Effects of Robot-Assisted Gait Training in Patients with Burn Injury on Lower Extremity: A Single-Blind, Randomized Controlled Trial
Abstract
:1. Background
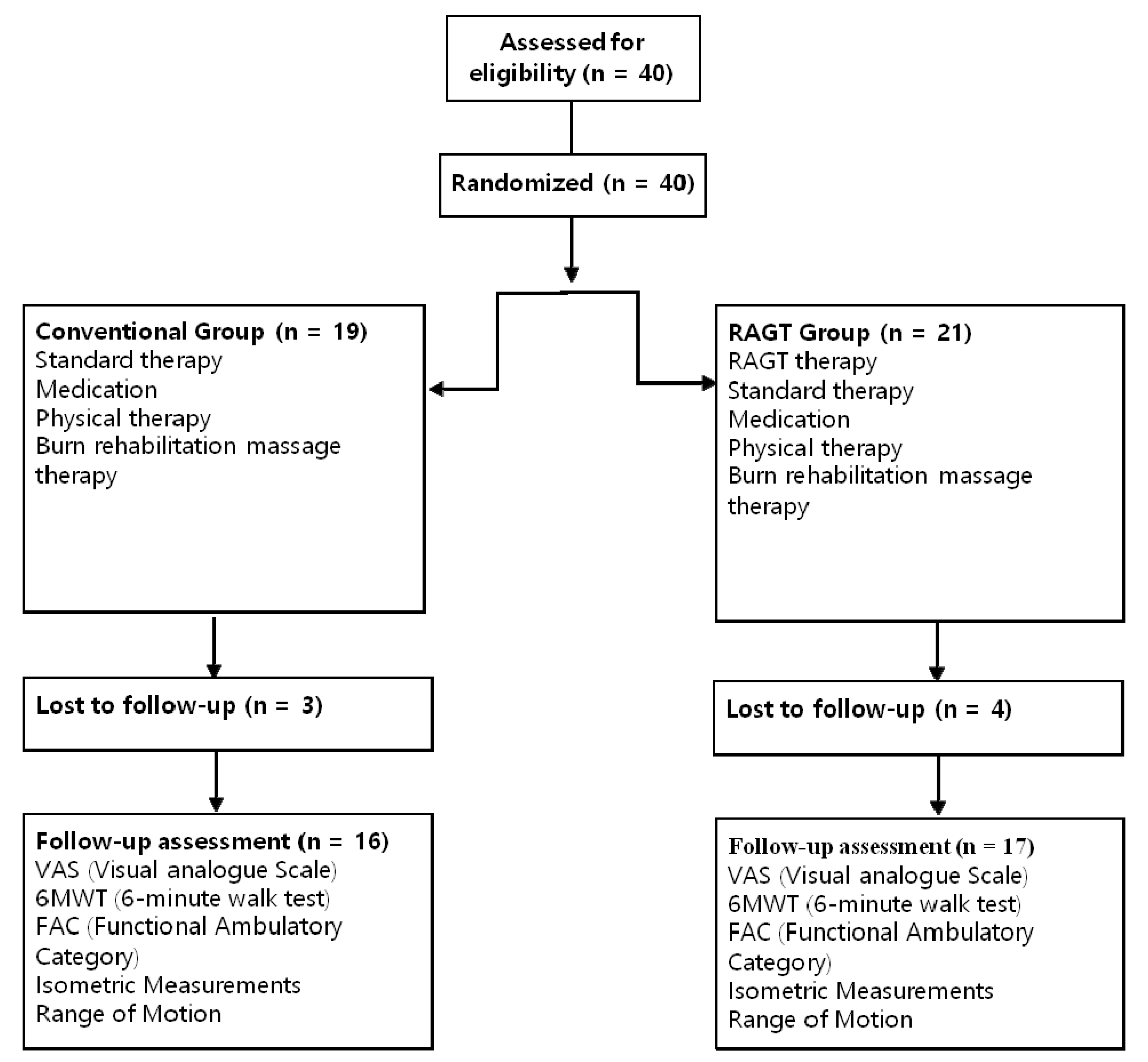
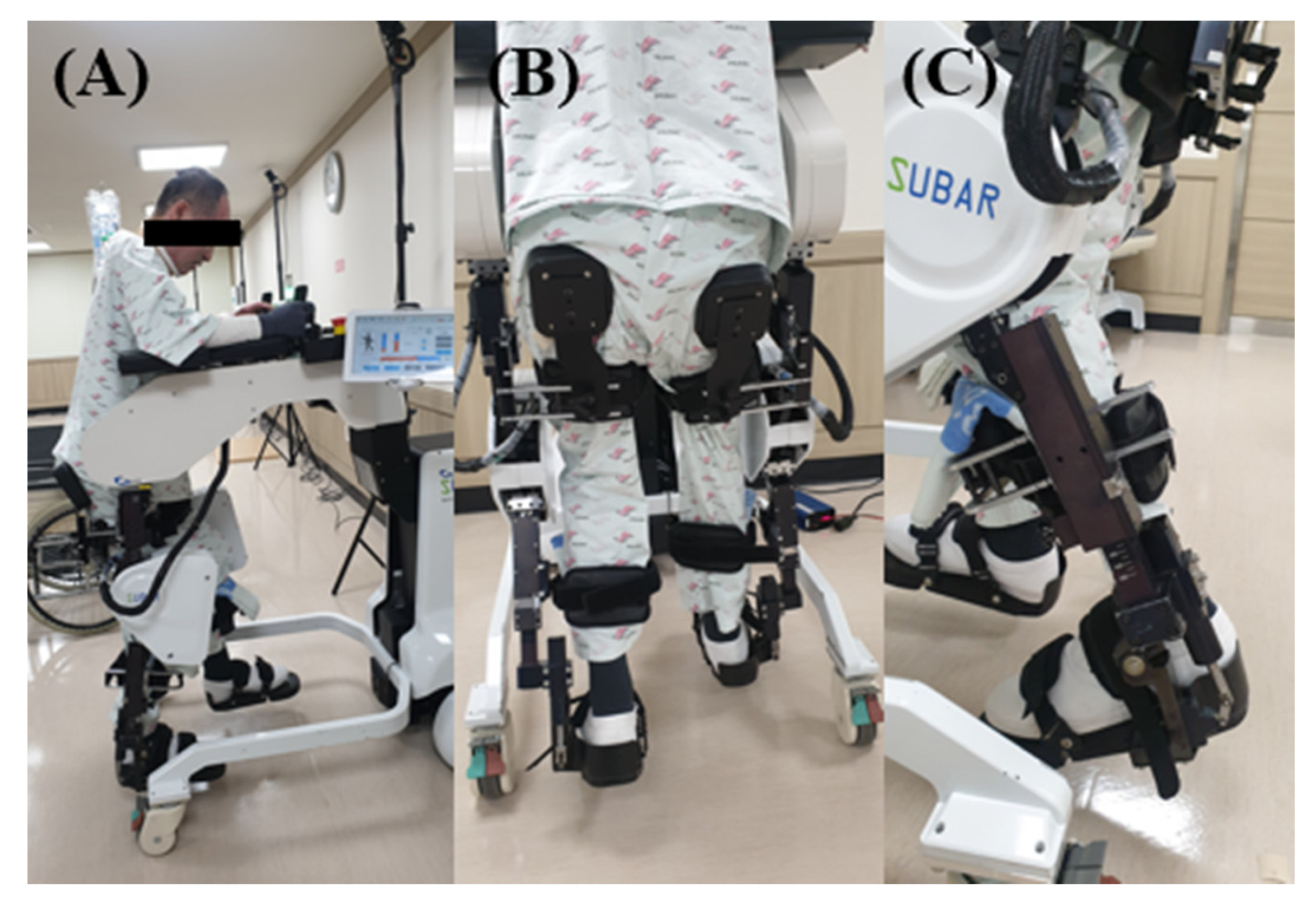
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bohannon, R.W.; Crouch, R. Minimal clinically important difference for change in 6-min walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef]
- Gawaziuk, J.P.; Peters, B.; Logsetty, S. Early ambulation after-grafting of lower extremity burns. Burn. J. Int. Soc. Burn Inj. 2018, 44, 183–187. [Google Scholar] [CrossRef]
- Tan, J.; Chen, J.; Zhou, J.; Song, H.; Deng, H.; Ao, M.; Luo, G.; Wu, J. Joint contractures in severe burn patients with early rehabilitation intervention in one of the largest burn intensive care unit in China: A descriptive analysis. Burns Trauma. 2019, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.G.; Yun, S.J.; Shin, H.I.; Kim, E.; Lee, H.H.; Oh, B.M.; Seo, H.G. Effects of robot-assisted gait training in patients with Parkinson’s disease: Study protocol for a randomized controlled trial. Trials 2019, 20, 15. [Google Scholar] [CrossRef]
- Johnson, M.J. Recent trends in robot-assisted therapy environments to improve real-life functional performance after stroke. J. Neuroeng. Rehabil. 2006, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.; Choi, S.; Kim, H.; Lee, G.; Han, C.; Ji, Y.; Shin, D.; Hwang, S.; Yun, D.; Jang, H.; et al. Feasibility of rehabilitation training with a newly Developed, portable, gait assistive robot for balance function in hemiplegic patients. Ann. Rehabil. Med. 2017, 41, 178–187. [Google Scholar] [CrossRef]
- Sczesny-Kaiser, M.; Höffken, O.; Aach, M.; Cruciger, O.; Grasmücke, D.; Meindl, R.; Schildhauer, T.A.; Schwenkreis, P.; Tegenthoff, M. HAL(R) exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J. Neuroeng. Rehabil. 2015, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Mehrholz, J.; Thomas, S.; Werner, C.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2017, 5, CD006185. [Google Scholar]
