Intraoperative Clinical Examination for Assessing Pelvic and Para-Aortic Lymph Node Involvement in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Data Sources and Search Strategy
2.3. Selection Criteria
2.4. Study Selection
2.5. Data Extraction and Quality Assessment
2.6. Statistical Analysis
3. Results
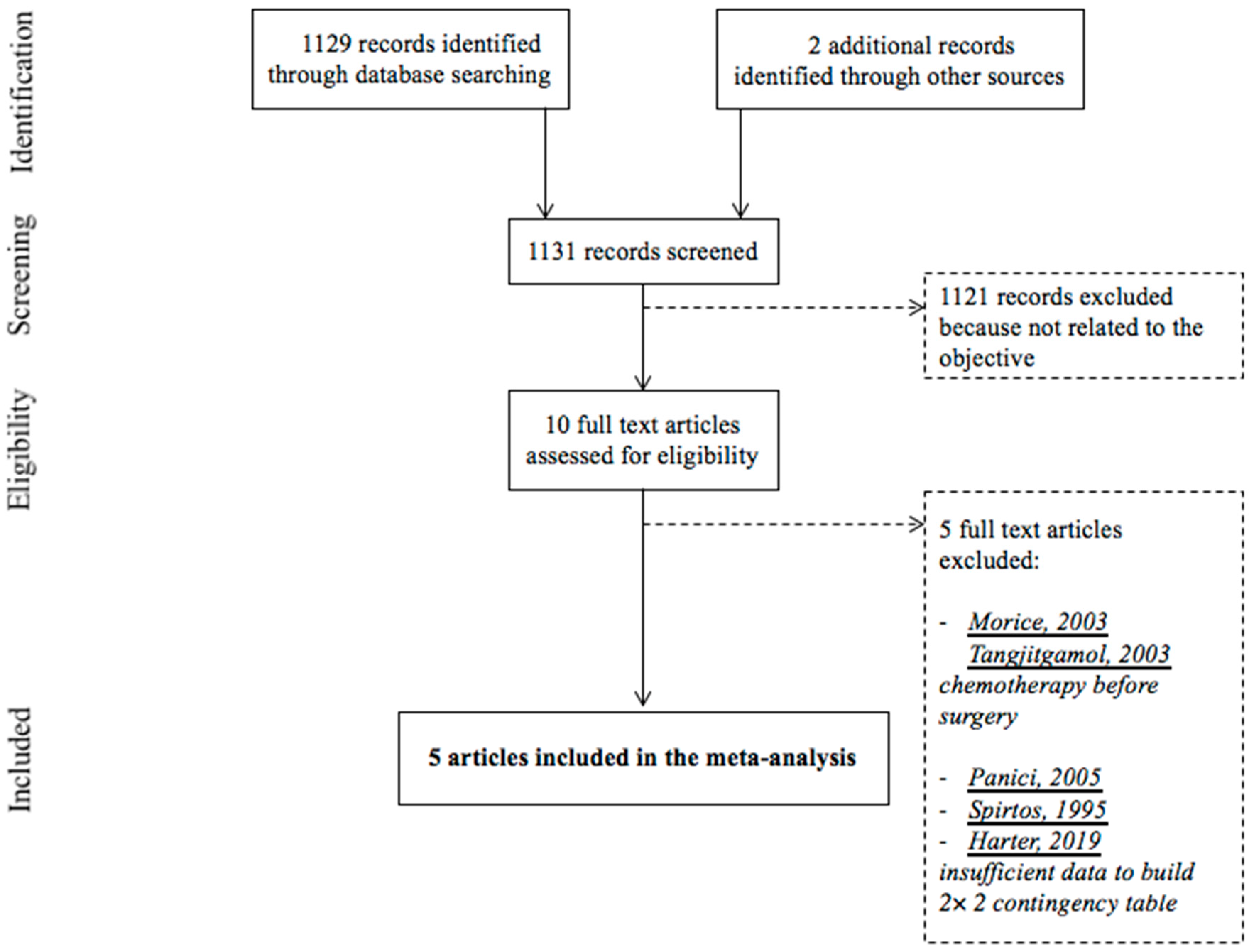
3.1. Study Selection
3.2. Study Description
3.3. Quality Assessment
3.4. Statistical Analysis
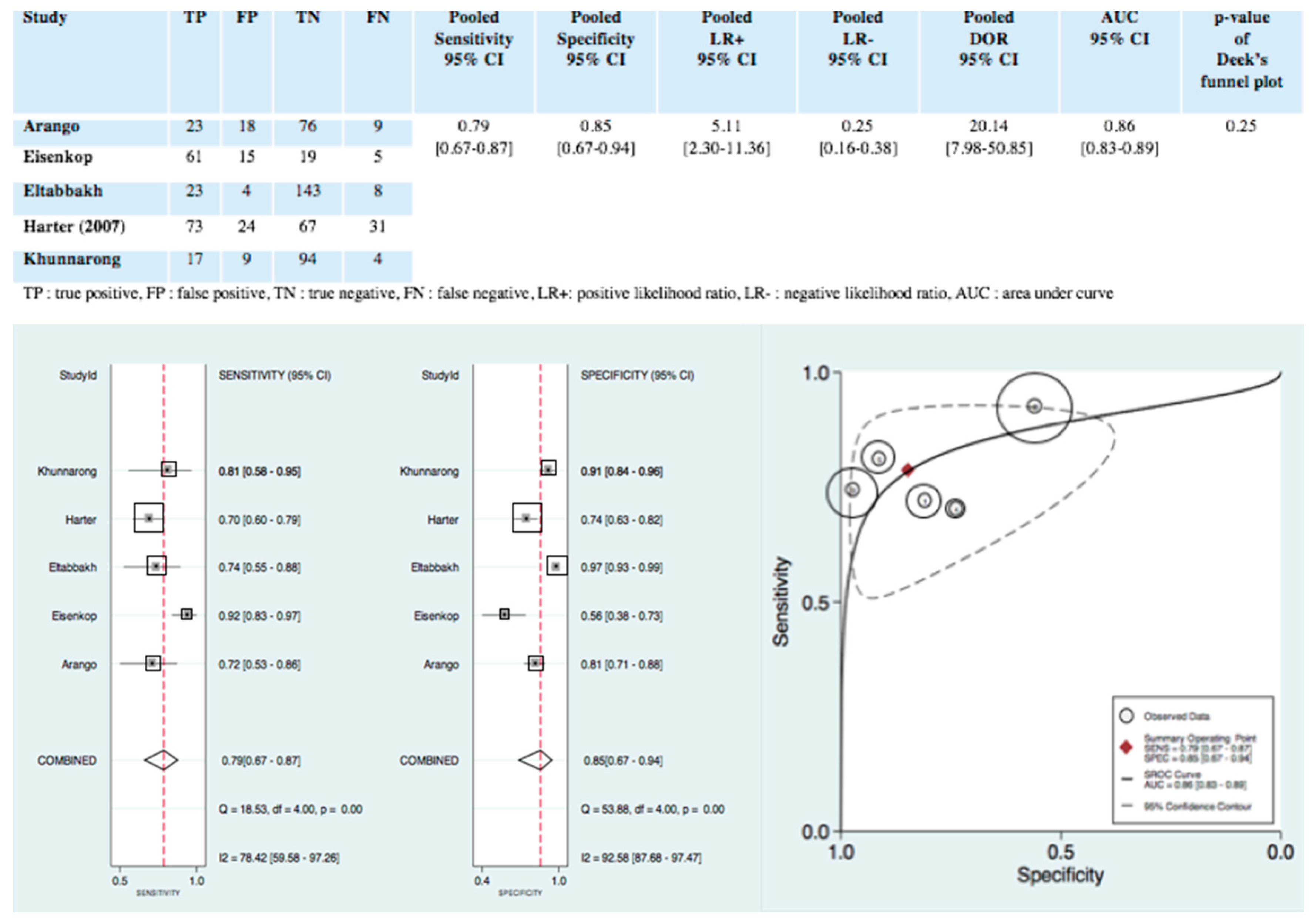
3.4.1. Diagnostic Accuracy of Intraoperative Clinical Examination
3.4.2. Exploration of Heterogeneity
3.4.3. Publication Bias
3.4.4. Clinical Utility
4. Discussion
- -
- Positive intraoperative clinical examination triages patients into a group at high risk of lymph node metastases, with a clear indication for pelvic and para-aortic lymphadenectomy, as the literature, and particularly the LION trial, recommends;
- -
- Negative intraoperative clinical examination triages patients into a group at low risk of lymph node metastases. However, it does not appear sufficient to conclusively rule them out, in view of the high number of false-negative of intraoperative clinical examination in the five studies included in our meta-analysis Spirtos et al. [28] and Harter et al. [13], which were excluded from the meta-analysis because their reports lacked the data to build a complete contingency 2 × 2 table, also found a high number of false-negative of intraoperative clinical examination—13/56 patients (23.2%) and 180/323 (55.7%) patients, respectively. Nonetheless, it must be noted that in the LION trial despite the 55.7% of a false-negative, no survival difference between the “lymphadenectomy” group and the “no lymphadenectomy” group was shown.
