Management of a Thin Endometrium by Hysteroscopic Instillation of Platelet-Rich Plasma Into The Endomyometrial Junction: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Methods for PRP Preparation
2.3. PRP Injection
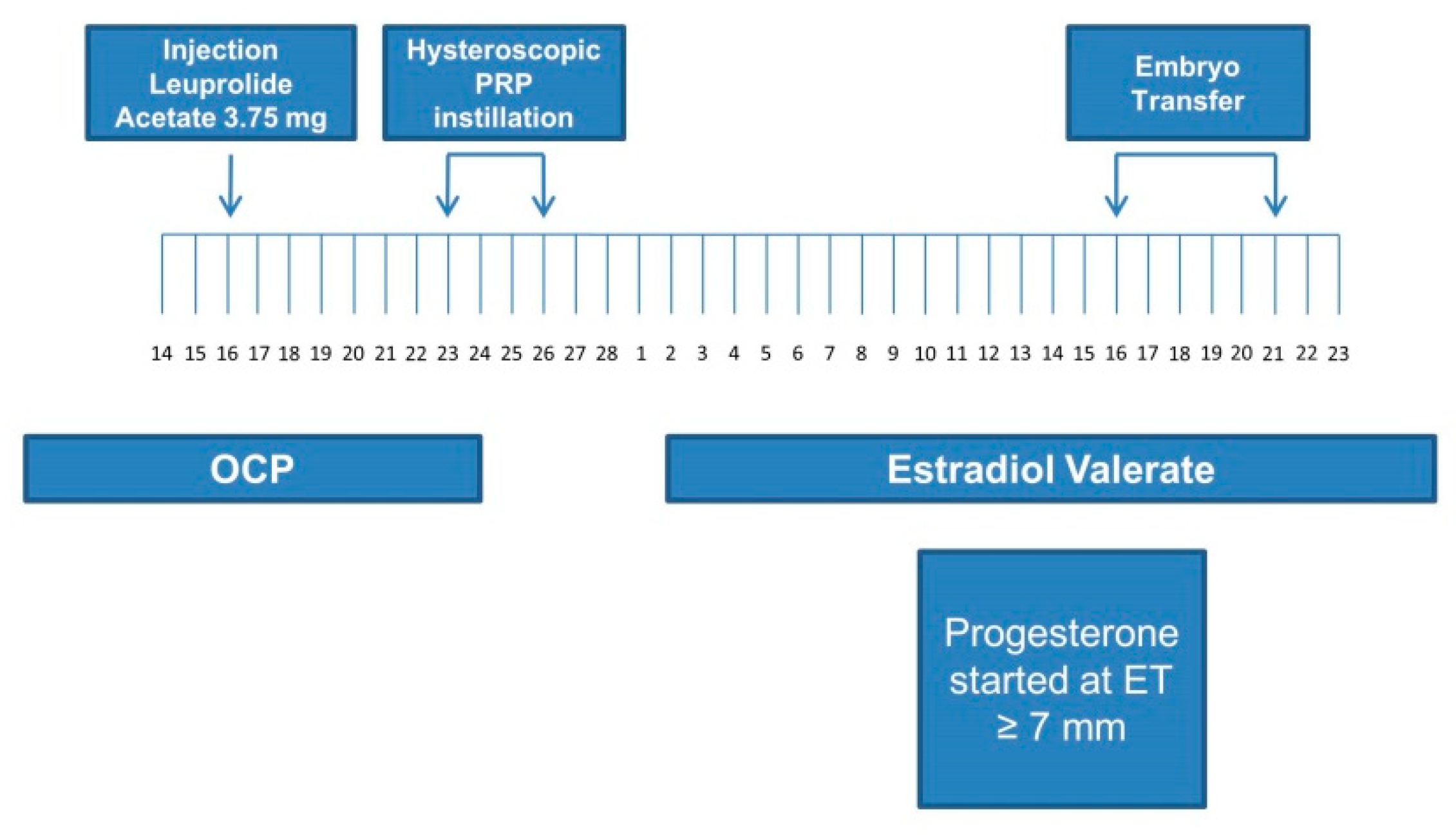
2.4. Endometrial Preparation and Embryo Transfer
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Toukhy, T.; Coomarasamy, A.; Khairy, M.; Sunkara, K.; Seed, P.; Khalaf, Y.; Braude, P. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil. Steril. 2008, 89, 832–839. [Google Scholar] [CrossRef]
- Richter, K.S.; Bugge, K.R.; Bromer, J.G.; Levy, M.J. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil. Steril. 2007, 87, 53–59. [Google Scholar] [CrossRef]
- Zadehmodarres, S.; Salehpour, S.; Saharkhiz, N.; Nazari, L. Treatment of thin endometrium with autologous platelet-rich plasma: A pilot study. JBRA Assist. Reprod. 2017, 21, 54–56. [Google Scholar] [CrossRef]
- Abdalla, H.I.; Brooks, A.A.; Johnson, M.R.; Kirkland, A.; Thomas, A.; Studd, J.W.W. Endometrial thickness: A predictor of implantation in ovum recipients? Hum. Reprod. 1994, 9, 363–365. [Google Scholar] [CrossRef]
- Alam, V.; Bernardini, L.; Gonzales, J.; Asch, R.H.; Balmaceda, J.P. A prospective study of echographic endometrial characteristics and pregnancy rates during hormonal replacement cycles. J. Assist. Reprod. Genet. 1993, 10, 215–219. [Google Scholar] [CrossRef]
- Gonen, Y.; Casper, R.F.; Jacobson, W.; Blankier, J. Endometrial thickness and growth during ovarian stimulation: A possible predictor of implantation in in vitro fertilization. Fertil. Steril. 1989, 52, 446–450. [Google Scholar] [CrossRef]
- Miwa, I.; Tamura, H.; Takasaki, A.; Yamagata, Y.; Shimamura, K.; Sugino, N. Pathophysiologic features of “thin” endometrium. Fertil. Steril. 2009, 91, 998–1004. [Google Scholar] [CrossRef]
- Hurst, B.S.; Bhojwani, J.T.; Marshburn, P.B.; Papadakis, M.A.; Loeb, T.A.; Matthews, M.L. Low-dose aspirin does not improve ovarian stimulation, endometrial response, or pregnancy rates for in vitro fertilization. J. Exp. Clin. Assist. Reprod. 2005, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.J.; Yang, J.H.; Peng, F.H.; Chen, S.U.; Ho, H.N.; Yang, Y.S. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J. Assist. Reprod. Genet. 2006, 23, 337–342. [Google Scholar] [CrossRef]
- Takasaki, A.; Tamura, H.; Miwa, I.; Taketani, T.; Shimamura, K.; Sugino, N. Endometrial growth and uterine blood flow: A pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil. Steril. 2010, 93, 1851–1858. [Google Scholar] [CrossRef]
- Tehraninejad, E.; Tanha, F.D.; Asadi, E.; Kamali, K.; Aziminikoo, E.; Rezayof, E. G-CSF Intrauterine for Thin Endometrium, and Pregnancy Outcome. J. Family Reprod. Health 2015, 9, 107–112. [Google Scholar]
