Low Body Weight Increases the Risk of Ischemic Stroke and Major Bleeding in Atrial Fibrillation: The COOL-AF Registry
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Study Protocol and Data Collection
2.3. Outcome Measurement
2.4. Statistical Analysis
3. Results
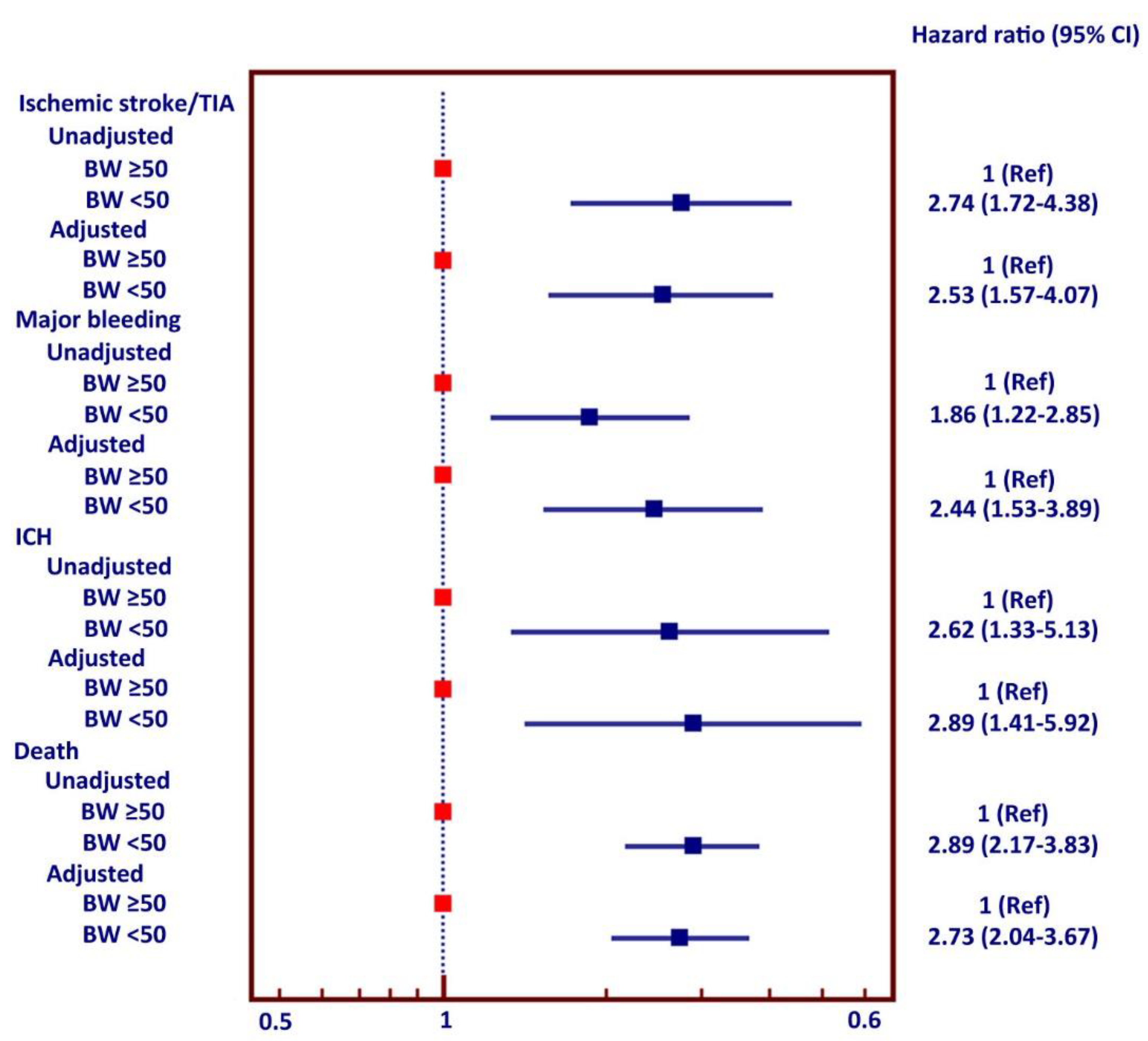
3.1. LBW and Outcomes
3.2. Subgroup Analyses
3.3. Multivariate Analysis
3.4. Body Weight as a Continuous Variable
3.5. Sensitivity Analysis
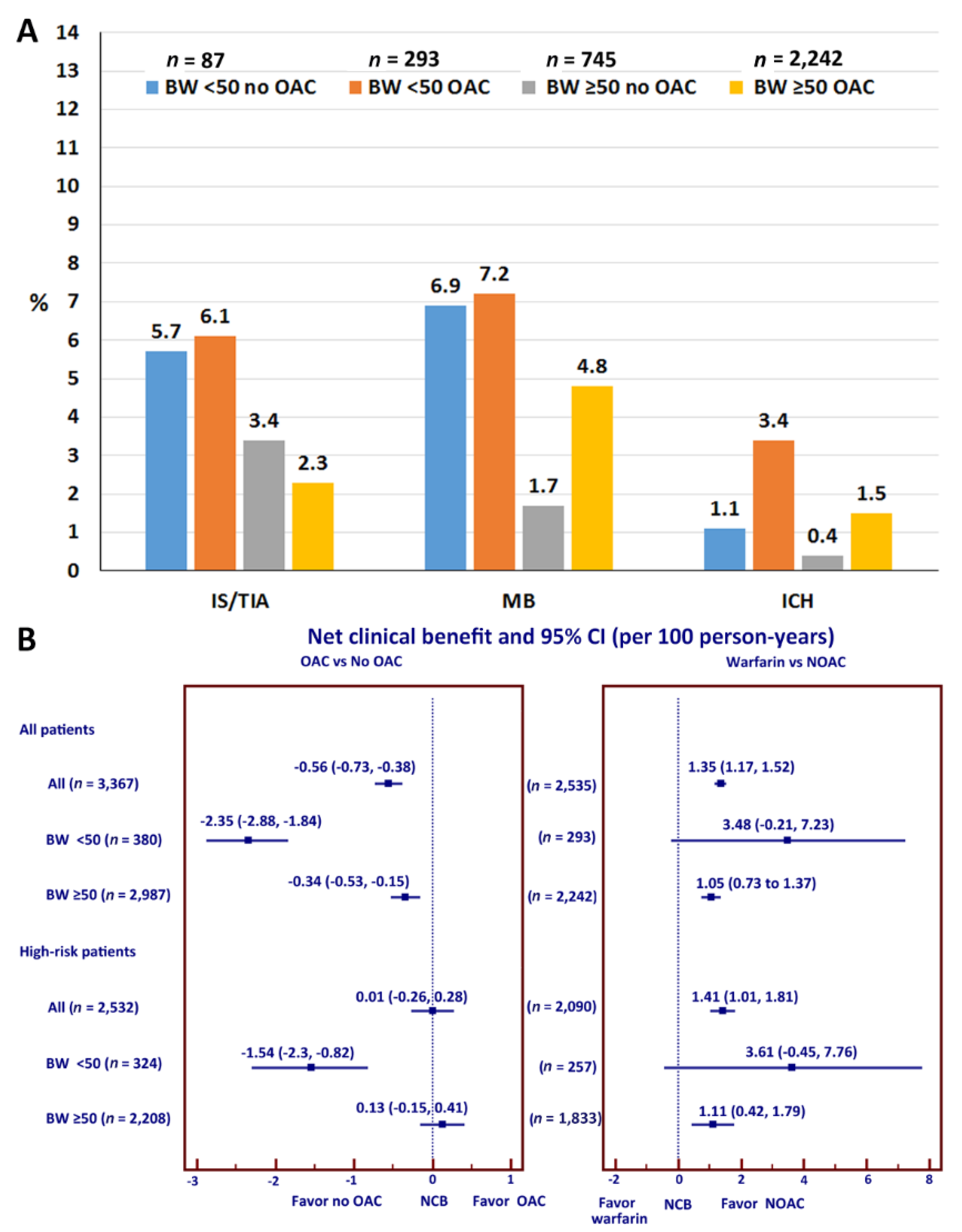
3.6. Effects of OAC on Clinical Outcomes
3.7. Net Clinical Benefit
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lip, G.; Freedman, B.; De Caterina, R.; Potpara, T.S. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017, 117, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [Green Version]
- Healey, J.S.; Oldgren, J.; Ezekowitz, M.; Zhu, J.; Pais, P.; Wang, J.; Commerford, P.; Jansky, P.; Avezum, A.; Sigamani, A.; et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: A cohort study. Lancet 2016, 388, 1161–1169. [Google Scholar] [CrossRef]
- Tse, H.F.; Wang, Y.J.; Ahmed Ai-Abdullah, M.; Pizarro-Borromeo, A.B.; Chiang, C.E.; Krittayaphong, R.; Singh, B.; Vora, A.; Wang, C.X.; Zubaid, M.; et al. Stroke prevention in atrial fibrillation—An Asian stroke perspective. Heart Rhythm 2013, 10, 1082–1088. [Google Scholar] [CrossRef]
- Shen, A.Y.; Yao, J.F.; Brar, S.S.; Jorgensen, M.B.; Chen, W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J. Am. Coll. Cardiol. 2007, 50, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Chao, T.F.; Liu, C.J.; Tuan, T.C.; Chen, S.J.; Wang, K.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chen, T.J.; et al. Comparisons of CHADS2 and CHA2DS2-VASc scores for stroke risk stratification in atrial fibrillation: Which scoring system should be used for Asians? Heart Rhythm 2016, 13, 46–53. [Google Scholar] [CrossRef]
- Chao, T.F.; Lip, G.Y.; Liu, C.J.; Tuan, T.C.; Chen, S.J.; Wang, K.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; et al. Validation of a Modified CHA2DS2-VASc Score for Stroke Risk Stratification in Asian Patients With Atrial Fibrillation: A Nationwide Cohort Study. Stroke 2016, 47, 2462–2469. [Google Scholar] [CrossRef] [Green Version]
