Impact of Lymphadenectomy on Survival of Patients with Serous Advanced Ovarian Cancer After Neoadjuvant Chemotherapy: A French National Multicenter Study (FRANCOGYN)
Abstract
1. Introduction
2. Materials and Methods
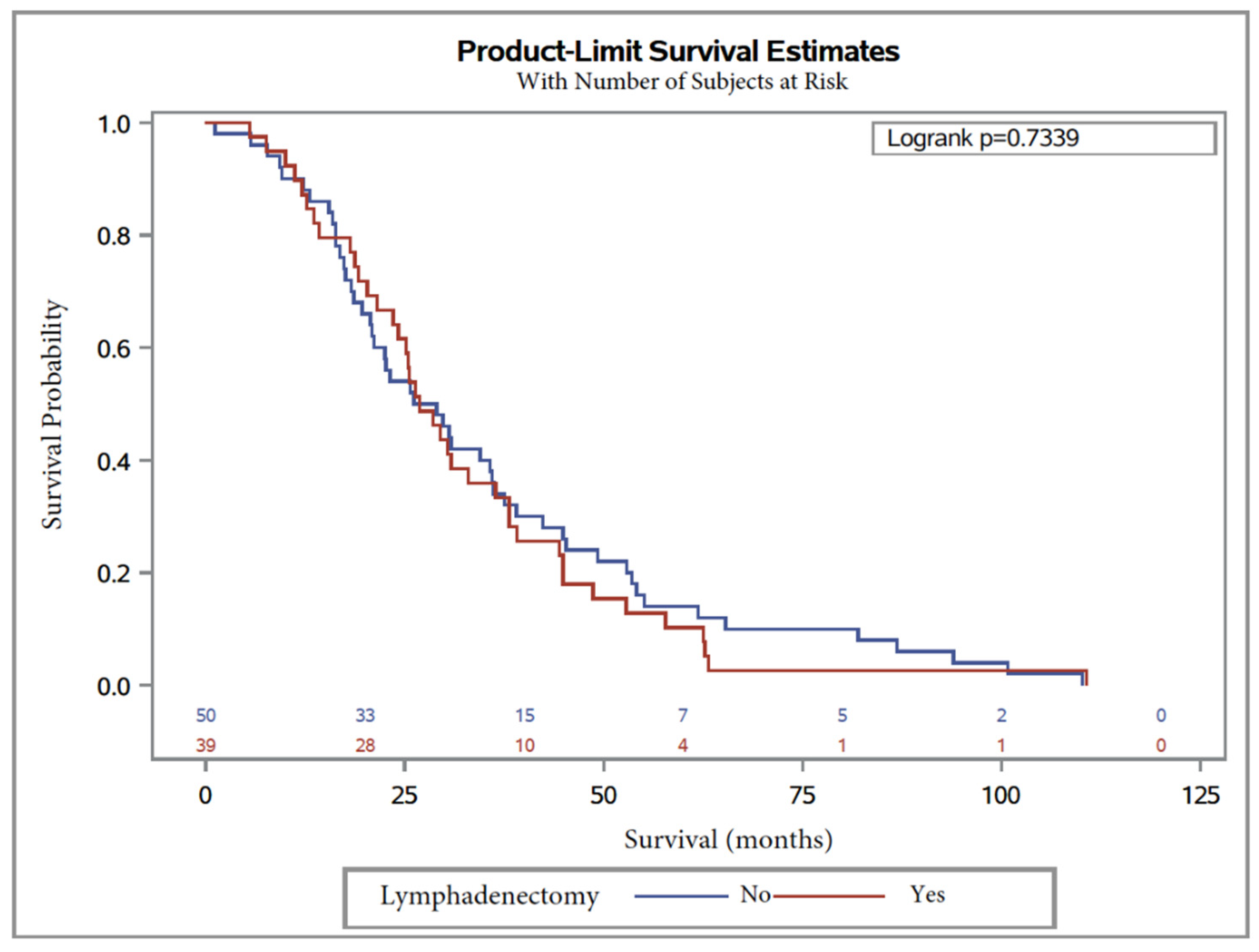
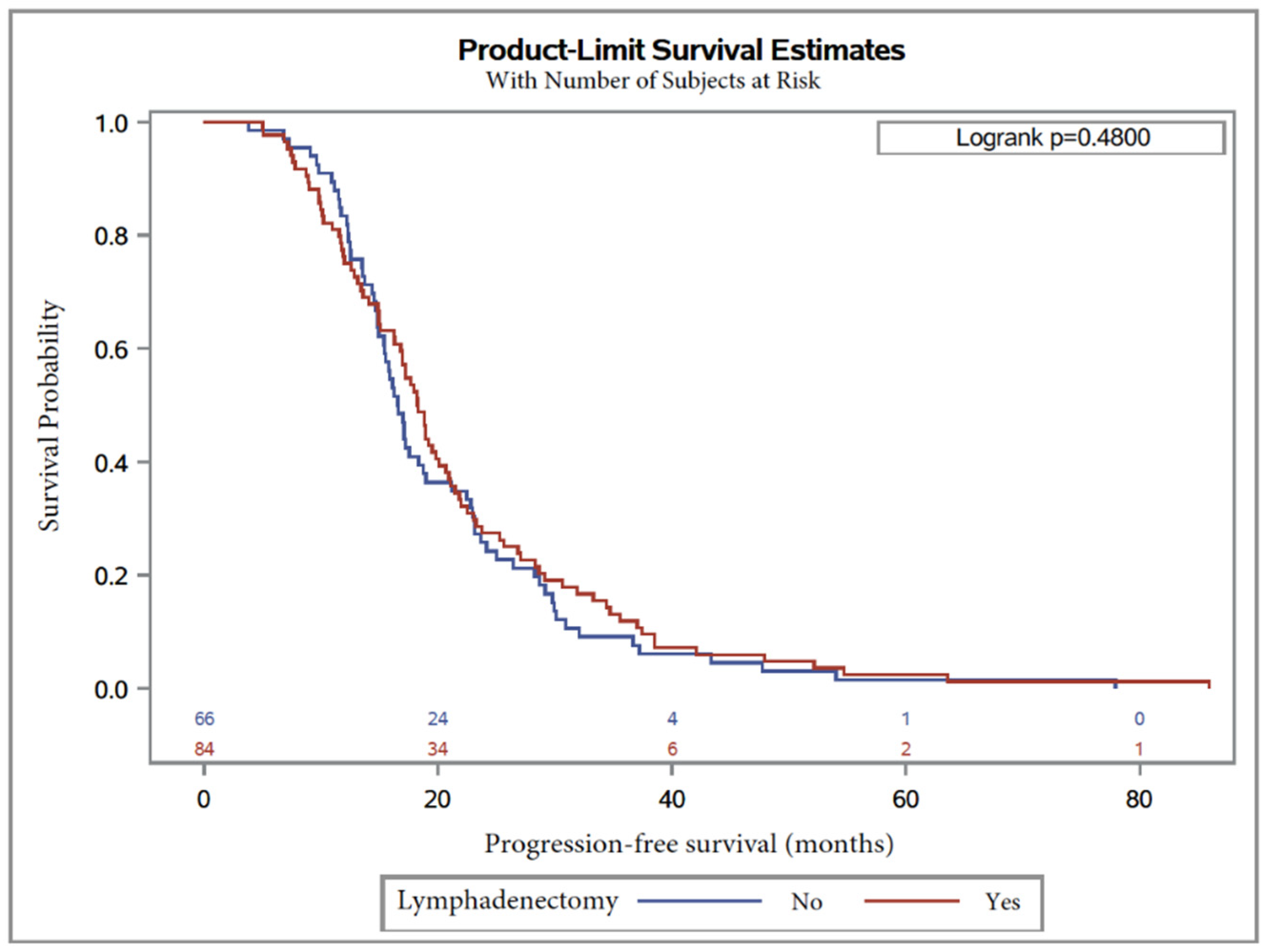
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Defossez, G.; Le Guyader-Peyrou, S.; Uhry, Z.; Grosclaude, P.; Remontet, L.; Colonna, M. Estimations Nationales de L’incidence et de la Mortalité Par Cancer en France Métropolitaine Entre 1990—2018; Étude à partir des registres des cancers du réseau Francim. Résultats préliminaires; Synthèse; Santé Publique: Saint Maurice, France, 2019; p. 20. [Google Scholar]
- Jemal, A.; Tiwari, R.C.; Murray, T.; Ghafoor, A.; Samuels, A.; Ward, E.; Feuer, E.J.; Thun, M.J.; Society, A.C. Cancer statistics, 2004. CA Cancer J. Clin. 2004, 54, 8–29. [Google Scholar] [CrossRef]
- Cowppli-Bony, A. Survie Des Personnes Atteintes de Cancer en France Métropolitaine 1989–2013—Partie 1—Tumeurs Solides; Partenariat Francim/HCL/ InVS/INCa; Institut de Veille Sanitaire: Saint-Maurice, France, 2016; p. 274. [Google Scholar]
- Du Bois, A.; Quinn, M.; Thigpen, T.; Vermorken, J.; Avall-Lundqvist, E.; Bookman, M.; Bowtell, D.; Brady, M.; Casado, A.; Cervantes, A.; et al. 2004 consensus statements on the management of ovarian cancer: Final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2005, 16. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.C.E.; Kitchener, H.; Bacon, M.; Dubois, A.; Friedlander, M.; Ledermann, J.; Marth, C.; Thigpen, T.; Trimble, E. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: Report from the Fourth Ovarian Cancer Consensus Conference. Int. J. Gynecol. Cancer 2011, 21, 750–755. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Stoeckle, E.; Paravis, P.; Floquet, A.; Thomas, L.; Tunon de Lara, C.; Bussières, E.; Macgrogan, G.; Picot, V.; Avril, A. Number of residual nodules, better than size, defines optimal surgery in advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2004, 14, 779–787. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijrn, R.H.M.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Morrison, J.; Haldar, K.; Kehoe, S.; Lawrie, T.A. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane. Database. Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.-W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Gnauert, K.; Hils, R.; Lehmann, T.G.; Fisseler-Eckhoff, A.; Traut, A.; du Bois, A. Pattern and clinical predictors of lymph node metastases in epithelial ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2007, 17, 1238–1244. [Google Scholar] [CrossRef]
- Morice, P.; Joulie, F.; Camatte, S.; Atallah, D.; Rouzier, R.; Pautier, P.; Pomel, C.; Lhomme, C.; Duvillard, P.; Castaigne, D. Lymph node involvement in epithelial ovarian cancer: Analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J. Am. Coll. Surg. 2003, 197, 198–205. [Google Scholar] [CrossRef]
- Isonishi, S.; Niimi, S.; Sasaki, H.; Ochiai, K.; Yasuda, M.; Tanaka, T. Drug sensitivity-related benefit of systematic lymphadenectomy during cytoreductive surgery in optimally debulked stages IIIc and IV ovarian cancer. Gynecol. Oncol. 2004, 93, 647–652. [Google Scholar] [CrossRef]
- Du Bois, A.; Reuss, A.; Harter, P.; Pujade-Lauraine, E.; Ray-Coquard, I.; Pfisterer, J. Potential role of lymphadenectomy in advanced ovarian cancer: A combined exploratory analysis of three prospectively randomized phase III multicenter trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1733–1739. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.; Podratz, K.C.; Cliby, W.A. Role of lymphadenectomy in the management of grossly apparent advanced stage epithelial ovarian cancer. Am. J. Obstet. Gynecol. 2006, 195, 1862–1868. [Google Scholar] [CrossRef]