Author Contributions
Funding
Conflicts of Interest
Appendix A

Appendix B
| Study | Surgeons Qualifications | Lymphadenectomy Protocol | Intraoperative Clinical Examination Protocol | ||
|---|---|---|---|---|---|
| Type | Indication | Limits/Regions | |||
| Arango | 3 obstetrician-gynecologist with subspecialty certification in gynecologic oncology from the American Board of Obstetrics and Gynecology, who had practiced gynecologic oncology for at least five years | -Pelvic or para-aortic lymph node samplings -Pelvic and para-aortic lymphadenectomies | -FIGO stage 1a and 1b cervical cancer had lymphadenectomies -Decision on whether to do lymphadenectomies or lymph node samplings in all other cancers were left to individual surgeons | -Pelvic lymphadenectomy: the deep circumflex iliac veins distally, the obturator nerves inferiorly, the internal iliac arteries medially and the aortic bifurcation proximally -Para-aortic lymphadenectomy: bifurcation of the aorta distally and the inferior mesenteric artery proximally, left and right sides were included | -Data of lymph node status were collected on gynecologic oncologist’s opinions by palpation of open retroperitoneal spaces -Nodes believed to be positive were sent separately |
| Eisenkop | - | Systematic pelvic and aortic lymphadenectomy | Advanced ovarian cancer + primary cytoreductive surgery | -Pelvic lymphadenectomy: removal of all identifiable nodal tissue bilaterally associated with the common iliac, external iliac, hypogastric vessels and the obturator fossa -Aortic lymphadenectomy: removal of all identifiable nodal tissue from the aortal-caval region, the lateral and anterior surface of the aorta and vena cava to the approximate levels of the renal vessels | Nodes were classified to be positive by palpation if recognized to be macroscopically involved by transperitoneal palpation, positive by inspection if recognized to be macroscopically involved by palpation after opening retroperitoneal area, and positive by dissection if recognized to be macroscopically involved any time after starting the actual process of lymph node dissection |
| Eltabbakh | One American board-certified gynecologist oncolgist | -Lymphadenectomy -Lymph node sampling -Lymph node biopsy | - | - | -Lymph nodes were evaluated after opening the retroperitoneal spaces in the pelvis and the peritoneum over the lower vena cava and aorta and identifying the major blood vessels -In all cases, three signs of possible lymph node involvement by metastatic disease were evaluated: enlargement, firmness and adherence to surrounding structures -In addition, the surgeon recorded an overall impression on the basis of these three signs as to whether the lymph nodes were involved |
| Harter (2007) | 3 Experienced gynecologist oncologists | Systematic pelvic and para-aortic lymphadenectomy | -Early ovarian cancer + primary complete cytoreductive surgery -Advanced ovarian cancer + primary complete cytoreductive surgery (residual disease smaller than 1 cm in 2000–2004 and macroscopic complete debulking after 2004) | -Resection of lymph nodes in the following regions: upper para-aortic region, lower para-aortic region, interaorto-caval region, para-caval region, iliaca communis, externa and interna regions and fossa obturatoria region | Intraoperative palpation |
| Khunnarong | Experienced gynecologist oncologists | -Lymphadenectomy -Lymph node sampling -Lymph node biopsy | - | -Pelvic lymphadenectomy: the deep circumflex iliac veins distally, the obturator nerves inferiorly, the internal iliac arteries medially and the aortic bifurcation proximally -Para-aortic lymphadenectomy: bifurcation of the aorta distally and the inferior mesenteric artery proximally | -Lymph nodes were evaluated after opening the retroperitoneal spaces in the pelvis and the peritoneum over the lower vena cava and aorta and identifying the major blood vessels -In all cases, the characteristics of lymph node metastasis that were evaluated included: size, consistency, shape and adherence to surrounding structures. These four characteristics were judged by individual surgeon. -In addition, the overall impression on the basis of these four characteristics as to whether the lymph nodes were involved were recorded |