- Gargett, C.E. Uterine stem cells: What is the evidence? Hum. Reprod. Updat. 2007, 13, 87–101. [Google Scholar] [CrossRef]
- Taylor, H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004, 292, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, T.; Petit, I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002, 30, 973–981. [Google Scholar] [CrossRef]
- Hufnagel, D.; Li, F.; Cosar, E.; Krikun, G.; Taylor, H.S. The Role of Stem Cells in the Etiology and Pathophysiology of Endometriosis. Semin. Reprod. Med. 2015, 33, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Cousins, F.L.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.; Chen, Y.; Wei, L.; Yang, X.; Shi, Y.; Liang, X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015, 8, 1286–1290. [Google Scholar]
- Smith, S.K. Angiogenesis, vascular endothelial growth factor and the endometrium. Hum. Reprod. Updat. 1998, 4, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.K. Angiogenesis and implantation. Hum. Reprod. 2000, 15 (Suppl. 6), 59–66. [Google Scholar]
- Sugino, N.; Suzuki, T.; Sakata, A.; Miwa, I.; Asada, H.; Taketani, T.; Yamagata, Y.; Tamura, H. Angiogenesis in the human corpus luteum: Changes in expression of angiopoietins in the corpus luteum throughout the menstrual cycle and in early pregnancy. J. Clin. Endocrinol. Metab. 2005, 90, 6141–6148. [Google Scholar] [CrossRef] [Green Version]
- Dreisler, E.; Kjer, J.J. Asherman’s syndrome: current perspectives on diagnosis and management. Int. J. Women’s Heal. 2019, 11, 191–198. [Google Scholar] [CrossRef]
- Sardo, A.D.S.; Calagna, G.; Scognamiglio, M.; O’Donovan, P.; Campo, R.; De Wilde, R.L. Prevention of intrauterine post-surgical adhesions in hysteroscopy. A systematic review. Eur. J. Obstet. Gynecol. Reprod. Boil. 2016, 203, 182–192. [Google Scholar] [CrossRef]
- Alfer, J.; Happel, L.; Dittrich, R.; Beckmann, M.W.; Hartmann, A.; Gaumann, A.; Buck, V.U.; Classen-Linke, I. Insufficient Angiogenesis: Cause of Abnormally Thin Endometrium in Subfertile Patients? Geburtshilfe und Frauenheilkd. 2017, 77, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Godinjak, Z.; Bilalovic, N. Estrogen and Progesterone Receptors in Endometrium in Women with Unexplained Infertility. Mater. Socio Medica 2014, 26, 52. [Google Scholar] [CrossRef]
- Kim, C.-H.; Chae, H.-D.; Huh, J.; Kang, B.M.; Chang, Y.-S.; Nam, J.-H. Relationship between endometrial estrogen and progesterone receptors, and sonographic endometrial appearance in the preovulatory phase. J. Obstet. Gynaecol. Res. 2000, 26, 95–101. [Google Scholar] [CrossRef]
