Anti-IL-6 Receptor Tocilizumab in Refractory Graves’ Orbitopathy: National Multicenter Observational Study of 48 Patients
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients, Enrollment Criteria and Study Design
2.2. Outcome Variables
2.3. Data Collection
2.4. Statistical Analysis
3. Results
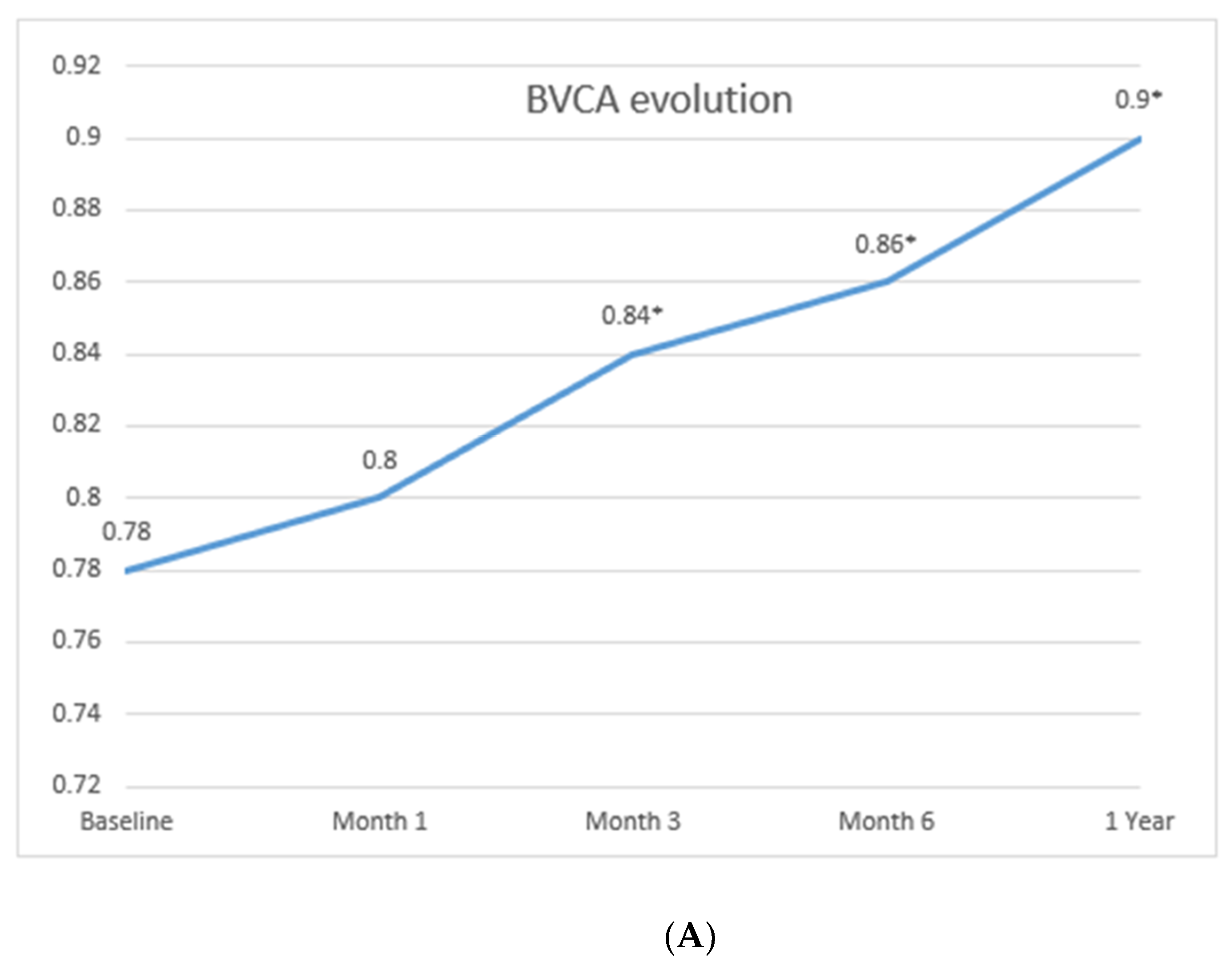
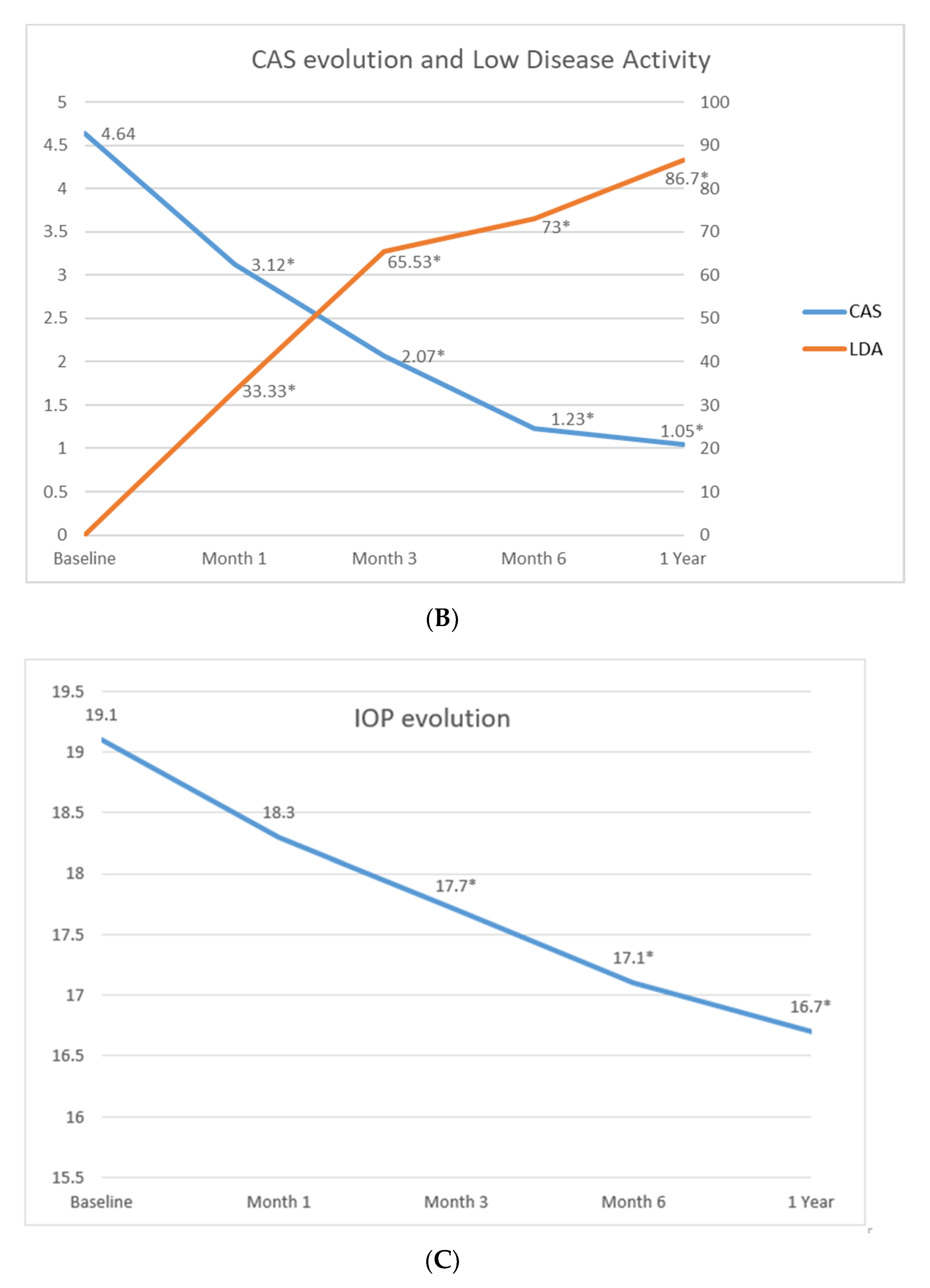
3.1. Main Clinical Features at Tocilizumab Onset
3.2. Treatment with Tocilizumab and Efficacy
3.3. Follow-Up and Side Effects of Tocilizumab Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Wang, Y.; Tooley, A.A.; Mehta, V.J.; Garrity, J.A.; Harrison, A.R.; Mettu, P. Thyroid Orbitopathy. Int. Ophthalmol. Clin. 2018, 58, 137–179. [Google Scholar] [CrossRef] [PubMed]
- Genere, N.; Stan, M.N. Current and Emerging Treatment Strategies for Graves’ Orbitopathy. Drugs 2019, 79, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Chandra Saha, B. Advances in the management of thyroid eye diseases: An overview. Int. Ophthalmol. 2018, 38, 2247–2255. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Baldeschi, L.; Dickinson, A.J.; Eckstein, A.; Kendall-Taylor, P.; Marcocci, C.; Mourits, M.P.; Perros, P.; Boboridis, K.G.; Boschi, A.; et al. Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid 2008, 18, 333–346. [Google Scholar] [CrossRef]
- Drui, D.; Du Pasquier Fediaevski, L.; Vignal Clermont, C.; Daumerie, C. Graves’ Orbitopathy: Diagnosis and treatment. Ann. Endocrinol. (Paris) 2018, 79, 656–664. [Google Scholar] [CrossRef]
- Li, Z.; Cestari, D.M.; Fortin, E. Thyroid eye disease: What is new to know? Curr. Opin. Ophthalmol. 2018, 29, 528–534. [Google Scholar] [CrossRef]
