The Influence of Hypertensive Therapies on Circulating Factors: Clinical Implications for SCFAs, FGF21, TNFSF14 and TNF-α
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Short-Chain Fatty Acids Analysis
2.3. Enzyme-Linked Immunosorbent Assays
2.4. Oxidative Stress Biomarkers
2.5. Biochemistry
2.6. Statistical Analysis
3. Results
3.1. Characterization of Study Population
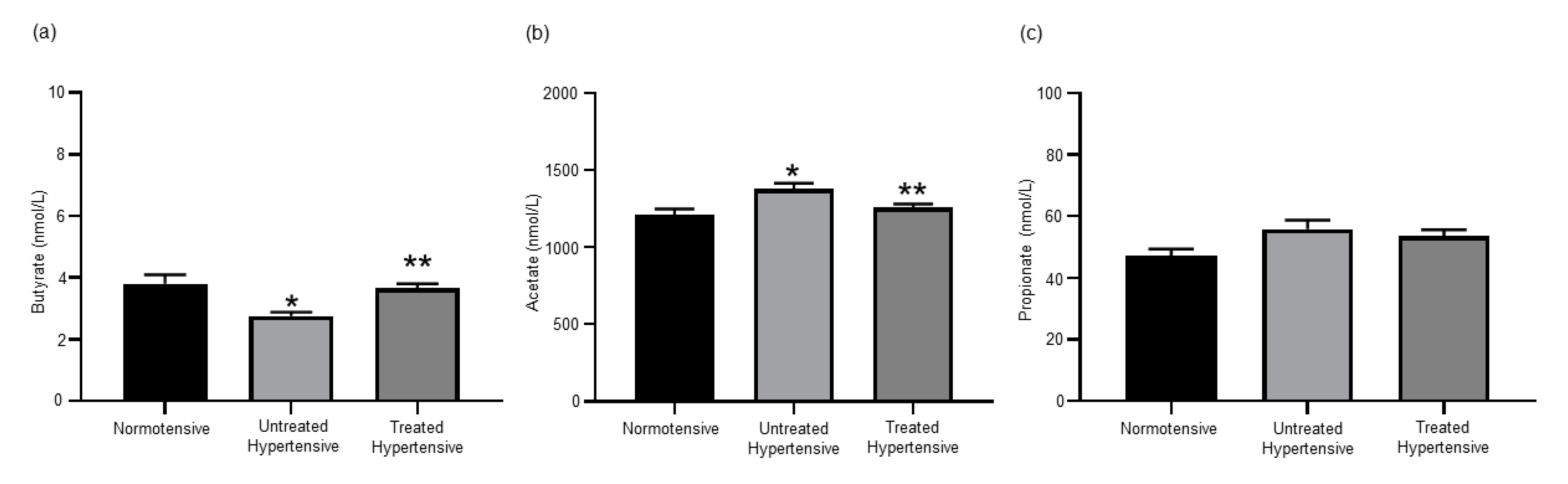
3.2. Effects of Antihypertensive Treatment on the Production of SCFAs
3.3. Antihypertensive Treatment Reduces Expression of FGF21
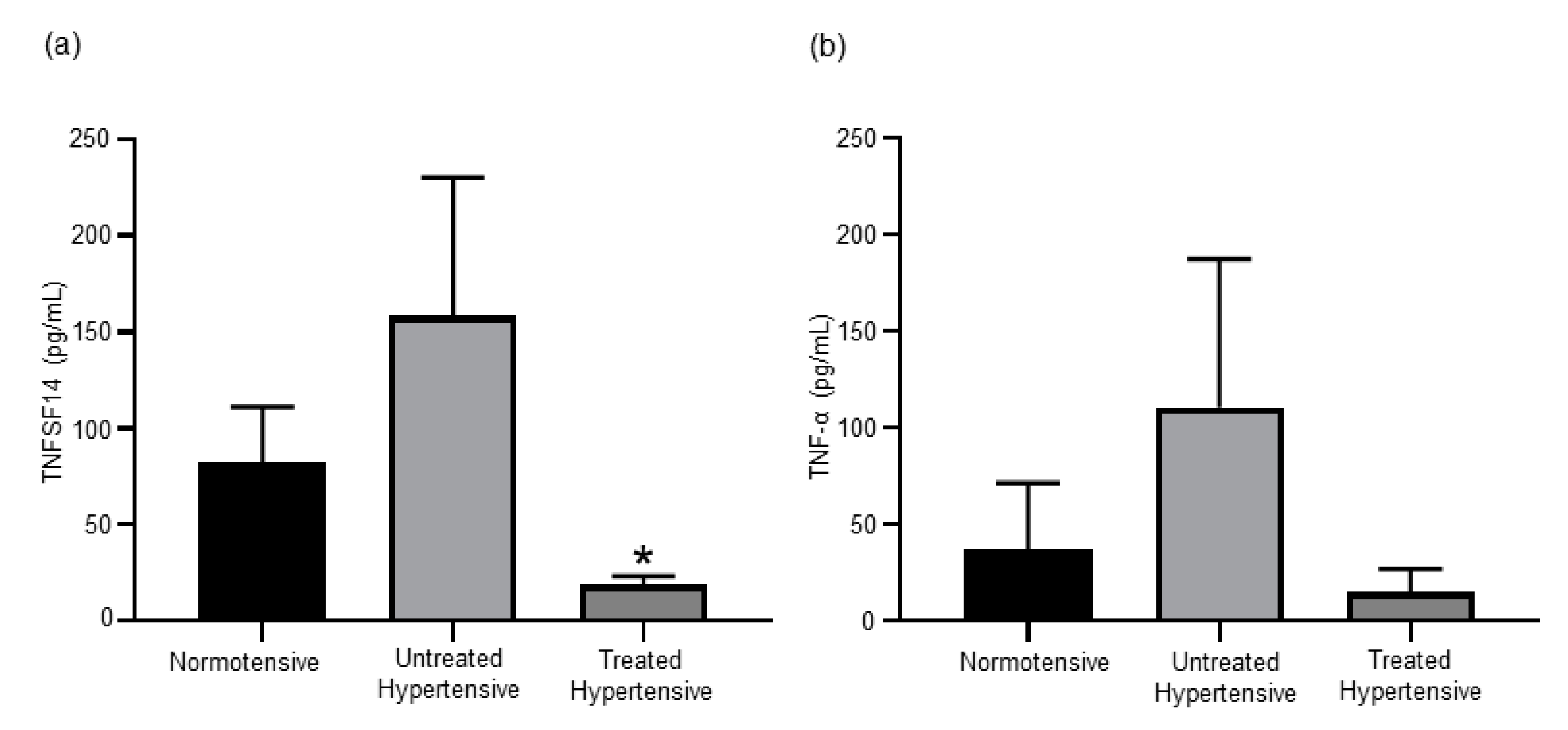
3.4. Antihypertensive Treatment Reduces Levels of TNF Superfamily Members
3.5. Effects of Antihypertensive Treatment on Oxidative Stress Biomarkers
3.6. Insulin Resistance in Hypertensive Subjects
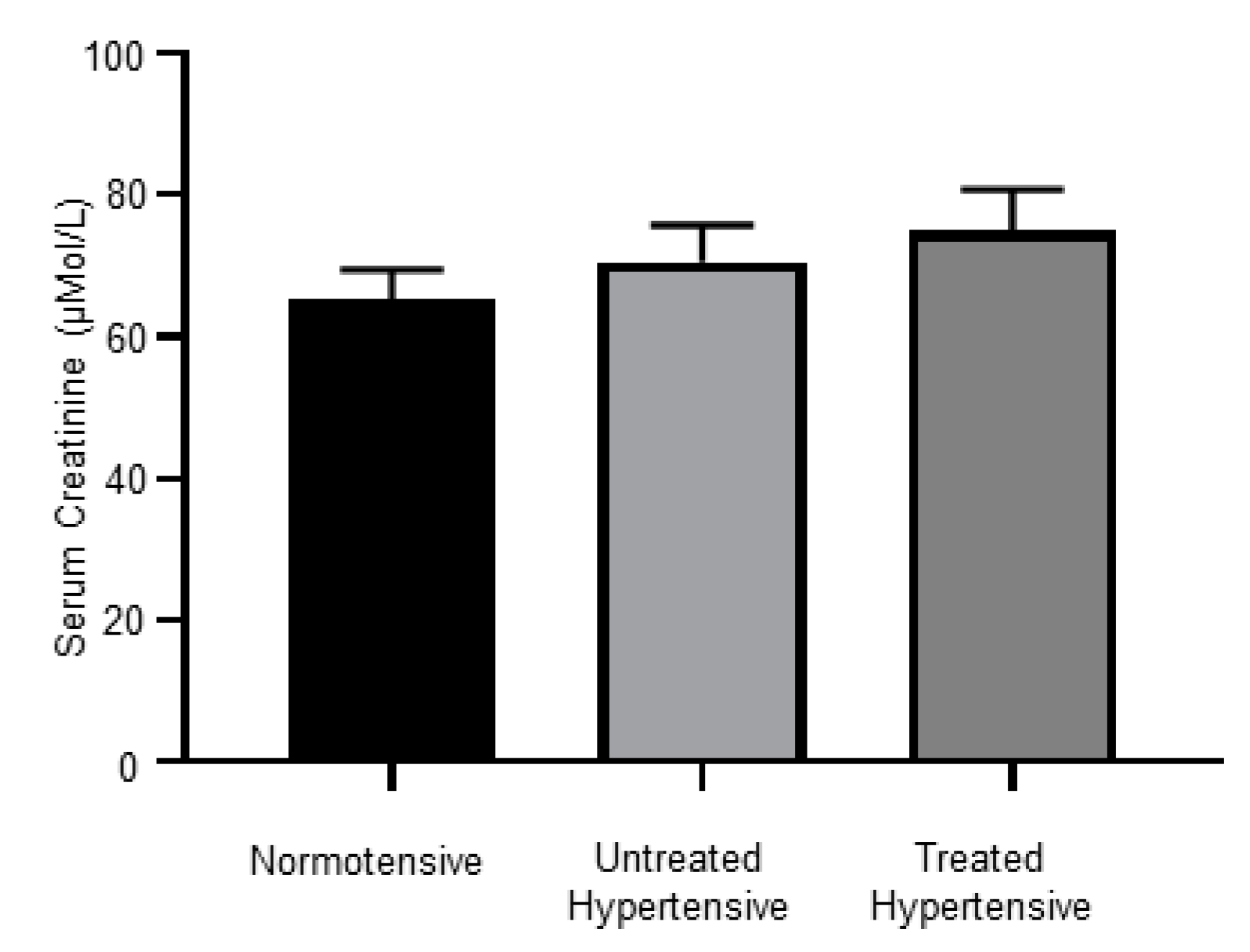
3.7. Kidney Dysfunction in Hypertensive Subjects
3.8. Antihypertensive Treatments Impact on Circulating Lipid Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sacco, R.L.; Roth, G.A.; Reddy, K.S.; Arnett, D.K.; Bonita, R.; Gaziano, T.A.; Heidenreich, P.A.; Huffman, M.D.; Mayosi, B.M.; Mendis, S.; et al. The Heart of 25 by 25: Achieving the Goal of Reducing Global and Regional Premature Deaths From Cardiovascular Diseases and Stroke: A Modeling Study From the American Heart Association and World Heart Federation. Circulation 2016, 133, e674–e690. [Google Scholar] [CrossRef] [PubMed]
- Liszka, H.A.; Mainous, A.G., 3rd; King, D.E.; Everett, C.J.; Egan, B.M. Prehypertension and cardiovascular morbidity. Ann. Fam. Med. 2005, 3, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Beevers, G.; Lip, G.Y.; O’Brien, E. ABC of hypertension: The pathophysiology of hypertension. BMJ 2001, 322, 912–916. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC Basic Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Kiuchi, M.G.; Jackson, K.L.; Carnagarin, R.; Head, G.A.; Schlaich, M.P.; Matthews, V.B. The Schlager mouse as a model of altered retinal phenotype. Neural Regen. Res. 2020, 15, 512–518. [Google Scholar] [CrossRef]