- Ohno, Y. Endometrial oestrogen and progesterone receptors and their relationship to sonographic appearance of the endometrium. Hum. Reprod. Updat. 1998, 4, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, R.; Taketani, T.; Mihara, Y.; Sato, S.; Okada, M.; Tamura, I.; Jozaki, K.; Kajimura, T.; Asada, H.; Takasaki, A. Thin endometrium transcriptome analysis reveals a potential mechanism of implantation failure. Reprod. Med. Biol. 2017, 16, 206–227. [Google Scholar] [CrossRef]
- Mirzaei, M.; Namiranian, N.; Firouzabadi, R.D.; Gholami, S. The prevalence of infertility in 20-49 years women in Yazd, 2014-2015: A cross-sectional study. Int. J. Reprod. Biomed. 2018, 16, 683–688. [Google Scholar]
- Punab, M.; Poolamets, O.; Paju, P.; Vihljajev, V.; Pomm, K.; Ladva, R.; Korrovits, P.; Laan, M. Causes of male infertility: A 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum. Reprod. 2016, 32, 18–31. [Google Scholar] [CrossRef]
- Scott, J.R.; Branch, D.W. Potential Alloimmune Factors and Immunotherapy in Recurrent Miscarriage. Clin. Obstet. Gynecol. 1994, 37, 761–767. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hosseini, M.S.; Hashemi Moghanjoughi, P. The effects of autologous platelet-rich plasma in repeated implantation failure: A randomized controlled trial. Hum. Fertil. (Camb) 2019, 1–5. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hoseini, S.; Zadehmodarres, S.; Azargashb, E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: A double-blind RCT. Int. J. Reprod. Biomed. (Yazd) 2019, 17, 443–448. [Google Scholar] [CrossRef]
- Maleki-Hajiagha, A.; Razavi, M.; Rouholamin, S.; Rezaeinejad, M.; Maroufizadeh, S.; Sepidarkish, M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 137, 103078. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Brosens, J.J.; Puttemans, P.; Benagiano, G.; Brosens, I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 2014, 20, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Lagana, A.S.; Sturlese, E.; Retto, G.; Sofo, V.; Triolo, O. Interplay between Misplaced Mullerian-Derived Stem Cells and Peritoneal Immune Dysregulation in the Pathogenesis of Endometriosis. Obstet. Gynecol. Int. 2013, 2013, 527041. [Google Scholar] [CrossRef]
- Rolla, E. Endometriosis: advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Research 2019, 8, 529. [Google Scholar] [CrossRef] [Green Version]
- Sapkota, Y.; Steinthorsdottir, V.; Morris, A.P.; Fassbender, A.; Rahmioglu, N.; De Vivo, I.; Buring, J.E.; Zhang, F.; Edwards, T.; Jones, S.; et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat. Commun. 2017, 8, 15539. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.G.; Chiantera, V.; Frangini, S.; Younes, S.; Köhler, C.; Taube, E.T.; Plendl, J.; Mechsner, S. Ultramicro-trauma in the endometrial-myometrial junctional zone and pale cell migration in adenomyosis. Fertil. Steril. 2015, 104, 1475–1483.e3. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.G.; Sillem, M.; Plendl, J.; Chiantera, V.; Sehouli, J.; Mechsner, S. Myofibroblasts Are Evidence of Chronic Tissue Microtrauma at the Endometrial-Myometrial Junctional Zone in Uteri with Adenomyosis. Reprod. Sci. 2017, 24, 1410–1418. [Google Scholar] [CrossRef]