- Kotwal, A.; Stan, M. Current and Future Treatments for Graves’ Disease and Graves’ Ophthalmopathy. Horm. Metab. Res. 2018, 50, 871–886. [Google Scholar] [CrossRef] [Green Version]
- Stan, M.N.; Garrity, J.A.; Carranza Leon, B.G.; Prabin, T.; Bradley, E.A.; Bahn, R.S. Randomized Controlled Trial of Rituximab in patients with Graves’ Orbitopathy. J. Clin. Endocrinol. Metab. 2015, 100, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Salvi, M.; Vannucchi, G.; Currò, N.; Campi, I.; Covelli, D.; Dazzi, D.; Simonetta, S.; Guastella, C.; Pignataro, L.; Avignone, S.; et al. Efficacy of B-cell Targeted Therapy with Rituximab in Patients with Active Moderate to Severe Graves’ Orbitopathy: A randomized controlled study. J. Clin. Endocrinol. Metab. 2015, 100, 422–431. [Google Scholar] [CrossRef]
- Smith, T.J.; Kahaly, G.J.; Ezra, D.G.; Fleming, J.C.; Dailey, R.A.; Tang, R.A.; Harris, G.J.; Antonelli, A.; Salvi, M.; Goldberg, R.; et al. Teprotumumab for Thyroid-Associated Ophthalmopathy. N. Engl. J. Med. 2017, 376, 1748–1761. [Google Scholar] [CrossRef]
- Perez-Moreiras, J.V.; Gomez-Reino, J.J.; Maneiro, J.R.; Pampin, E.P.; Lopez, A.R.; Alvarez, F.M.R.; Laguarta, J.M.C.; Cabello, A.D.E.; Sorroche, M.G.; Gregori, E.E.; et al. Efficacy of Tocilizumab in Patients With Moderate-to-Severe Corticosteroid-Resistant Graves’ Orbitopathy: A Randomized Clinical Trial. Am. J. Ophthalmol. 2018, 195, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Slowik, M.; Urbaniak-Kujda, D.; Bohdanowicz-Pawlak, A.; Kapelko-Slowik, K.; Dybko, J.; Wolowiec, D.; Jaźwiec, B.; Daroszewski, J. CD8+CD28-lymphocytes in Peripheral Blood and Serum Concentrations of Soluble Interleukin 