- Hamatani, Y.; Yamashita, Y.; Esato, M.; Chun, Y.H.; Tsuji, H.; Wada, H.; Hasegawa, K.; Abe, M.; Lip, G.Y.; Akao, M. Predictors for Stroke and Death in Non-Anticoagulated Asian Patients with Atrial Fibrillation: The Fushimi AF Registry. PLoS ONE 2015, 10, e0142394. [Google Scholar] [CrossRef]
- Boonyawat, K.; Caron, F.; Li, A.; Chai-Adisaksopha, C.; Lim, W.; Iorio, A.; Lopes, R.D.; Garcia, D.; Crowther, M.A. Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: A systematic review and meta-analysis. J. Thromb. Haemost. 2017, 15, 1322–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamatani, Y.; Ogawa, H.; Uozumi, R.; Iguchi, M.; Yamashita, Y.; Esato, M.; Chun, Y.H.; Tsuji, H.; Wada, H.; Hasegawa, K.; et al. Low Body Weight Is Associated With the Incidence of Stroke in Atrial Fibrillation Patients—Insight From the Fushimi AF Registry. Circ. J. 2015, 79, 1009–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upreti, V.V.; Wang, J.; Barrett, Y.C.; Byon, W.; Boyd, R.A.; Pursley, J.; LaCreta, F.P.; Frost, C.E. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br. J. Clin. Pharm. 2013, 76, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Winijkul, A.; Methavigul, K.; Wongtheptien, W.; Wongvipaporn, C.; Wisaratapong, T.; Kunjara-Na-Ayudhya, R.; Boonyaratvej, S.; Komoltri, C.; Kaewcomdee, P.; et al. Risk profiles and pattern of antithrombotic use in patients with non-valvular atrial fibrillation in Thailand: A multicenter study. BMC Cardiovasc. Disord. 2018, 18, 174. [Google Scholar] [CrossRef] [Green Version]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific; Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Singer, D.E.; Chang, Y.; Fang, M.C.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Go, A.S. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann. Intern. Med. 2009, 151, 297–305. [Google Scholar] [CrossRef]
- Pan, W.H.; Yeh, W.T. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: An extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 2008, 17, 370–374. [Google Scholar]
- Pijl, H.; Meinders, A.E. Bodyweight change as an adverse effect of drug treatment. Mechanisms and management. Drug Saf. 1996, 14, 329–342. [Google Scholar] [CrossRef]
- Eliasson, E. Ethnicity and adverse drug reactions. BMJ 2006, 332, 1163–1164. [Google Scholar] [CrossRef]