- Lefmann, S.; Russo, R.; Hillier, S. The effectiveness of robotic-assisted gait training for paediatric gait disorders: Systematic review. J. Neuroeng. Rehabil. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Goto, K.; Morishita, T.; Kamada, S.; Saita, K.; Fukuda, H.; Shiota, E.; Sankai, Y.; Inoue, T. Feasibility of rehabilitation using the single-joint hybrid assistive limb to facilitate early recovery following total knee arthroplasty: A pilot study. Assist Technol. 2017, 29, 197–201. [Google Scholar] [CrossRef]
- Tanaka, Y.; Oka, H.; Nakayama, S.; Ueno, T.; Matsudaira, K.; Miura, T.; Tanaka, K.; Tanaka, S. Improvement of walking ability during postoperative rehabilitation with the hybrid assistive limb after total knee arthroplasty: A randomized controlled study. SAGE Open Med. 2017, 5, 2050312117712888. [Google Scholar] [CrossRef]
- Zhang, M.; Davies, T.C.; Xie, S. Effectiveness of robot-assisted therapy on ankle rehabilitation—A systematic review. J. Neuroeng. Rehabil. 2013, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Cordo, P.; Lutsep, H.; Cordo, L.; Wright, W.G.; Cacciatore, T.; Skoss, R. Assisted movement with enhanced sensation (AMES): Coupling motor and sensory to remediate motor deficits in chronic stroke patients. Neurorehabil. Neural. Repair. 2009, 23, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Maggioni, S.; Melendez-Calderon, A.; Van Asseldonk, E.; Klamroth-Marganska, V.; Lünenburger, L.; Riener, R.; Van Der Kooij, H. Robot-aided assessment of lower extremity functions: A review. J. Neuroeng. Rehabil. 2016, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.M.; Cheng, M.S.; Smith, A.R., Jr.; Kolber, M.J. Intrarater reliability of hand held dynamometry in measuring lower extremity isometric strength using a portable stabilization device. Musculoskelet Sci. Pract. 2017, 27, 137–141. [Google Scholar] [CrossRef]
- Krewer, C.; Muller, F.; Husemann, B.; Heller, S.; Quintern, J.; Koenig, E. The influence of different Lokomat walking conditions on the energy expenditure of hemiparetic patients and healthy subjects. Gait Posture 2007, 26, 372–377. [Google Scholar] [CrossRef]
- Ditor, D.S.; Kamath, M.V.; MacDonald, M.J.; Bugaresti, J.; McCartney, N.; Hicks, A.L. Effects of body weight-supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. J. Appl. Physiol. 2005, 98, 1519–1525. [Google Scholar] [CrossRef]