6 Receptor are Increased in Patients with Graves’ Orbitopathy and Correlate with Disease Activity. Endocr. Res. 2012, 37, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bossowski, A.; Urban, M.; Koput, A.; Gardziejczyk, M.; Wysocka, J.; Kreczko, S. Serum Levels of Interleukin 6 (IL-6) and Soluble IL-6 Receptor (sIL-6R) in Children and Adolescents with Graves-Basedow Disease. Endokrynol. Diabetol. Choro- Przemiany Materii Wieku Rozw. 1999, 5, 85–93. [Google Scholar]
- Salvi, M.; Girasole, G.; Pedrazzoni, M. Increased serum concentrations of IL-6 and sIL-6 receptor in patients with Graves’ disease. J. Clin. Endocrinol. Metabol. 1996, 81, 2976–2984. [Google Scholar]
- Pérez-Moreiras, J.V.; Álvarez-López, A.; Gómez, E.C. Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Bégaud, B.; Atry, P.; Miremont-Salamé, G.; Fourrier, A.; Moore, N. Differences between Clinical trials and postmarketing use. Br. J. Clin. Pharmacol. 2004, 57, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Stuart, E.A.; Cole, S.R.; Bradshaw, C.P.; Leaf, P.J. The use of propensity scores to assess the generalizability of results from randomized trials. J. R. Stat. Soc. Ser. A Stat. Soc. 2001, 174, 369–386. [Google Scholar] [CrossRef] [Green Version]
- Canas, C.A.; Bonilla-Abadia, F.; Vallejo, K.; Rengifo, H.M.; Gallon, M.A.; Tobon, G.J. Successful Treatment for Severe Thyroid associated Ophthalmopathy with Tocilizumab. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 665–667. [Google Scholar] [CrossRef]