- Hori, M.; Matsumoto, M.; Tanahashi, N.; Momomura, S.; Uchiyama, S.; Goto, S.; Izumi, T.; Koretsune, Y.; Kajikawa, M.; Kato, M.; et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—The J-ROCKET AF study. Circ. J. 2012, 76, 2104–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lip, G.Y.; Wang, K.L.; Chiang, C.E. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: Time for a reappraisal. Int. J. Cardiol. 2015, 180, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Goto, S.; Accetta, G.; Angchaisuksiri, P.; Camm, A.J.; Cools, F.; Haas, S.; Kayani, G.; Koretsune, Y.; Lim, T.W.; et al. Vitamin K antagonist control in patients with atrial fibrillation in Asia compared with other regions of the world: Real-world data from the GARFIELD-AF registry. Int. J. Cardiol. 2016, 223, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Phrommintikul, A.; Detnuntarat, P.; Prasertwitayakij, N.; Wongcharoen, W. Prevalence of atrial fibrillation in Thai elderly. J. Geriatr. Cardiol. 2016, 13, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Pengpid, S.; Peltzer, K. Underweight and overweight or obesity and associated factors among school-going adolescents in five ASEAN countries, 2015. Diabetes Metab. Syndr. 2019, 13, 3075–3080. [Google Scholar] [CrossRef] [PubMed]
- Kongbunkiat, K.; Kasemsap, N.; Travanichakul, S.; Thepsuthammarat, K.; Tiamkao, S.; Sawanyawisuth, K. Hospital mortality from atrial fibrillation associated with ischemic stroke: A national data report. Int. J. Neurosci. 2015, 125, 924–928. [Google Scholar] [CrossRef]
- Lee, S.R.; Choi, E.K.; Park, C.S.; Han, K.D.; Jung, J.H.; Oh, S.; Lip, G.Y.H. Direct Oral Anticoagulants in Patients with Nonvalvular Atrial Fibrillation and Low Body Weight. J. Am. Coll. Cardiol. 2019, 73, 919–931. [Google Scholar] [CrossRef]
- Malik, A.H.; Yandrapalli, S.; Shetty, S.; Aronow, W.S.; Jain, D.; Frishman, W.H.; Cooper, H.A.; Panza, J.A.; Investigators, M. Impact of weight on the efficacy and safety of direct-acting oral anticoagulants in patients with non-valvular atrial fibrillation: A meta-analysis. Europace 2020, 22, 361–367. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Montori, V.M.; Somers, V.K.; Korinek, J.; Thomas, R.J.; Allison, T.G.; Mookadam, F.; Lopez-Jimenez, F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: A systematic review of cohort studies. Lancet 2006, 368, 666–678. [Google Scholar] [CrossRef]