Appendix C

Appendix D

References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Bois, A.; Quinn, M.A.; Thigpen, T.; Vermorken, J.; Avall-Lundqvist, E.; Bookman, M.; Bowtell, D.; Brady, M.; Casado, A.; Cervantes, A.; et al. 2004 consensus statements on the management of ovarian cancer: Final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann. Oncol. 2005, 16, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, É.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [PubMed]
- Panici, P.B.; Maggioni, A.; Hacker, N.; Landoni, F.; Ackermann, S.; Campagnutta, E.; Tamussino, K.; Winter, R.; Pellegrino, A.; Greggi, S.; et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: A randomized clinical trial. J. Natl. Cancer Inst. 2005, 97, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Magrina, J.F.; Rey, V.; Cortes, M.; Magtibay, P.M. Pelvic and aortic lymph node metastasis in epithelial ovarian cancer. Gynecol. Oncol. 2007, 105, 604–608. [Google Scholar] [CrossRef]
- Fournier, M.; Stoeckle, E.; Guyon, F.; Brouste, V.; Thomas, L.; MacGrogan, G.; Floquet, A. Lymph node involvement in epithelial ovarian cancer: Sites and risk factors in a series of 355 patients. Int. J. Gynecol. Cancer 2009, 19, 1307–1313. [Google Scholar] [CrossRef]
- Chan, J.K.; Urban, R.; Hu, J.M.; Shin, J.Y.; Husain, A.; Teng, N.N.; Berek, J.S.; Osann, K.; Kapp, D.S. The potential therapeutic role of lymph node resection in epithelial ovarian cancer: A study of 13918 patients. Br. J. Cancer 2007, 96, 1817–1822. [Google Scholar] [CrossRef] [Green Version]
- Du Bois, A.; Reuss, A.; Harter, P.; Pujade-Lauraine, E.; Ray-Coquard, I.; Pfisterer, J. Potential role of lymphadenectomy in advanced ovarian cancer: A combined exploratory analysis of three prospectively randomized phase III multicenter trials. J. Clin. Oncol. 2010, 28, 1733–1739. [Google Scholar] [CrossRef] [Green Version]
- Aletti, G.; Dowdy, S.; Podratz, K.C.; Cliby, W.A. Role of lymphadenectomy in the management of grossly apparent advanced stage epithelial ovarian cancer. Am. J. Obstet. Gynecol. 2006, 195, 1862–1868. [Google Scholar] [CrossRef]
- di Re, F.; Baiocchi, G.; Fontanelli, R.; Grosso, G.; Cobellis, L.; Raspagliesi, F.; di Re, E. Systematic pelvic and paraaortic lymphadenectomy for advanced ovarian cancer: Prognostic significance of node metastases. Gynecol. Oncol. 1996, 62, 360–365. [Google Scholar] [CrossRef]
- Scarabelli, C.; Gallo, A.; Visentin, M.; Canzonieri, V.; Carbone, A.; Zarrelli, A. Systematic pelvic and para-aortic lymphadenectomy in advanced ovarian cancer patients with no residual intraperitoneal disease. Int. J. Gynecol. Cancer 1997, 7, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Bergzoll, C.; Brun, J.-L.; Dubernard, G.; Selle, F.; Uzan, S.; Pomel, C.; Daraï, E. The role of lymph node resection in ovarian cancer: Analysis of the Surveillance, Epidemiology, and End Results (SEER) database. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Lorusso, M.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.-W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Management of Epithelial Cancer of the Ovary, Fallopian Tube, and Primary Peritoneum. 2019. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30936027 (accessed on 29 August 2020).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.; Rutjes, A.W.S.; Westwood, M.E.; Leeflang, M.M.; Sterne, J.A.C.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.S.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef]
- Walter, S.D. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat. Med. 2002, 21, 1237–1256. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Fagan, T.J. Letter: Nomogram for Bayes theorem. N. Engl. J. Med. 1975, 293, 257. [Google Scholar]
- Dwamena, B. MIDAS: Stata Module for Meta-Analytical Integration of Diagnostic Test Accuracy Studies. 2009. Available online: https://ideas.repec.org/c/boc/bocode/s456880.html (accessed on 29 August 2020).