- de-Pablo-Gómez-de-Liaño, L.; Fernández-Vigo, J.I.; Troyano-Rivas, J.; Niño-Rueda, C.; Romo-López, Á.; Gómez-de-Liaño, R. Response to Tocilizumab Treatment in Graves’ Ophthalmopathy by Measuring Rectus Muscle Thickness and Chemosis Using Optical Coherence Tomography. Evaluación del grosor de los músculos extraoculares y la quemosis tras tocilizumab en oftalmopatía de Graves mediante tomografía de coherencia óptica. Arch. Soc. Esp. Oftalmol. 2018, 93, 386–391. [Google Scholar] [CrossRef]
- Maldiney, T.; Deschasse, C.; Bielefeld, P. Tocilizumab for the Management of Corticosteroid-Resistant Mild to Severe Graves’ Ophthalmopathy, a Report of Three Cases. Ocul. Immunol. Inflamm. 2020, 28, 281–284. [Google Scholar] [CrossRef]
- Sy, A.; Eliasieh, K.; Silkiss, R.Z. Clinical Response to Tocilizumab in Severe Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Gómez Rodríguez, L.; Cárdenas Aranzana, M.J.; Avilés Mora, C. Effectiveness and safety of Tocilizumab in corticoid refractory Graves’ Orbitopathy. Farm Hosp. 2014, 38, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.J.; Wagner, L.H.; Seiff, S.R. Tocilizumab as a steroid sparing agent for the treatment of Graves’ orbitopathy. Am. J. Ophthalmol. Case Rep. 2017, 7, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, M.R.; Boelaert, K. Knowledge of Thyroid Eye Disease in Graves’ Disease Patients with and Without Orbitopathy. Thyroid 2019, 29, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, M.; Schott, M.; Allelein, S. Graves’ Disease in Clinical Perspective. Front. Biosci 2019, 24, 35–47. [Google Scholar] [CrossRef]
- Davies, M.D.; Henry Burch, M.D. Clinical Features and Diagnosis of Graves’ Orbitopathy (Ophthalmopathy). Post, T.W., ed.; UpToDate. Waltham, MA: UpToDate Inc. Available online: https://www.uptodate.com (accessed on 9 May 2020).