- Lancefield, T.; Clark, D.J.; Andrianopoulos, N.; Brennan, A.L.; Reid, C.M.; Johns, J.; Freeman, M.; Charter, K.; Duffy, S.J.; Ajani, A.E.; et al. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc. Interv. 2010, 3, 660–668. [Google Scholar] [CrossRef] [Green Version]
- Uretsky, S.; Messerli, F.H.; Bangalore, S.; Champion, A.; Cooper-Dehoff, R.M.; Zhou, Q.; Pepine, C.J. Obesity paradox in patients with hypertension and coronary artery disease. Am. J. Med. 2007, 120, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Momin, A.U.; Melikian, N.; Shah, A.M.; Grieve, D.J.; Wheatcroft, S.B.; John, L.; El Gamel, A.; Desai, J.B.; Nelson, T.; Driver, C.; et al. Leptin is an endothelial-independent vasodilator in humans with coronary artery disease: Evidence for tissue specificity of leptin resistance. Eur. Heart J. 2006, 27, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Kimura, M.; Noma, K.; Sasaki, S.; Hara, K.; Matsuura, H.; Goto, C.; Oshima, T.; et al. Low body mass index is a risk factor for impaired endothelium-dependent vasodilation in humans: Role of nitric oxide and oxidative stress. J. Am. Coll. Cardiol. 2003, 42, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.A.; Neutel, J.M.; Smith, D.H. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J. Am. Coll. Cardiol. 2001, 37, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Mak, K.H.; Bhatt, D.L.; Shao, M.; Haffner, S.M.; Hamm, C.W.; Hankey, G.J.; Johnston, S.C.; Montalescot, G.; Steg, P.G.; Steinhubl, S.R.; et al. The influence of body mass index on mortality and bleeding among patients with or at high-risk of atherothrombotic disease. Eur. Heart J. 2009, 30, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Yamaoka, H.; Morita, K.; Ebata, M.; Eguchi, S.; Muneyuki, T.; Munakata, H. Elderly people with low body weight may have subtle low-grade inflammation. Obesity (Silver Spring) 2009, 17, 803–808. [Google Scholar] [CrossRef]