- Di Re, F.; Baiocchi, G.; Fontanelli, R.; Grosso, G.; Cobellis, L.; Raspagliesi, F.; di Re, E. Systematic pelvic and paraaortic lymphadenectomy for advanced ovarian cancer: Prognostic significance of node metastases. Gynecol. Oncol. 1996, 62, 360–365. [Google Scholar] [CrossRef]
- Scarabelli, C.; Gallo, A.; Visentin, M.C.; Canzonieri, V.; Carbone, A.; Zarrelli, A. Systematic pelvic and para-aortic lymphadenectomy in advanced ovarian cancer patients with no residual intraperitoneal disease. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer. Soc. 1997, 7, 18–26. [Google Scholar] [CrossRef]
- Chan, J.K.; Urban, R.; Hu, J.M.; Shin, J.Y.; Husain, A.; Teng, N.N.; Berek, J.S.; Osann, K.; Kapp, D.S. The potential therapeutic role of lymph node resection in epithelial ovarian cancer: A study of 13 918 patients. Br. J. Cancer 2007, 96, 1817–1822. [Google Scholar] [CrossRef]
- Panici, P.B.; Maggioni, A.; Hacker, N.; Landoni, F.; Ackermann, S.; Campagnutta, E.; Tamussino, K.; Winter, R.; Pellegrino, A.; Greggi, S.; et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: A randomized clinical trial. J. Natl. Cancer. Inst. 2005, 97, 560–566. [Google Scholar] [CrossRef]
- Morice, P.; Joulie, F.; Rey, A.; Atallah, D.; Camatte, S.; Pautier, P.; Thoury, A.; Lhomme, C.; Duvillard, P.; Castaigne, D. Are nodal metastases in ovarian cancer chemoresistant lesions? Analysis of nodal involvement in 105 patients treated with preoperative chemotherapy. Eur. J. Gynaecol. Oncol. 2004, 25, 169–174. [Google Scholar]
- Lavoue, V.; Huchon, C.; Akladios, C.; Alfonsi, P.; Bakrin, N.; Ballester, M.; Bendifallah, S.; Bolze, P.A.; Bonnet, C.; Bourgin, C.; et al. Management of epithelial ovarian cancer. Short text drafted from the French joint recommendations of FRANCOGYN, CNGOF, SFOG, GINECO–ARCAGY and endorsed by INCa. Bull. Cancer 2019, 106, 354–370. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- ASA Physical Status Classification System n.d. Available online: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (accessed on 7 April 2020).