- Urman, B.; Boza, A.; Balaban, B. Platelet-rich plasma another add-on treatment getting out of hand? How can clinicians preserve the best interest of their patients? Hum. Reprod. 2019, 34, 2099–2103. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.E.; Koo, H.S.; Kwon, H.; Choi, D.H.; Kim, J.H. (Effect of Autologous Platelet-Rich Plasma Treatment on Refractory Thin Endometrium during the Frozen Embryo Transfer Cycle: A Pilot Study. Front. Endocrinol. (Lausanne) 2019, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Moraes, V.Y.; Lenza, M.; Tamaoki, M.J.; Faloppa, F.; Belloti, J.C. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst. Rev. 2014, 4, CD010071. [Google Scholar] [CrossRef]



| Endometrial Thickness | Number of Patients |
|---|---|
| ≥7 mm (on the day of progesterone start and embryo transfer done) | 24 (75%) |
| 6–7 mm (embryo transfer not done) | 4 (12.5%) |
| <6 mm no improvement (embryo transfer not done) | 4 (12.5%) |
| Pregnancy Outcome | Number of Patients (24 in Total) |
|---|---|
| Beta-hCG positive | 12 (50%) |
| Clinical pregnancy | 10 (41.66%) |
| Biochemical pregnancy | 2 (8.33%) |
| Ongoing pregnancy | 3 (12.5%) |
| Live birth | 5 (20.83%) |
| Missed abortion | 2 (8.33%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, M.; Mettler, L.; Jain, S.; Meshram, S.; Günther, V.; Alkatout, I. Management of a Thin Endometrium by Hysteroscopic Instillation of Platelet-Rich Plasma Into The Endomyometrial Junction: A Pilot Study. J. Clin. Med. 2020, 9, 2795. https://doi.org/10.3390/jcm9092795
Agarwal M, Mettler L, Jain S, Meshram S, Günther V, Alkatout I. Management of a Thin Endometrium by Hysteroscopic Instillation of Platelet-Rich Plasma Into The Endomyometrial Junction: A Pilot Study. Journal of Clinical Medicine. 2020; 9(9):2795. https://doi.org/10.3390/jcm9092795
Chicago/Turabian StyleAgarwal, Meenu, Liselotte Mettler, Smita Jain, Sandhya Meshram, Veronika Günther, and Ibrahim Alkatout. 2020. "Management of a Thin Endometrium by Hysteroscopic Instillation of Platelet-Rich Plasma Into The Endomyometrial Junction: A Pilot Study" Journal of Clinical Medicine 9, no. 9: 2795. https://doi.org/10.3390/jcm9092795
APA StyleAgarwal, M., Mettler, L., Jain, S., Meshram, S., Günther, V., & Alkatout, I. (2020). Management of a Thin Endometrium by Hysteroscopic Instillation of Platelet-Rich Plasma Into The Endomyometrial Junction: A Pilot Study. Journal of Clinical Medicine, 9(9), 2795. https://doi.org/10.3390/jcm9092795