- Ward, N.C.; Hodgson, J.M.; Puddey, I.B.; Mori, T.A.; Beilin, L.J.; Croft, K.D. Oxidative stress in human hypertension: Association with antihypertensive treatment, gender, nutrition, and lifestyle. Free Radic. Biol. Med. 2004, 36, 226–232. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; Duarte, J. Microbiota and hypertension: Role of the sympathetic nervous system and the immune system. Am. J. Hypertens. 2020. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, A.; Cheung, B.M.Y. The Potential Role of Fibroblast Growth Factor 21 in Lipid Metabolism and Hypertension. Curr. Hypertens Rep. 2017, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Crasto, C.; Strait, J.; Sun, K.; Schaumberg, D.A.; Ferrucci, L. Elevated serum fibroblast growth factor 21 is associated with hypertension in community-dwelling adults. J. Hum. Hypertens 2013, 27, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.R.; Bando, Y.; Miyawaki, K.; Shikama, Y.; Kosugi, C.; Aki, N.; Funaki, M.; Noji, S. Correlation of fibroblast growth factor 21 serum levels with metabolic parameters in Japanese subjects. J. Med. Investig. 2014, 61, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Parchim, N.F.; Iriyama, T.; Luo, R.; Zhao, C.; Liu, C.; Irani, R.A.; Zhang, W.; Ning, C.; Zhang, Y.; et al. Excess LIGHT contributes to placental impairment, increased secretion of vasoactive factors, hypertension, and proteinuria in preeclampsia. Hypertension 2014, 63, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Dorffel, Y.; Latsch, C.; Stuhlmuller, B.; Schreiber, S.; Scholze, S.; Burmester, G.R.; Scholze, J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension 1999, 34, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Zinman, B.; Hanley, A.J.; Harris, S.B.; Kwan, J.; Fantus, I.G. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 1999, 84, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Caparros-Martin, J.A.; Lareu, R.R.; Ramsay, J.P.; Peplies, J.; Reen, F.J.; Headlam, H.A.; Ward, N.C.; Croft, K.D.; Newsholme, P.; Hughes, J.D.; et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome 2017, 5, 95. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Caparros-Martin, J.A.; Matthews, V.B.; Koch, H.; O’Gara, F.; Croft, K.D.; Ward, N.C. Isoquercetin and inulin synergistically modulate the gut microbiome to prevent development of the metabolic syndrome in mice fed a high fat diet. Sci. Rep. 2018, 8, 10100. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.A.; Croft, K.D.; Puddey, I.B.; Beilin, L.J. An improved method for the measurement of urinary and plasma F2-isoprostanes using gas chromatography-mass spectrometry. Anal. Biochem. 1999, 268, 117–125. [Google Scholar] [CrossRef]