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Iwase, H.; Takada, T.; Iitsuka, C.; Nomura, H.; Abe, A.; Taniguchi, T.; Takizawa, K. Clinical significance of systematic retroperitoneal lymphadenectomy during interval debulking surgery in advanced ovarian cancer patients. J. Gynecol. Oncol. 2015, 26, 303–310. [Google Scholar] [CrossRef]
- Song, N.; Gao, Y. Therapeutic value of selective lymphadenectomy in interval debulking surgery for stage IIIc and IV epithelial ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2019, 29, 761–767. [Google Scholar] [CrossRef]
- Eoh, K.J.; Yoon, J.W.; Lee, I.; Lee, J.-Y.; Kim, S.; Kim, S.W.; Kim, Y.T.; Nam, E.J. The efficacy of systematic lymph node dissection in advanced epithelial ovarian cancer during interval debulking surgery performed after neoadjuvant chemotherapy. J. Surg. Oncol. 2017, 116, 329–336. [Google Scholar] [CrossRef]
- Kaban, A.; Topuz, S.; Saip, P.; Sozen, H.; Celebi, K.; Salihoglu, Y. Poor Prognostic Factors in Patients Undergoing Surgery After Neoadjuvant Chemotherapy for Ovarian, Tubal, or Peritoneal Cancer. J. Obstet. Gynaecol. Can. JOGC J. Obstet. Gynecol. Can. JOGC 2017, 39, 1163–1170. [Google Scholar] [CrossRef]
- Schwartz, L.; Schrot-Sanyan, S.; Brigand, C.; Baldauf, J.-J.; Wattiez, A.; Akladios, C. Impact of Pelvic and Para-aortic Lymphadenectomy in Advanced Ovarian Cancer After Neoadjuvant Chemotherapy. Anticancer. Res. 2015, 35, 5503–5509. [Google Scholar]
- Fagotti, A.; De Iaco, P.; Fanfani, F.; Vizzielli, G.; Perelli, F.; Pozzati, F.; Perrone, A.M.; Turco, L.C.; Scambia, G. Systematic pelvic and aortic lymphadenectomy in advanced ovarian cancer patients at the time of interval debulking surgery: A double-institution case-control study. Ann. Surg. Oncol. 2012, 19, 3522–3527. [Google Scholar] [CrossRef]
- Chang, S.-J.; Bristow, R.E.; Ryu, H.-S. Prognostic significance of systematic lymphadenectomy as part of primary debulking surgery in patients with advanced ovarian cancer. Gynecol. Oncol. 2012, 126, 381–386. [Google Scholar] [CrossRef]
- Sakai, K.; Kajiyama, H.; Umezu, T.; Shibata, K.; Mizuno, M.; Suzuki, S.; Kawai, M.; Nagasaka, T.; Kikkawa, F. Is there any association between retroperitoneal lymphadenectomy and survival benefit in advanced stage epithelial ovarian carcinoma patients? J. Obstet. Gynaecol. Res. 2012, 38, 1018–1023. [Google Scholar] [CrossRef]
- Gao, J.; Yang, X.; Zhang, Y. Systematic lymphadenectomy in the treatment of epithelial ovarian cancer: A meta-analysis of multiple epidemiology studies. Jpn. J. Clin. Oncol. 2015, 45, 49–60. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, G.; Chen, Y. The effect of lymphadenectomy on survival and recurrence in patients with ovarian cancer: A systematic review and meta-analysis. Jpn. J. Clin. Oncol. 2016, 46, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ju, W.; Jee, B.C.; Kim, Y.B.; Park, N.H.; Song, Y.S.; Kim, S.C.; Kang, S.-B.; Kim, J.W. Systematic lymphadenectomy for survival in epithelial ovarian cancer: A meta-analysis. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2010, 20, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, F.; Song, Z.; Wang, X.; Zhang, C.; Ouyang, L. Prognostic Significance of Systematic Lymphadenectomy in Patients With Optimally Debulked Advanced Ovarian Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Kauderer, J.; Tewari, K.S.; Mannel, R.S.; Bristow, R.E.; O’Malley, D.M.; Rubin, S.C.; Glaser, G.E.; Hamilton, C.A.; Fujiwara, K.; et al. Correlation between Surgeon’s assessment and radiographic evaluation of residual disease in women with advanced stage ovarian cancer reported to have undergone optimal surgical cytoreduction: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2018, 149, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Pelissier, A.; Bonneau, C.; Chéreau, E.; de La Motte Rouge, T.; Fourchotte, V.; Daraï, E.; Rouzier, R. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol. Oncol. 2014, 135, 542–546. [Google Scholar] [CrossRef]
- Luyckx, M.; Leblanc, E.; Filleron, T.; Morice, P.; Darai, E.; Classe, J.-M.; FERRON, G.; STOECKLE, E.; POMEL, C.; VINET, B.; et al. Maximal cytoreduction in patients with FIGO stage IIIC to stage IV ovarian, fallopian, and peritoneal cancer in day-to-day practice: A Retrospective French Multicentric Study. Int. J. Gynecol. Cancer Off. J Int. Gynecol. Cancer Soc. 2012, 22, 1337–1343. [Google Scholar] [CrossRef]
- Bakrin, N.; Gladieff, L. Malignant epithelial ovarian cancer: Role of intra peritoneal chemotherapy and hyperthermic intra peritoneal chemotherapy (HIPEC): Article drafted from the French Guidelines in oncology entitled “Initial management of patients with epithelial ovarian cancer” developed by FRANCOGYN, CNGOF, SFOG, GINECO–ARCAGY under the aegis of CNGOF and endorsed by INCa. Gynecol. Obstet. Fertil. Senol. 2019, 47, 214–221. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sonke, G.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 1363–1364. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. It’s what the surgeon doesn’t see that kills the patient. J. Nippon. Med. Sch. Nippon. Ika. Daigaku. Zasshi. 2000, 67, 5–8. [Google Scholar] [CrossRef]
- Ceresoli, M.; Verrengia, A.; Montori, G.; Busci, L.; Coccolini, F.; Ansaloni, L.; Frigerio, L. Effect of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on relapse pattern in primary epithelial ovarian cancer: A propensity score based case-control study. J. Gynecol. Oncol. 2018, 29, e53. [Google Scholar] [CrossRef] [PubMed]
- Joulie, F.; Morice, P.; Rey, A.; Thoury, A.; Camatte, S.; Pautier, P.; Lhomme, C.; Haie-Meder, C.; Duvillard, P.; Castaigne, D. Are nodal metastases in ovarian cancer chemoresistant lesions? Comparative study of initial lymphadenectomy or after chemotherapy. Gynecol. Obstet. Fertil. 2004, 32, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Ferrandina, G.; Vizzielli, G.; Conte, C.; Lucidi, A.; Costantini, B.; De Rose, A.M.; Giorgio, A.D.; Zannoni, G.F.; Fagotti, A.; et al. Hepatoceliac Lymph Node Involvement in Advanced Ovarian Cancer Patients: Prognostic Role and Clinical Considerations. Ann. Surg. Oncol. 2017, 24, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Gallotta, V.; Fanfani, F.; Fagotti, A.; Chiantera, V.; Legge, F.; Alletti, S.G.; Nero, C.; Margariti, P.; Papa, V.; Alfieri, S.; et al. Mesenteric Lymph Node Involvement in Advanced Ovarian Cancer Patients Undergoing Rectosigmoid Resection: Prognostic Role and Clinical Considerations. Ann. Surg. Oncol. 2014, 21, 2369–2375. [Google Scholar] [CrossRef]

| Group 1: Nonlymphadenectomy | Group 2: Lymphadenectomy | p-Value | |
