results in a buildup of residual stresses, leading to crack propagation in the printed part. Due to the layer-by-layer fabrication method, each new layer is overlaid on top of the previous layer before material solidification from the melt occurs, resulting in volume shrinkage in the previous layer. The volume shrinkage contributes to weak interlayer bonding, and therefore, the structural failures are often confined to the interface between the layers [
34]. All these effects invariably contribute towards a requirement of optimum thermal management during the PEEK printing process, along with a consideration of the principles of design for additive manufacturing.
Until now, PEEK PSIs have been manufactured by external MedTech companies. This production method sometimes takes several weeks and requires numerous meetings between clinicians and biomedical engineers. Furthermore, the expenses related to the manufacturing of customized PEEK cranial implants are high and depend on the defect size and shape [
35]. For example, the average cost for the Group 1 and Group 2 PEEK PSIs procured from external companies is around 7000–10,000 € and 3000–4000 €, respectively. Nonetheless, the use of 3D printing technology in hospitals would be very advantageous. This could significantly reduce the production lead times and treatment times, thereby increasing patients’ satisfaction and surgical outcomes.
As the AM of PSIs is gradually moving towards in-house or POC manufacturing, clinicians need to understand the various factors that can affect the quality of the fabricated implant. As per the guidelines published in “Additively Manufactured Medical Products—The FDA Perspective,” like any external service provider, hospital-based 3D printing set-ups should provide the same efficacy and manufacturing quality for medical devices [
36]. Besides, organizations such as the American Society for Testing and Materials (ASTM) International, the International Organization for Standardization (ISO), and the Association for the Advancement of Medical Instrumentation (AAMI) have proposed standard technical consensus for PEEK medical devices [
37]. Furthermore, standardized operational measures such as quality management protocols should be implemented in the hospital environment to assess whether the intended 3D printed part conforms to its clinical application [
38]. One aspect of these protocols is part verification, which was analyzed in this study. Although the dimensional accuracy of PSIs fabricated in both the groups were within the clinically acceptable range; however, attention must be paid to the temperature control during the printing process to ensure that it is well regulated to fabricate implants of consistent crystallinity.
AM processing of PEEK thermoplastic polymer for the fabrication of large-sized, complex cranial implants presents significant challenges due to the limitations associated with large thermal gradients, residual stress buildup, and the inability of the 3D printer to provide the required ambient temperatures consistently. A beta version of an updated software from the FFF PEEK 3D printer’s manufacturer, which promises a layer-by-layer incremental increase in the airflow temperature, is in the developmental stage. Therefore, these results will need to be revisited to access the improved performance of the FFF PEEK printing process for medical implants at the POC manufacturing. Another aspect that needs attention is the anisotropic behavior of the FFF 3D printed PEEK cranial implants. Due to the layer-by-layer fabrication method, the specific anisotropic performance needs to be tested in future experiments addressing the biomechanical properties of the FFF 3D printed PEEK cranial implants.