- Luo, R.; Liu, C.; Elliott, S.E.; Wang, W.; Parchim, N.; Iriyama, T.; Daugherty, P.S.; Tao, L.; Eltzschig, H.K.; Blackwell, S.C.; et al. Transglutaminase is a Critical Link Between Inflammation and Hypertension. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Takeuchi, T.; Kotani, T.; Yamamoto, N.; Hata, K.; Nagai, K.; Shoda, T.; Takai, S.; Makino, S.; Hanafusa, T. Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J. Hum. Hypertens 2014, 28, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Passalacqua, W.; Araya, J.; Orellana, M.; Rivera, G. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J. Cardiovasc. Pharm. 2003, 42, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Dolegowska, B.; Blogowski, W.; Kedzierska, K.; Safranow, K.; Jakubowska, K.; Olszewska, M.; Rac, M.; Chlubek, D.; Ciechanowski, K. Platelets arachidonic acid metabolism in patients with essential hypertension. Platelets 2009, 20, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Guichard, C.; Bachler, J.P. Relationship between oxidative stress and essential hypertension. Hypertens Res. 2007, 30, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Karlafti, E.F.; Hatzitolios, A.I.; Karlaftis, A.F.; Baltatzi, M.S.; Koliakos, G.G.; Savopoulos, C.G. Effects of moxonidine on sympathetic nervous system activity: An update on metabolism, cardio, and other target-organ protection. J. Pharm. Bioallied. Sci. 2013, 5, 253–256. [Google Scholar] [CrossRef]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front Cell Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef] [Green Version]
- Herat, L.Y.; Ward, N.; Magno, A.L.; Rakoczy, E.P.; Kiuchi, M.G.; Schlaich, M.P.; Matthews, V.B. Sodium glucose co-transporter 2 inhibition reduces succinate levels in diabetic mice. World J. Gastroenterol. 2020, 26, 3225–3235. [Google Scholar] [CrossRef]
- Jama, H.A.; Kaye, D.M.; Marques, F.Z. The gut microbiota and blood pressure in experimental models. Curr. Opin. Nephrol. Hypertens 2019, 28, 97–104. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Liu, Y.B.; Hu, H. Metabolic role of fibroblast growth factor 21 in liver, adipose and nervous system tissues. Biomed. Rep. 2017, 6, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Xu, B.; Feng, Y.; Li, K.X.; Liu, Z.; Sun, X.; Lu, X.L.; Wang, L.Q.; Chen, Y.W.; Fan, X.X.; et al. FGF-21 ameliorates essential hypertension of SHR via baroreflex afferent function. Brain Res. Bull. 2020, 154, 9–20. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Zhao, M.; Xia, J.J.; Guan, J.; Liu, Y.; Wang, L.Q.; Song, D.X.; Qu, M.Y.; Zuo, M.; Wen, X.; et al. FGF21 ameliorates the neurocontrol of blood pressure in the high fructose-drinking rats. Sci. Rep. 2016, 6, 29582. [Google Scholar] [CrossRef]