|---|---|---|---|
| Number of patients | 100 | 155 | |
| Age at diagnosis (years) | <0.0001 | ||
| ≤4/9 | 15 (20.3%) | 59 (79.7%) | <0001 |
| 50–69 | 43 (37.7%) | 71 (62.3%) | 0.009 |
| ≥70 | 42 (62.7%) | 25 (37.3%) | 0.04 |
| Median (range) | 67.5 (31–83) | 59 (31–82) | |
| BMI a (kg/m2) | 0.63 | ||
| <25 | 38 (49.4%) | 70 (54.3%) | |
| 25–30 | 27 (35%) | 37 (28.7%) | |
| >30 Missing | 12 (15.6%) 23 | 22 (17.1%) 26 | |
| Geographic origin | 0.09 | ||
| Caucasian | 74 (93.7%) | 74 (89.2%) | |
| North Africa | 1 (1.3%) | 7 (8.4%) | |
| Afro-Caribbean | 2 (2.5%) | 2 (2.4%) | |
| Asia | 2 (2.5%) | 0 | |
| South America | 0 | 0 | |
| Missing | 21 | 72 | |
| ASA b score | 0.03 | ||
| 0 | 0 | 2 (100%) | / |
| 1 | 15 (24.6%) | 46 (75.4%) | <0.0001 |
| 2 | 39 (45.9%) | 46 (54.1%) | 0.45 |
| 3 | 21 (47.7%) | 23 (52.3%) | 0.76 |
| 4 | 0 | 1 (100%) | / |
| Missing | 25 | 37 | |
| Charlson index | 0.003 | ||
| 0 | 11 (18.6%) | 48 (81.4%) | <0.0001 |
| ≥1 | 25 (44.6%) | 31 (55.4%) | 0.4 |
| Missing | 64 | 76 | |
| Gynecological-cancer history | 0.9 | ||
| Personal | 3 (3%) | 5 (3%) | |
| None | 97 (97%) | 150 (97%) | |
| Genetic mutations | 0.19 | ||
| No mutation | 2 (25%) | 7 (21.2%) | |
| Known mutation | 2 (25%) | 19 (57.6%) | |
| No research | 4 (50%) | 7 (21.2%) | |
| Missing | 92 | 122 |
| Group 1: Nonlymphadenectomy | Group 2: Lymphadenectomy | p-Value | |
|---|---|---|---|
| Number of patients | 100 | 155 | |
| FIGO Stage | 0.4 | ||
| III | 78 (78%) | 127 (81.9%) | 0.0008 |
| IV | 22 (22%) | 28 (18.1%) | 0.47 |
| Grade | 0.8 | ||
| 1–2 | 10 (17.9%) | 23 (19.3%) | |
| 3 | 46 (82.1%) | 96 (80.7%) | |
| Missing | 44 | 36 | |
| Preoperative CA125 (U/mL) | 0.36 | ||
| ≤1500 | 58 (65.2%) | 102 (70.8%) | |
| >1500 | 31 (34.8%) | 42 (29.2%) | |
| Missing | 11 | 11 | |
| NAC a | |||
| Number of cycles | 0.02 | ||
| ≤3 | 5 (5.1%) | 25 (16.7%) | |
| 4–6 | 74 (76.3%) | 107 (71.3%) | |
| ≥7 | 18 (18.6%) | 18 (12%) | |
| Missing | 3 | 5 | |
| Response to NAC | 0.08 | ||
| Complete | 5 (27.8%) | 19 (52.8%) | 0.007 |
| Partial | 13 (72.2%) | 17 (47.2%) | 0.58 |
| Missing | 82 | 119 |
| Group 1: Nonlymphadenectomy | Group 2: Lymphadenectomy | p-Value | |
|---|---|---|---|
| Number of patients | 100 | 155 | |
| Interval bebulking surgery | 0.01 | ||
| Laparoscopy | 15 (16.3%) | 51 (33.3%) | <0.0001 |
| Laparoscopy with laparoconversion | 6 (6.5%) | 6 (4%) | 1 |
| Laparotomy | 71 (77.2%) | 96 (62.7%) | 0.06 |
| Missing | 8 | 2 | |
| Operative time (min) | 242 (155–405) | 383 (170–660) | <0.0001 |
| Number of transfused patients | 0.7 | ||
| Yes | 9 (60%) | 16 (55.2%) | |
| No | 6 (40%) | 13 (44.8%) | |
| Missing | 85 | 126 | |
| Number of transfused red-blood-cell units | 1.9 (0–6) | 2.2 (0–4) | 0.45 |
| Postoperative residual disease (R) | <0.0001 | ||
| 0 | 50 (50%) | 137 (88.4%) | <0.0001 |
| 1 | 23 (23%) | 9 (5.8%) | 0.02 |
| 2 | 27 (27%) | 9 (5.8%) | 0.004 |
| Intraoperative complications | 0.003 | ||
| Yes | 6 (6.5%) | 28 (20.9%) | |
| No | 86 (93.5%) | 106 (79.1%) | |
| Missing | 8 | 21 | |
| Postoperative complications | 0.01 | ||
| Yes | 12 (12.8%) | 39 (26.7%) | |
| No | 82 (87.2%) | 107 (73.3%) | |
| Missing | 6 | 9 | |
| Dindo–Clavien score | 0.15 | ||
| 0 | 81 (85.3%) | 107 (73.8%) | |
| 1–2 | 10 (10.5%) | 29 (20%) | |
| 3–4 | 4 (4.2%) | 9 (6.2%) | |