- Hansen, H.; Beyer, N.; Frolich, A.; Godtfredsen, N.; Bieler, T. Intra- and inter-rater reproducibility of the 6-min walk test and the 30-s sit-to-stand test in patients with severe and very severe COPD. Int. J. Chron. Obstruct Pulmon. Dis. 2018, 13, 3447–3457. [Google Scholar] [CrossRef] [Green Version]
- Sale, P.; Franceschini, M.; Waldner, A.; Hesse, S. Use of the robot assisted gait therapy in rehabilitation of patients with stroke and spinal cord injury. Eur. J. Phys. Rehabil. Med. 2012, 48, 111–121. [Google Scholar]
- Sale, P.; Stocchi, F.; Galafate, D.; De Pandis, M.F.; Le Pera, D.; Sova, I.; Galli, M.; Foti, C.; Franceschini, M. Effects of robot assisted gait training in progressive supranuclear palsy (PSP): A preliminary report. Front Hum. Neurosci. 2014, 8, 207. [Google Scholar] [CrossRef]
- Chisholm, A.E.; Alamro, R.A.; Williams, A.M.; Lam, T. Overground vs. treadmill-based robotic gait training to improve seated balance in people with motor-complete spinal cord injury: A case report. J. Neuroeng. Rehabil. 2017, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Yang, Y.R.; Cheng, S.J.; Wang, R.Y. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch. Phys. Med. Rehabil. 2006, 87, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kubota, S.; Sugaya, H.; Hyodo, K.; Ogawa, K.; Taniguchi, Y.; Kanamori, A.; Sankai, Y.; Yamazaki, M. Robotic device-assisted knee extension training during the early postoperative period after opening wedge high tibial osteotomy: A case report. J. Med. Case Rep. 2017, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.J.; Brown, D.A.; Klassen, T.; Mulroy, S.; Ge, T.; Azen, S.P.; Winstein, C.J. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: Results of the STEPS randomized clinical trial. Phys. Ther. 2007, 87, 1580–1602. [Google Scholar] [CrossRef] [Green Version]
- Waldman, G.; Yang, C.Y.; Ren, Y.; Liu, L.; Guo, X.; Harvey, R.L.; Roth, E.J.; Zhang, L.Q. Effects of robot-guided passive stretching and active movement training of ankle and mobility impairments in stroke. NeuroRehabilitation 2013, 32, 625–634. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, J.Y.; Park, H.K.; Kim, N.Y. Effect of robotic-assisted gait training in patients with incomplete spinal cord injury. Ann. Rehabil. Med. 2014, 38, 719–725. [Google Scholar] [CrossRef]