- Pan, X.; Shao, Y.; Wu, F.; Wang, Y.; Xiong, R.; Zheng, J.; Tian, H.; Wang, B.; Wang, Y.; Zhang, Y.; et al. FGF21 Prevents Angiotensin II-Induced Hypertension and Vascular Dysfunction by Activation of ACE2/Angiotensin-(1-7) Axis in Mice. Cell Metab. 2018, 27, 1323–1337.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.J.; Hong, H.C.; Choi, H.Y.; Yoo, H.J.; Cho, G.J.; Hwang, T.G.; Baik, S.H.; Choi, D.S.; Kim, S.M.; Choi, K.M. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin. Endocrinol. 2011, 75, 464–469. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fam, K.D.; Chia, K.L.; Yap, M.M.C.; Goh, J.; Yeo, K.P.; Yap, E.P.H.; Chotirmall, S.H.; Lim, C.L. Age-related bone loss is associated with FGF21 but not IGFBP1 in healthy adults. Exp. Physiol. 2020, 105, 622–631. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Cao, Z.B.; Oshima, S.; Ise, R.; Sakamoto, S.; Higuchi, M. Cardiorespiratory fitness and visceral fat are key determinants of serum fibroblast growth factor 21 concentration in Japanese men. J. Clin. Endocrinol. Metab. 2014, 99, E1877–E1884. [Google Scholar] [CrossRef] [Green Version]
- Dushay, J.; Chui, P.C.; Gopalakrishnan, G.S.; Varela-Rey, M.; Crawley, M.; Fisher, F.M.; Badman, M.K.; Martinez-Chantar, M.L.; Maratos-Flier, E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010, 139, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Mraz, M.; Bartlova, M.; Lacinova, Z.; Michalsky, D.; Kasalicky, M.; Haluzikova, D.; Matoulek, M.; Dostalova, I.; Humenanska, V.; Haluzik, M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. 2009, 71, 369–375. [Google Scholar] [CrossRef]
- Staiger, H.; Keuper, M.; Berti, L.; Hrabe de Angelis, M.; Haring, H.U. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr. Rev. 2017, 38, 468–488. [Google Scholar] [CrossRef]