| 5 | 0 | 0 | |
| Missing | 5 | 10 | |
| Number of cycles of adjuvant chemotherapy | 6.5 (1–15) | 6.9 (2–31) | 0.2 |
| Missing | 71 | 106 |
| KERRYPNX | UnivariateAnalysis | MultivariateAnalysis * | ||||
|---|---|---|---|---|---|---|
| Variables | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value |
| Center | 0.4 | |||||
| 1 (ref) | 1 | |||||
| 2 | 1.6 | 0–123.3 | ||||
| 3 | 1 | 0–60.6 | ||||
| 4 | 0.1 | 0–1616.8 | ||||
| 5 | 1.5 | 0–107.8 | ||||
| 6 | 0 | 0 | ||||
| 7 | 1.8 | 0–115.6 | ||||
| 8 | 0.7 | 0–43 | ||||
| 9 | 0 | 0 | ||||
| Lymphadenectomy: Number of removed lymph nodes | ||||||
| 0 (ref) | 1 | 1 | ||||
| 1–15 | 0.8 | 0.4–1.6 | 0.4 | 0.7 | 0.4–1.6 | 0.4 |
| 16–30 | 1.2 | 0.6–2.3 | 0.6 | 1.1 | 0.5–2.2 | 0.7 |
| 31–50 | 1.6 | 0.8–3.3 | 0.1 | 1.8 | 0.7–4.3 | 0.06 |
| Age (years) ≤49 (ref) 50–69 ≥ 70 | ||||||
| 1 | ||||||
| 0.8 | 0.4–1.7 | 0.7 | ||||
| 0.8 | 0.4–1.8 | 0.7 | ||||
| BMI a (kg/m2) <25 (ref) 25–30 >30 | ||||||
| 1 | ||||||
| 0.9 | 0.5–1.5 | 0.3 | ||||
| 1.5 | 0.7–3.3 | 0.2 | ||||
| Charlson index | 0.4 | |||||
| 0 (ref) | 1 | |||||
| ≥1 | 1.3 | 0.7–2.5 | ||||
| ASA | ||||||
| 0 (ref) | 1 | |||||
| 1 | 0.1 | 0–22.4 | 0.4 | |||
| 2 | 0.1 | 0–39.1 | 0.8 | |||
| 3 | 0.1 | 0–26.9 | 0.5 | |||
| 4 | 0 | 0 | 0 | |||
| FIGO stage | ||||||
| III (ref) | 1 | |||||
| IV | 1.2 | 0.8–2 | 0.4 | |||
| Grade | 0.8 | |||||
| 1–2 (ref) | 1 | |||||
| 3 | 0.9 | 0.4–1.9 | ||||
| Preoperative CA125 (U/mL) | 0.07 | 0.01 | ||||
| ≤1500 | 1 | 1 | ||||
| >1500 | 1.5 | 1–2.4 | 1.9 | 1.1–3.1 | ||
| Number of cycles | ||||||
| ≤3 | 1 | |||||
| 4–6 | 0.9 | 0.4–2 | 0.3 | |||
| ≥7 | 1.3 | 0.5–3.4 | 0.3 | |||
| NAC b response | ||||||
| Complete (ref) | 1 | |||||
| Partial | 1.5 | 0.5–4.5 | 0.4 | |||
| Surgery debulking | ||||||
| Laparoscopy (ref) | 1 | |||||
| Laparoscopy with laparoconversion | 1.7 | 0.2–12.6 | 0.5 | |||
| Laparotomy | 0.6 | 0.4–1.1 | 0.2 | |||
| Postoperative residual disease | ||||||
| R0 (ref) | 1 | 1 | ||||
| R1 | 1.6 | 0.9–2.8 | 0.09 | 1.8 | 0.9–3.4 | 0.07 |
| R2 | 1 | 0.6–1.6 | 0.3 | 1 | 0.6–1.9 | 0.4 |
| Number of invaded lymph nodes | ||||||
| 0–5 (ref) | 1 | 1 | ||||
| 6–10 | 1.2 | 0.1–21.1 | 0.4 | 1.3 | 0.1–25 | 0.5 |
| 11–20 | 6.4 | 0.2–199 | 0.1 | 9.6 | 0.3–320.7 | 0.06 |
| 21–30 | 1.1 | 0–33.8 | 0.6 | 0.9 | 0–28.9 | 0.4 |
| ≥31 | 0 | / | / | 0 | / | / |
| Univariate Analysis | Multivariate Analysis * | |||||
|---|---|---|---|---|---|---|
| Variables | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value |
| Center | 0.8 | |||||
| 1 (ref) | 1 | |||||
| 2 | 1.6 | 0.1–36.9 | ||||
| 3 | 0.6 | 0–11.2 | ||||
| 4 | 0.2 | 0–2282.7 | ||||
| 5 | 0.7 | 0–16.3 | ||||
| 6 | 0.5 | 0–14.1 | ||||
| 7 | 0.7 | 0–13.3 | ||||
| 8 | 0.7 | 0–13.5 | ||||
| 9 | 0 | 0 | ||||
| Lymphadenectomy: Number of removed lymph nodes | ||||||
| 0 (ref) | 1 | 1 | ||||
| 1–15 | 1.2 | 0.7–2 | 0.2 | 0.2 | 0.06–0.7 | 0.01 |
| 16–30 | 0.9 | 0.6–1.4 | 0.7 | 0.2 | 0.05–0.8 | 0.03 |
| 31–50 | 0.6 | 0.4–1.1 | 0.1 | 0.03 | 0.04–0.2 | 0.0005 |
| Age (years) | ||||||
| ≤49 (ref) | 1 | |||||
| 50–69 | 0.8 | 0.5–1.4 | 0.5 | |||
| ≥70 | 0.9 | 0.5–1.6 | 0.9 | |||
| BMI a (kg/m2) | ||||||
| <25 (ref) | 1 | |||||
| 25–30 | 1.2 | 0.8–1.8 | 0.4 | |||
| >30 | 1 | 0.6–1.7 | 0.7 | |||
| Charlson index | 0.3 | |||||
| 0 (ref) | 1 | |||||
| ≥1 | 0.8 | 0.5–1.2 | ||||
| ASA | ||||||