- Alcobendas-Maestro, M.; Esclarín-Ruz, A.; Casado-López, R.M.; Muñoz-González, A.; Pérez-Mateos, G.; González-Valdizán, E.; Martín, J.L. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: Randomized controlled trial. Neurorehabil. Neural. Repair. 2012, 26, 1058–1063. [Google Scholar] [CrossRef]
- Bae, Y.H.; Ko, Y.J.; Chang, W.H.; Lee, J.H.; Lee, K.B.; Park, Y.J.; Ha, H.G.; Kim, Y.H. Effects of robot-assisted gait training combined with functional electrical stimulation on recovery of locomotor mobility in chronic stroke patients: A randomized controlled trial. J. Phys. Ther. Sci. 2014, 26, 1949–1953. [Google Scholar] [CrossRef] [Green Version]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Shustack, S.; Giladi, N.; Hausdorff, J.M. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017, 115, 41–46. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Xu, Z.; Gu, X. A pilot study of post-total knee replacement gait rehabilitation using lower limbs robot-assisted training system. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 203–208. [Google Scholar] [CrossRef]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquenazi, A.; Lee, S.; Wikoff, A.; Packel, A.; Toczylowski, T.; Feeley, J. A comparison of locomotor therapy interventions: Partial-body weight-supported treadmill, lokomat, and G-EO Training in people with traumatic brain injury. PM R 2017, 9, 839–846. [Google Scholar] [CrossRef] [PubMed]


| Robot Training (n = 17) | Conventional Training (n = 16) | p | |
|---|---|---|---|
| Male:female | 14:3 | 13:3 | 0.64 |
| Age (years) | 53. 94 ± 9.51 | 49.06 ± 15.11 | 0.49 |
| TBSA (%) | 33.94 ± 14.64 | 23.19 ± 14.50 | 0.13 |
| Mechanism of burn,
n FB:EB:SB:CB | 9:3:4:1 | 7:3:2:4 | 0.37 |
| Duration (days) between burn injury and therapy | 82.29 ± 31.50 | 74.19 ± 44.75 | 0.07 |
| VAS | 8.06 ± 0.66 | 8.00 ± 1.21 | 0.93 |
| FAC | 1.76 ± 0.56 | 1.75 ± 0.58 | 0.96 |
| 6MWT (m) | 204.41 ± 85.60 | 220.44 ± 10.90 | 0.61 |
| Isometric Measurements (Nm) | |||
| Hip flexor, right | 22.71 ± 4.79 | 28.44 ± 10.90 | 0.08 |
| Hip flexor, left | 23.59 ± 7.87 | 26.69 ± 11.75 | 0.38 |
| Hip extensor, right | 16.41 ± 4.21 | 18.38 ± 7.46 | 0.36 |
| Hip extensor, left | 18.29 ± 7.86 | 15.81 ± 5.95 | 0.32 |
| Knee flexor, right | 15.94 ± 3.03 | 17.63 ± 2.92 | 0.12 |