- Saunders, B.M.; Rudnicka, C.; Filipovska, A.; Davies, S.; Ward, N.; Hricova, J.; Schlaich, M.P.; Matthews, V.B. Shining LIGHT on the metabolic role of the cytokine TNFSF14 and the implications on hepatic IL-6 production. Immunol. Cell Biol. 2018, 96, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens 2017, 35, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Swislocki, A.L. Effects of antihypertensives on glucose metabolism. Metab. Syndr. Relat. Disord. 2007, 5, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Li, Z.L.; Ding, A.; Yang, H.; Zhu, W.P.; Cui, T.X.; Zhang, H.T.; Zhang, H. Olmesartan Medoxomil, An Angiotensin II-Receptor Blocker, Ameliorates Renal Injury In db/db Mice. Drug Des. Devel. Ther. 2019, 13, 3657–3667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.M.; Hsu, Y.M.; Ying, M.C.; Tsai, F.J.; Tsai, C.H.; Chung, J.G.; Yang, J.S.; Tang, C.H.; Cheng, L.Y.; Su, P.H.; et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr. Metab. 2019, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.O.; Meng, J.; Tang, H.; Yin, K. High-density lipoprotein-mediated cardioprotection in heart failure. Heart Fail Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Allard-Ratick, M.P.; Kindya, B.R.; Khambhati, J.; Engels, M.C.; Sandesara, P.B.; Rosenson, R.S.; Sperling, L.S. HDL: Fact, fiction, or function? HDL cholesterol and cardiovascular risk. Eur. J. Prev. Cardiol. 2019, 2047487319848214. [Google Scholar] [CrossRef]
- Herat, L.; Rudnicka, C.; Okada, Y.; Mochizuki, S.; Schlaich, M.; Matthews, V. The Metalloproteinase ADAM28 Promotes Metabolic Dysfunction in Mice. Int. J. Mol. Sci. 2017, 18, 884. [Google Scholar] [CrossRef]
- Fu, Y.C.; Chi, C.S.; Yin, S.C.; Hwang, B.; Chiu, Y.T.; Hsu, S.L. Norepinephrine induces apoptosis in neonatal rat cardiomyocytes through a reactive oxygen species-TNF alpha-caspase signaling pathway. Cardiovasc. Res. 2004, 62, 558–567. [Google Scholar] [CrossRef]
- Jowett, J.B.; Okada, Y.; Leedman, P.J.; Curran, J.E.; Johnson, M.P.; Moses, E.K.; Goring, H.H.; Mochizuki, S.; Blangero, J.; Stone, L.; et al. ADAM28 is elevated in humans with the metabolic syndrome and is a novel sheddase of human tumour necrosis factor-alpha. Immunol. Cell Biol. 2012, 90, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, N.; Figarola, J.L.; Li, Y.; Swiderski, P.M.; Rahbar, S.; Natarajan, R. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes 2008, 57, 879–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.N.; Gollapudi, S.; Sharman, E.H.; Jia, Z.; Gupta, S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell 2010, 9, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Rizzo, M.R.; Mazziotti, G.; Tagliamonte, M.R.; Gambardella, A.; Rotondi, M.; Carella, C.; Giugliano, D.; Varricchio, M.; D’Onofrio, F. Advancing age and insulin resistance: Role of plasma tumor necrosis factor-alpha. Am. J. Physiol. 1998, 275, E294–E299. [Google Scholar] [CrossRef] [PubMed]