- Barrio-Barrio, J.; Sabater, A.L.; Bonet-Farriol, E.; Velázquez-Villoria, Á.; Galofré, J.C. Graves’ Ophthalmopathy: VISA versus EUGOGO Classification, Assessment, and Management. J. Ophthalmol. 2015, 249125. [Google Scholar] [CrossRef] [Green Version]
- Martín-Varillas, J.L.; Calvo-Río, V.; Beltran, E.; Sánchez-Bursón, J.; Mesquida, M.; Adan, A.; Hernández, M.V.; Garfella, M.H.; Pascual, E.V.; Martínez-Costa, L.; et al. Successful Optimization of Adalimumab Therapy in Refractory Uveitis Due to Behçet’s Disease. Ophthalmology 2018, 125, 1444–1451. [Google Scholar] [CrossRef] [Green Version]
- Riancho-Zarrabeitia, L.; Calvo-Río, V.; Blanco, R.; Mesquida, M.; Adan, A.M.; Herreras, J.M.; Aparicio, A.; Peiteado-Lopez, D.; Cordero-Coma, M.; Serrano, J.L.G.; et al. Anti-TNF-α therapy in refractory uveitis associated with sarcoidosis: Multicenter study of 17 patients. Semin. Arthritis Rheum. 2015, 45, 361–368. [Google Scholar] [CrossRef]
- Calvo-Río, V.; Blanco, R.; Santos-Gómez, M.; Rubio-Romero, E.; Cordero-Coma, M.; Gallego-Flores, A.; Veroz, R.; Torre, I.; Hernández, F.F.; Atanes, A.; et al. Golimumab in Refractory Uveitis Related to Spondyloarthritis. Multicenter Study of 15 Patients. Semin. Arthritis Rheum. 2016, 46, 95–101. [Google Scholar] [CrossRef]
- Calderón-Goercke, M.; Loricera, J.; Aldasoro, V.; Castañeda, S.; Villa, I.; Humbría, A.; Moriano, C.; Romero-Yuste, S.; Narváez, J.; Gómez-Arango, C.; et al. Tocilizumab in Giant Cell Arteritis. Observational, Open-Label Multicenter Study of 134 Patients in Clinical Practice. Semin. Arthritis Rheum. 2019, 49, 126–135. [Google Scholar] [CrossRef]
- Levenson, J.H.; Kozarsky, A. Visual Acuity. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; Chapter 115. [Google Scholar]
- Atienza-Mateo, B.; Calvo-Río, V.; Beltran, E.; Martínez-Costa, L.; Valls-Pascual, È.; Hernández-Garfella, M.; Atanes, A.; Cordero-Coma, M.; Nolla, J.M.; Carrasco-Cubero, C.; et al. Anti-interleukin 6 receptor tocilizumab in refractory uveitis associated with Behçet’s disease: Multicentre retrospective study. Rheumatology 2018, 57, 856–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Río, V.; De La Hera, D.; Beltrán-Catalán, E.; Blanco, R.; Hernandez, M.; Martínez-Costa, L.; Loricera, J.; Cañal, J.; Ventosa, J.; Ortiz-Sanjuán, F.; et al. Tocilizumab in uveitis refractory to other biologic drugs: A study of 3 cases and a literature review. Clin. Exp. Rheumatol. 2014, 32 (Suppl. 84), S54–S57. [Google Scholar]
- Santos-Gómez, M.; Calvo-Río, V.; Blanco, R.; Beltrán, E.; Mesquida, M.; Adán, A.; Cordero-Coma, M.; García-Aparicio, A.M.; Valls Pascual, E.; Martínez-Costa, L.; et al. The Effect of Biologic Therapy Different from Infliximab or Adalimumab in patients with Refractory Uveitis due to Behçet’s Disease: Results of a Multicentre Open-label Study. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 102), S34–S44. [Google Scholar]