| Knee flexor, left | 15.12 ± 4.54 | 16.06 ± 5.23 | 0.58 |
| Knee extensor, right | 17.00 ± 6.15 | 20.38 ± 6.34 | 0.13 |
| Knee extensor, left | 16.71 ± 8.47 | 21.13 ± 7.14 | 0.12 |
| Ankle dorsiflexor, right | 15.06 ± 8.42 | 17.75 ± 7.39 | 0.34 |
| Ankle dorsiflexor, left | 14.01 ± 6.26 | 14.50 ± 8.69 | 0.99 |
| Ankle plantarflexor, right | 15.21 ± 8.84 | 18.06 ± 7.22 | 0.14 |
| Ankle plantarflexor, left | 16.21 ± 8.05 | 17.19 ± 8.56 | 0.74 |
| Range of Motion (degree) | |||
| Hip flexion, right | 97.06 ± 6.14 | 100.00 ± 3.65 | 0.28 |
| Hip flexion, left | 95.00 ± 11.18 | 98.75 ± 5.00 | 0.40 |
| Hip extension, right | 16.94 ± 5.92 | 19.75 ± 6.07 | 0.19 |
| Hip extension, left | 18.35 ± 6.10 | 19.75 ± 6.07 | 0.23 |
| Knee flexion, right | 119.41 ± 16.67 | 126.19 ± 18.28 | 0.27 |
| Knee flexion, left | 111.71 ± 27.12 | 122.00 ± 31.98 | 0.14 |
| Knee extension, right | −5.94 ± 8.93 | 0.13 ± 2.99 | 0.05 |
| Knee extension, left | −2.47 ± 5.39 | −0.81 ± 3.15 | 0.09 |
| Ankle dorsiflexion, right | 16.29 ± 8.04 | 18.31 ± 7.94 | 0.51 |
| Ankle dorsiflexion, left | 15.82 ± 6.28 | 14.19 ± 7.34 | 0.71 |
| Ankle plantarflexion, right | 28.35 ± 11.89 | 35.69 ± 8.73 | 0.12 |
| Ankle plantarflexion, left | 29.88 ± 11.48 | 30.56 ± 11.28 | 0.85 |
| Robot Training (n = 17) | p | Conventional Training (n = 16) | p | |||
|---|---|---|---|---|---|---|
| Before Training | After Training | Before Training | After Training | |||
| VAS | 8.06 ± 0.66 | 4.41 ± 1.18 | <0.001 * | 8.00 ± 1.21 | 5.00 ± 1.03 | <0.001 * |
| FAC | 1.76 ± 0.56 | 4.18 ± 0.39 | <0.001 ** | 1.75 ± 0.58 | 3.81 ± 1.05 | <0.001 ** |
| 6MWT | 204.41 ± 85.60 | 298.53 ± 47.75 | <0.001 * | 220.94 ± 116.88 | 272.19 ± 110.14 | 0.005 * |
| Isometric measurements (Nm) | ||||||
| Hip flexor, right | 22.71 ± 6.79 | 25.24 ± 7.55 | 0.08 | 28.44 ± 10.90 | 29.38 ± 10.28 | 0.40 |
| Hip flexor, left | 23.59 ± 7.87 | 25.00 ± 7.24 | 0.32 | 26.69 ± 11.75 | 27.31 ± 11.88 | 0.49 |
| Hip extensor, right | 16.41 ± 4.21 | 18.24 ± 5.47 | 0.02 * | 18.38 ± 7.46 | 18.81 ± 6.91 | 0.68 |
| Hip extensor, left | 18.29 ± 7.86 | 18.59 ± 6.43 | 0.84 | 15.81 ± 5.95 | 16.00 ± 5.91 | 0.80 |
| Knee flexor, right | 15.94 ± 3.03 | 17.71 ± 5.92 | 0.04 ** | 17.63 ± 2.92 | 18.94 ± 6.06 | 0.34 |
| Knee flexor, left | 15.12 ± 4.54 | 19.76 ± 5.30 | 0.001 * | 16.06 ± 5.23 | 16.13 ± 6.96 | 0.96 |