- Bryhni, B.; Arnesen, E.; Jenssen, T.G. Associations of age with serum insulin, proinsulin and the proinsulin-to-insulin ratio: A cross-sectional study. BMC Endocr. Disord. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | Normotensive | Untreated Hypertensive | Treated Hypertensive |
|---|---|---|---|
| Gender (M/F) | 3/7 | 11/4 | 22/17 |
| Age (years) | 54.50 ± 2.15 | 55.60 ± 2.97 | 58.87 ± 1.40 |
| BMI (kg/m2) | 27.38 ± 1.11 | 26.70 ± 0.72 | 26.64 ± 0.58 |
| SBP (mmHg) | 130.40 ± 2.40 | 138.13 ± 2.30 | 131.36 ± 1.90 |
| DBP (mmHg) | 75.40 ± 6.89 | 82.47 ± 2.37 | 80.21 ± 1.47 |
| Parameter | Normotensive | Untreated Hypertensive | Treated Hypertensive |
|---|---|---|---|
| Gender (M/F) | 8/10 | 8/4 | 8/10 |
| Age (years) | 61.3 ± 2.21 | 53.92 ± 2.57 | 63.06 ± 1.39 1 |
| BMI (kg/m2) | 27.19 ± 0.50 | 28.67 ± 1.01 | 29.23 ± 0.99 |
| SBP (mmHg) | 123.7 ± 4.18 | 146.08 ± 4.30 2 | 131.3 ± 2.82 3 |
| DBP (mmHg) | 74.4 ± 2.27 | 90.25 ± 3.65 4 | 79.7 ± 2.01 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magno, A.L.; Herat, L.Y.; Kiuchi, M.G.; Schlaich, M.P.; Ward, N.C.; Matthews, V.B. The Influence of Hypertensive Therapies on Circulating Factors: Clinical Implications for SCFAs, FGF21, TNFSF14 and TNF-α. J. Clin. Med. 2020, 9, 2764. https://doi.org/10.3390/jcm9092764
Magno AL, Herat LY, Kiuchi MG, Schlaich MP, Ward NC, Matthews VB. The Influence of Hypertensive Therapies on Circulating Factors: Clinical Implications for SCFAs, FGF21, TNFSF14 and TNF-α. Journal of Clinical Medicine. 2020; 9(9):2764. https://doi.org/10.3390/jcm9092764
Chicago/Turabian StyleMagno, Aaron L., Lakshini Y. Herat, Márcio G. Kiuchi, Markus P. Schlaich, Natalie C. Ward, and Vance B. Matthews. 2020. "The Influence of Hypertensive Therapies on Circulating Factors: Clinical Implications for SCFAs, FGF21, TNFSF14 and TNF-α" Journal of Clinical Medicine 9, no. 9: 2764. https://doi.org/10.3390/jcm9092764
APA StyleMagno, A. L., Herat, L. Y., Kiuchi, M. G., Schlaich, M. P., Ward, N. C., & Matthews, V. B. (2020). The Influence of Hypertensive Therapies on Circulating Factors: Clinical Implications for SCFAs, FGF21, TNFSF14 and TNF-α. Journal of Clinical Medicine, 9(9), 2764. https://doi.org/10.3390/jcm9092764