- Vegas-Revenga, N.; Calvo-Río, V.; Mesquida, M.; Adán, A.; Hernández, M.V.; Beltrán, E.; Pascual, E.V.; Díaz-Valle, D.; Cordovés, G.D.; Hernandez-Garfella, M.; et al. Anti-IL6-Receptor Tocilizumab in Refractory and Noninfectious Uveitic Cystoid Macular Edema: Multicenter Study of 25 Patients. Am. J. Ophthalmol. 2019, 200, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Stem, M.S.; Todorich, B.; Faia, L.J. Ocular Pharmacology for Scleritis: Review of Treatment and a Practical Perspective. J. Ocul. Pharmacol. Ther. 2017, 33, 240–246. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Ma, L.; Lasave, A.F.; Foster, C.S. Rituximab Induction and Maintenance Treatment in Patients with Scleritis and Granulomatosis with Polyangiitis (Wegener’s). Ocul. Immunol. Inflamm. 2018, 26, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, W.; Wu, J.; Zhang, H.; Zhou, H. Peripheral Ulcerative Keratitis Associated with Autoimmune Disease: Pathogenesis and Treatment. J. Ophthalmol. 2017, 2017, 7298026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, S.; Hashemi, K.; Catanese, M.; Candil, M.; Zufferey, P.; Gabison, E.; Guex-Crosier, Y. Necrotising Scleritis and Peripheral Ulcerative Keratitis Associated with Rheumatoid Arthritis Treated with Rituximab. Klinische Monatsblätter für Augenheilkunde 2017, 234, 567–570. [Google Scholar] [CrossRef]
- Abad, S.; Héran, F.; Terrada, C.; Bielefeld, P.; Sène, D.; Trad, S.; Saadoun, D.; Sève, P. Management of Orbital Inflammation in Internal Medicine. Proposal for a diagnostic work-up. Rev. Med. Interne 2018, 39, 746–754. [Google Scholar] [CrossRef]
- Mourits, M.P.; Prummel, M.F.; Wiersinga, W.M.; Koornneef, L. Clinical Activity Score as a Guide in the Management of Patients with Graves’ Ophthalmopathy. Clin. Endocrinol. 1997, 47, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, E.H.; Bernasconi, C.; Aassi, M.; Molina, J.F.; Epis, O.M. Treatment of Rheumatoid Arthritis with Anti-Tumor Necrosis Factor or Tocilizumab Therapy as First Biologic Agent in a Global Comparative Observational Study. Arthritis Care Res. (Hoboken) 2017, 69, 1484–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, C.; Emery, P.; Van Vollenhoven, R.; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. Tocilizumab Monotherapy Versus Adalimumab Monotherapy for treatment of Rheumatoid Arthritis (ADACTA): A Randomised, Double-Blind, Controlled Phase 4 Trial. Lancet 2013, 381, 1541–1550. [Google Scholar] [CrossRef]

| Number of Patients/Eyes Affected, n/n | 48/95 * |
|---|---|
| Age, mean (SD), years | 50.96 (11.8) |
| Sex, men/women, n/n (%) | 10/38 (20.8/79.2) |
| Duration GD in years before TCZ, median (IQR) | 1.8 (0.7–4.0) |
| Duration GO in years before TCZ, median (IQR) | 0.9 (0.0–2.4) |
| Thyroidopathy family history, n (%) | 12 (25) |
| Smoking, n (%) | 25 (52.1) |
| Comorbidities, n (%) | |
| Hypertension | 14 (29.2) |
| Diabetes mellitus | 6 (12.5) |
| Dyslipidemia | 12 (25) |
| Others | 16 (33.3) |
| Hormones, mean (SD) | |
| T4 (ref. 0.8–1.8 ng/dL) | 4.04 (8.08) |
| T3 (ref. 2.3–4.2 pg/mL) | 4.18 (4.26) |
| TSH (ref. 0.55–4.78 mIU/L) | 4.46 (18.98) |
| Antithyroid antibodies, (%)/mean (SD) mcg/dL | |
| TSI/TRAb (ref. 0–0.7 U/L) | 77%/17.3 (19.3) 1 |