| Knee extensor, right | 17.00 ± 6.15 | 23.00 ± 6.21 | 0.003 * | 20.38 ± 6.34 | 25.19 ± 9.17 | 0.04 * |
| Knee extensor, left | 16.71 ± 8.47 | 23.29 ± 7.19 | 0.002 * | 21.13 ± 7.14 | 21.25 ± 8.62 | 0.93 |
| Ankle dorsiflexor, right | 15.06 ± 8.42 | 17.71 ± 8.45 | 0.04 ** | 17.75 ± 7.39 | 18.44 ± 6.67 | 0.89 |
| Ankle dorsiflexor, left | 14.01 ± 6.26 | 17.24 ± 7.94 | 0.02 ** | 14.50 ± 8.69 | 14.19 ± 8.38 | 0.79 |
| Ankle plantarflexor, right | 15.21 ± 8.84 | 20.35 ± 8.90 | 0.001 ** | 18.06 ± 7.22 | 19.63 ± 7.08 | 0.18 |
| Ankle plantarflexor, left | 16.21 ± 8.05 | 20.88 ± 9.16 | 0.008 * | 17.19 ± 8.56 | 16.06 ± 6.55 | 0.22 |
| Range of motion (degree) | ||||||
| Hip flexion, right | 97.06 ± 6.14 | 99.76 ± 0.97 | 0.07 | 100.00 ± 3.65 | 99.63 ± 1.50 | 0.66 |
| Hip flexion, left | 95.00 ± 11.18 | 99.76 ± 0.97 | 0.07 | 98.75 ± 5.00 | 99.50 ± 2.00 | 0.32 |
| Hip extension, right | 16.94 ± 5.92 | 17.94 ± 6.65 | 0.27 | 19.75 ± 6.07 | 21.25 ± 7.02 | 0.78 |
| Hip extension, left | 18.35 ± 6.10 | 19.94 ± 5.13 | 0.16 | 20.63 ± 4.50 | 20.13 ± 9.46 | 0.81 |
| Knee flexion, right | 119.41 ± 16.67 | 115.88 ± 20.21 | 0.26 | 126.19 ± 18.28 | 132.63 ± 16.32 | 0.22 |
| Knee flexion, left | 111.71 ± 27.12 | 117.47 ± 19.65 | 0.21 | 122.00 ± 31.98 | 121.44 ± 25.51 | 0.88 |
| Knee extension, right | −5.94 ± 8.93 | −1.18 ± 3.64 | 0.03 ** | 0.13 ± 2.99 | −0.19 ± 0.75 | 0.66 |
| Knee extension, left | −2.47 ± 5.39 | −1.12 ± 2.03 | 0.12 | −0.81 ± 3.15 | −0.69 ± 1.54 | 0.66 |
| Ankle dorsiflexion, right | 16.29 ± 8.04 | 16.65 ± 5.93 | 0.44 | 18.31 ± 7.94 | 16.88 ± 7.27 | 0.53 |
| Ankle dorsiflexion, left | 15.82 ± 6.28 | 16.59 ± 6.01 | 0.34 | 14.19 ± 7.34 | 13.19 ± 11.09 | 0.40 |
| Ankle plantarflexion, right | 28.35 ± 11.89 | 36.18 ± 8.00 | 0.008 ** | 35.69 ± 8.73 | 39.63 ± 1.50 | 0.14 |
| Ankle plantarflexion, left | 29.88 ± 11.48 | 36.12 ± 7.70 | 0.03 ** | 30.56 ± 11.28 | 34.63 ± 7.56 | 0.09 |
| Robot Training (n = 17) | Conventional Training (n = 16) | p | |
|---|---|---|---|
| VAS | −3.65 ± 1.50 | −3.00 ± 1.51 | 0.23 |
| FAC | 2.41 ± 0.62 | 2.06 ± 0.77 | 0.14 |
| 6MWT | 94.12 ± 61.23 | 51.25 ± 61.55 | 0.05 |
| Isometric measurements (Nm) | |||
| Hip flexor, right | 2.53 ± 5.60 | 0.94 ± 3.62 | 0.23 |
| Hip flexor, left | 1.41 ± 5.62 | 0.63 ± 3.50 | 0.64 |