- Arango, H.A.; Hoffman, M.S.; Roberts, W.S.; DeCesare, S.L.; Fiorica, J.V.; Drake, J. Accuracy of lymph node palpation to determine need for lymphadenectomy in gynecologic malignancies. Obstet. Gynecol. 2000, 95, 553–556. [Google Scholar] [PubMed]
- Eisenkop, S.M.; Spirtos, N.M. The clinical significance of occult macroscopically positive retroperitoneal nodes in patients with epithelial ovarian cancer. Gynecol. Oncol. 2001, 82, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Eltabbakh, G.H. Intraoperative clinical evaluation of lymph nodes in women with gynecologic cancer. Am. J. Obstet. Gynecol. 2001, 184, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Khunnarong, J.; Inthasorn, P.; Boriboonhirunsarn, D. Accuracy of intraoperative clinical evaluation of lymph nodes in women with gynecologic cancer. J. Med. Assoc. Thail. 2004, 87, 80–84. [Google Scholar]
- Harter, P.; Gnauert, K.; Hils, R.; Lehmann, T.; Traut, A.; Du Bois, A.; Fisseler-Eckhoff, A. Pattern and clinical predictors of lymph node metastases in epithelial ovarian cancer. Int. J. Gynecol. Cancer 2007, 17, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Spirtos, N.M.; Gross, G.M.; Freddo, J.L.; Ballon, S.C. Cytoreductive surgery in advanced epithelial cancer of the ovary: The impact of aortic and pelvic lymphadenectomy. Gynecol. Oncol. 1995, 56, 345–352. [Google Scholar] [CrossRef]
- Tangjitgamol, S.; Manusirivithaya, S.; Sheanakul, C.; Leelahakorn, S.; Sripramote, M.; Thawaramara, T.; Kaewpila, N. Can we rely on the size of the lymph node in determining nodal metastasis in ovarian carcinoma? Int. J. Gynecol. Cancer 2003, 13, 297–302. [Google Scholar] [CrossRef]
- Morice, P.; Joulie, F.; Camatte, S.; Atallah, D.; Rouzier, R.; Pautier, P.; Pomel, C.; Lhommé, C.; Duvillard, P.; Castaigne, D. Lymph node involvement in epithelial ovarian cancer: Analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J. Am. Coll. Surg. 2003, 197, 198–205. [Google Scholar] [CrossRef]
- Buckley, R.G.; King, K.J.; Disney, J.D.; Ambroz, P.K.; Gorman, J.D.; Klausen, J.H. Derivation of a clinical prediction model for the emergency department diagnosis of ectopic pregnancy. Acad. Emerg. Med. 1998, 5, 951–960. [Google Scholar] [CrossRef]


| Author Year Country | Study Design | Number of Centers | Inclusion Intervals | Type of Cancer | Histology | FIGO Stage | Initial/Complete Surgery | Gold Standard | Number of Patients/Nodes | Patients’ Mean Age (Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| Arango 2000 US | Prospective | 1 | August 1995–June 1997 | -Ovarian (21%) -Cervical (43%) -Uterine (31%) -Vaginal (2%) | - | - | - | Histology | 126/2138 | 55 (range, 18–83) |
| Eisenkop 2001 US | Prospective | 1 | 1997–2000 | Ovarian | Epithelial | IIIC and IV | Primary complete cytoreductive surgery | Histology | 100/- | 61.4 (range, 24.2–88.3) |
| Eltabbakh 2001 US | Prospective | 1 | February 1998–September 1999 | -Ovarian (30.9%) -Endometrial (41%) -Cervical or vaginal (19.1%) -Vulvar (9%) | - | - | - | Histology | 178/2158 | 56.6 (range, 18–90) |
| Harter 2007 Germany | Retrospective | 1 | 2000–2005 | Ovarian | Epithelial | -Early ovarian cancer (36%) -Advanced ovarian cancer (IIIb to IV) (64%) | Primary complete cytoreductive surgery | Histology | 195/- | 60 (range, 22–80) |
| Khunnarong 2004 Thailand | Prospective | 1 | May 2003–April 2004 | -Ovarian (17%) -Cervical (48%) -Endometrial (33%) -Vulvar (2%) | - | - | - | Histology | 124/1609 | 51 ± 11.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mimoun, C.; Benifla, J.L.; Fauconnier, A.; Huchon, C. Intraoperative Clinical Examination for Assessing Pelvic and Para-Aortic Lymph Node Involvement in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2793. https://doi.org/10.3390/jcm9092793
Mimoun C, Benifla JL, Fauconnier A, Huchon C. Intraoperative Clinical Examination for Assessing Pelvic and Para-Aortic Lymph Node Involvement in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(9):2793. https://doi.org/10.3390/jcm9092793
Chicago/Turabian StyleMimoun, Camille, Jean Louis Benifla, Arnaud Fauconnier, and Cyrille Huchon. 2020. "Intraoperative Clinical Examination for Assessing Pelvic and Para-Aortic Lymph Node Involvement in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 9: 2793. https://doi.org/10.3390/jcm9092793
APA StyleMimoun, C., Benifla, J. L., Fauconnier, A., & Huchon, C. (2020). Intraoperative Clinical Examination for Assessing Pelvic and Para-Aortic Lymph Node Involvement in Advanced Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(9), 2793. https://doi.org/10.3390/jcm9092793