| 0 (ref) | 1 | |||||
| 1 | 3.4 | 0–951.4 | 0.7 | |||
| 2 | 2.9 | 0–801.9 | 0.9 | |||
| 3 | 3.9 | 0–1103.6 | 0.7 | |||
| 4 | 0 | 0 | 0 | |||
| FIGO stage | ||||||
| III (ref) | 1 | |||||
| IV | 1.1 | 0.8–1.7 | 0.5 | |||
| Grade | 0.7 | |||||
| 1–2 (ref) | 1 | |||||
| 3 | 1.1 | 0.7–1.8 | ||||
| Preoperative CA125 (U/mL) | 0.1 | 0.1 | ||||
| ≤1500 | 1 | 1 | ||||
| >1500 | 1.3 | 0.9–1.8 | 2.8 | 0.8–9.9 | ||
| Number of cycles | ||||||
| ≤3 | 1 | |||||
| 4–6 | 1.2 | 0.7–2.1 | 0.4 | |||
| ≥7 | 1.1 | 0.6–2 | 0.9 | |||
| NAC response | 0.03 | 0.7 | ||||
| Complete (ref) | 1 | 2 | ||||
| Partial | 2.3 | 1.1–5 | 1.3 | 0.3–4.7 | ||
| Surgery debulking | ||||||
| Laparoscopy (ref) | 1 | 1 | ||||
| Laparoscopy with laparoconversion | 2.4 | 0.9–6.4 | 0.07 | 154.8 | 5.4–441.1 | 0.003 |
| Laparotomy | 1.1 | 0.7–1.6 | 0.2 | 1.4 | 0.4–4.6 | 0.5 |
| Postoperative residual disease | ||||||
| R0 (ref) | 1 | 1 | ||||
| R1 | 1.7 | 1.05–2.8 | 0.1 | 0.8 | 0.2–4.4 | 0.8 |
| R2 | 1.3 | 0.8–2.1 | 0.9 | 1.3 | 0.1–10.1 | 0.8 |
| Number of invaded lymph nodes | ||||||
| 0–5 (ref) | 1 | |||||
| 6–10 | 0.9 | 0.5–1.7 | 0.4 | |||
| 11–20 | 1.5 | 0.5–4.6 | 0.7 | |||
| 21–30 | 1.9 | 0.4–7.6 | 0.5 | |||
| ≥31 | 1.0 | 0.1–7.4 | 0.8 |
| Group 1: Nonlymphadenectomy | Group 2: Lymphadenectomy | p-Value | |
|---|---|---|---|
| Recurrence | |||
| Pelvic | 0 | 0 | |
| Intraperitoneal | 25 (44%) | 36 (61%) | 0.2 |
| Retroperitoneal | 10 (15%) | 13 (22%) | 0.67 |
| Remote metastasis | 23 (41%) | 20 (34%) | 0.76 |
| Missing | 42 | 86 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bund, V.; Lecointre, L.; Velten, M.; Ouldamer, L.; Bendifallah, S.; Koskas, M.; Bolze, P.-A.; Collinet, P.; Canlorbe, G.; Touboul, C.; et al. Impact of Lymphadenectomy on Survival of Patients with Serous Advanced Ovarian Cancer After Neoadjuvant Chemotherapy: A French National Multicenter Study (FRANCOGYN). J. Clin. Med. 2020, 9, 2427. https://doi.org/10.3390/jcm9082427
Bund V, Lecointre L, Velten M, Ouldamer L, Bendifallah S, Koskas M, Bolze P-A, Collinet P, Canlorbe G, Touboul C, et al. Impact of Lymphadenectomy on Survival of Patients with Serous Advanced Ovarian Cancer After Neoadjuvant Chemotherapy: A French National Multicenter Study (FRANCOGYN). Journal of Clinical Medicine. 2020; 9(8):2427. https://doi.org/10.3390/jcm9082427
Chicago/Turabian StyleBund, Virginie, Lise Lecointre, Michel Velten, Lobna Ouldamer, Sofiane Bendifallah, Martin Koskas, Pierre-Adrien Bolze, Pierre Collinet, Geoffroy Canlorbe, Cyril Touboul, and et al. 2020. "Impact of Lymphadenectomy on Survival of Patients with Serous Advanced Ovarian Cancer After Neoadjuvant Chemotherapy: A French National Multicenter Study (FRANCOGYN)" Journal of Clinical Medicine 9, no. 8: 2427. https://doi.org/10.3390/jcm9082427
APA StyleBund, V., Lecointre, L., Velten, M., Ouldamer, L., Bendifallah, S., Koskas, M., Bolze, P.-A., Collinet, P., Canlorbe, G., Touboul, C., Huchon, C., Coutant, C., Faller, E., Boisramé, T., Gantzer, J., Demarchi, M., Baldauf, J.-J., Ballester, M., Lavoué, V., & Akladios, C. (2020). Impact of Lymphadenectomy on Survival of Patients with Serous Advanced Ovarian Cancer After Neoadjuvant Chemotherapy: A French National Multicenter Study (FRANCOGYN). Journal of Clinical Medicine, 9(8), 2427. https://doi.org/10.3390/jcm9082427