| Hip Extensor, right | 1.82 ± 3.00 | 0.44 ± 2.66 | 0.13 |
| Hip extensor, left | 0.29 ± 4.55 | 0.19 ± 2.97 | 0.94 |
| Knee flexor, right | 1.76 ± 5.24 | 1.31 ± 5.34 | 0.81 |
| Knee flexor, left | 4.65 ± 4.61 | 0.06 ± 4.45 | 0.01 * |
| Knee extensor, right | 6.00 ± 6.91 | 4.81 ± 8.79 | 0.67 |
| Knee extensor, left | 6.59 ± 7.13 | 0.13 ± 5.66 | 0.01 * |
| Ankle dorsiflexor, right | 2.65 ± 4.39 | 0.69 ± 6.01 | 0.14 |
| Ankle dorsiflexor, left | 3.22 ± 4.69 | −0.31 ± 4.53 | 0.02 ** |
| Ankle plantarflexor, right | 5.15 ± 6.22 | 1.56 ± 5.32 | 0.09 |
| Ankle plantarflexor, left | 4.67± 6.35 | −1.13 ± 3.52 | 0.003 * |
| Range of motion (degree) | |||
| Hip flexion, right | 2.71 ± 5.71 | −0.38 ± 2.75 | 0.26 |
| Hip flexion, left | 4.76 ± 10.77 | 0.75 ± 3.00 | 0.40 |
| Hip extension, right | 1.00 ± 5.74 | 1.50 ± 5.98 | 0.33 |
| Hip extension, left | 1.59 ± 4.39 | −0.50 ± 9.45 | 0.33 |
| Knee flexion, right | −3.53 ± 12.50 | 6.44 ± 19.98 | 0.09 |
| Knee flexion, left | 5.76 ± 20.44 | −0.56 ± 14.23 | 0.31 |
| Knee extension, right | 4.76 ± 7.78 | −0.31 ± 2.87 | 0.09 |
| Knee extension, left | 1.35 ± 4.33 | 0.13 ± 3.30 | 0.14 |
| Ankle dorsiflexion, right | 0.35 ± 7.07 | −1.44 ± 7.08 | 0.38 |
| Ankle dorsiflexion, left | 0.76 ± 2.99 | −1.00 ± 6.16 | 0.33 |
| Ankle plantarflexion, right | 7.82 ± 10.24 | 3.94 ± 8.87 | 0.10 |
| Ankle plantarflexion, left | 6.24 ± 9.90 | 4.06 ± 8.87 | 0.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, S.Y.; Lee, S.Y.; Cho, Y.S.; Lee, K.J.; Seo, C.H. Effects of Robot-Assisted Gait Training in Patients with Burn Injury on Lower Extremity: A Single-Blind, Randomized Controlled Trial. J. Clin. Med. 2020, 9, 2813. https://doi.org/10.3390/jcm9092813
Joo SY, Lee SY, Cho YS, Lee KJ, Seo CH. Effects of Robot-Assisted Gait Training in Patients with Burn Injury on Lower Extremity: A Single-Blind, Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(9):2813. https://doi.org/10.3390/jcm9092813
Chicago/Turabian StyleJoo, So Young, Seung Yeol Lee, Yoon Soo Cho, Kuem Ju Lee, and Cheong Hoon Seo. 2020. "Effects of Robot-Assisted Gait Training in Patients with Burn Injury on Lower Extremity: A Single-Blind, Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 9: 2813. https://doi.org/10.3390/jcm9092813