| GO complications present before treatment, n (eyes) (%) | |
| Exophthalmos | 57 (60) |
| Strabismus | 40 (42.1) |
| Muscle fibrosis | 41 (43.2) |
| DON | 7 (14.7) |
| Previous treatment to TCZ onset, n (%) | |
| Pulses of IVMP | 43 (89.6) |
| Selenium | 11 (22.9) |
| Decompressive surgery | 7 (14.58) |
| Regimen of TCZ therapy | |
| Monotherapy/combined treatment, n (%) | 45/3 (93.8/6.2) |
| AZA | 1 (2.1) |
| MTX | 2 (4.2) |
| TCZ dosage, n (%) | |
| 8 mg/kg/IV/4 weeks | 43 (89.6) |
| 162 mg/s.c./week | 5 (10.4) |
| Follow-up on TCZ therapy, mean (SD), months | 16.05 ± 2.06 |
| Low disease activity, n (%) | 79 (83.2) |
| Discontinuation treatment, n (%) | 29 (60.4) |
| Low disease activity | 25 (86.2) |
| Inefficacy | 4 (13.8) |
| Side effects/toxicity | 0 |
| Relapses number | 0 |
| Severe side effects, n (per 100 patients/year) | 0 |
| Baseline n = 95 | Month 1 n = 87 | Month 3 n = 85 | Month 6 n = 85 | Month 12 n = 83 | |
|---|---|---|---|---|---|
| Low disease activity | NA | 29 (33.33) * | 54 (63.53) * | 62 (72.94) * | 72 (86.75) * |
| Clinical response in CAS | NA | 43 (49.42) * | 64 (75.29) * | 76 (89.41) * | 79 (95.18) * |
| Spontaneous retroocular pain | 42 (44.21) | 28 (32.18) | 11 (12.94) * | 5 (5.88) * | 0 (0) * |
| Pain on eye movement | 52 (54.73) | 41 (47.13) | 36 (42.35) | 18 (21.17) * | 2 (2.41) * |
| Eyelids redness | 42 (44.21) | 35 (40.23) | 28 (32.94) | 12 (14.12) * | 9 (10.84) * |
| Conjunctival redness | 74 (77.89) | 56 (64.37) | 57 (67.05) | 33 (38.83) * | 16 (19.28) * |
| Caruncular edema | 44 (46.31) | 38 (43.67) | 16 (18.82) * | 13 (15.29) * | 7 (8.43) * |
| Eyelids edema | 77 (81.05) | 64 (73.56) | 69 (81.17) | 57 (67.05) * | 47 (56.63) * |
| Outcome in chemosis | 54 (56.84) | 37 (42.53) | 40 (47.05) | 22 (25.88) * | 14 (16.87) * |
| Proptosis (>2 mm) | ND | 25 (28.74) | 16 (18.82) | 11 (12.64) * | 5 (6.02) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bilbao, L.; Martínez-López, D.; Revenga, M.; López-Vázquez, Á.; Valls-Pascual, E.; Atienza-Mateo, B.; Valls-Espinosa, B.; Maiz-Alonso, O.; Blanco, A.; Torre-Salaberri, I.; et al. Anti-IL-6 Receptor Tocilizumab in Refractory Graves’ Orbitopathy: National Multicenter Observational Study of 48 Patients. J. Clin. Med. 2020, 9, 2816. https://doi.org/10.3390/jcm9092816
Sánchez-Bilbao L, Martínez-López D, Revenga M, López-Vázquez Á, Valls-Pascual E, Atienza-Mateo B, Valls-Espinosa B, Maiz-Alonso O, Blanco A, Torre-Salaberri I, et al. Anti-IL-6 Receptor Tocilizumab in Refractory Graves’ Orbitopathy: National Multicenter Observational Study of 48 Patients. Journal of Clinical Medicine. 2020; 9(9):2816. https://doi.org/10.3390/jcm9092816
Chicago/Turabian StyleSánchez-Bilbao, Lara, David Martínez-López, Marcelino Revenga, Ángel López-Vázquez, Elia Valls-Pascual, Belén Atienza-Mateo, Beatriz Valls-Espinosa, Olga Maiz-Alonso, Ana Blanco, Ignacio Torre-Salaberri, and et al. 2020. "Anti-IL-6 Receptor Tocilizumab in Refractory Graves’ Orbitopathy: National Multicenter Observational Study of 48 Patients" Journal of Clinical Medicine 9, no. 9: 2816. https://doi.org/10.3390/jcm9092816