- Conway, D.S.; Buggins, P.; Hughes, E.; Lip, G.Y. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J. Am. Coll. Cardiol. 2004, 43, 2075–2082. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.D.; Kandula, N.R.; Lin, F.; Allison, M.A.; Carr, J.; Herrington, D.; Liu, K.; Kanaya, A.M. Less favorable body composition and adipokines in South Asians compared with other US ethnic groups: Results from the MASALA and MESA studies. Int. J. Obes. (Lond.) 2016, 40, 639–645. [Google Scholar] [CrossRef] [Green Version]



| Characteristics | All (n = 3367) | BW < 50 (n = 380) | BW ≥ 50 (n = 2987) | p |
|---|---|---|---|---|
| Age (years) | 67.2 ± 11.2 | 74.1 ± 10.2 | 66.4 ± 11.1 | <0.001 |
| Female | 1397 (41.5%) | 284 (74.7%) | 1113 (37.3%) | <0.001 |
| Time after NVAF diagnosis (years) | 3.4 ± 4.3 | 3.2 ± 3.9 | 3.4 ± 4.4 | 0.362 |
| Type of NVAF | 0.070 | |||
| - Paroxysmal | 1131 (33.6%) | 115 (30.3%) | 1016 (34.0%) | |
| - Persistent | 642 (19.1%) | 64 (16.8%) | 578 (19.4%) | |
| - Permanent | 1594 (47.3%) | 201 (52.9%) | 1393 (46.6%) | |
| Symptomatic NVAF | 2600 (77.2%) | 292 (76.8%) | 2308 (77.3%) | 0.852 |
| History of heart failure | 897 (26.6%) | 93 (24.5%) | 804 (26.9%) | 0.310 |
| History of coronary artery disease | 543 (16.1%) | 44 (11.6%) | 499 (16.7%) | 0.010 |
| CIED | 329 (9.8%) | 45 (11.8%) | 284 (9.5%) | 0.149 |
| History of ischemic stroke/TIA | 573 (17.0%) | 72 (18.9%) | 501 (16.8%) | <0.001 |
| Hypertension | 2299 (68.3%) | 229 (60.3%) | 2070 (69.3%) | <0.001 |
| Diabetes mellitus | 834 (24.8%) | 44 (11.6%) | 790 (26.4%) | <0.001 |
| Current smoker | 676 (20.1%) | 34 (8.9%) | 642 (21.5%) | <0.001 |
| Dyslipidemia | 1901 (56.5%) | 163 (42.9%) | 1738 (58.2%) | <0.001 |
| Renal replacement therapy | 38 (1.1%) | 3 (0.8%) | 35 (1.2%) | 0.795 |
| Dementia | 28 (0.8%) | 4 (1.1%) | 24 (0.8%) | 0.549 |
| History of bleeding | 322 (9.6%) | 45 (11.8%) | 277 (9.3%) | 0.109 |
| CHA2DS2-VASc score | <0.001 | |||
| - 0 | 198 (5.9%) | 4 (1.1%) | 194 (6.5%) | |
| - 1 | 422 (12.5%) | 21 (5.5%) | 401 (13.4%) | |
| - ≥2 | 2747 (81.6%) | 355 (93.4%) | 2392 (80.1%) | |
| HAS-BLED score | <0.001 | |||
| - 0 | 489 (14.5%) | 25 (6.6%) | 464 (15.5%) | |
| - 1–2 | 2354 (69.9%) | 281 (73.9%) | 2073 (69.4%) | |
| - ≥3 | 524 (15.6%) | 74 (19.5%) | 450 (15.1%) | |
| Antiplatelet | 882 (26.2%) | 81 (21.3%) | 801 (26.8%) | 0.022 |
| Anticoagulant | 2535 (75.3%) | 293 (77.1%) | 2242 (75.1%) | 0.384 |
| - Warfarin | 2310 (68.6%) | 276 (72.6%) | 2034 (68.1%) | 0.073 |
| - NOACs | 225 (6.7%) | 17 (4.5%) | 208 (7.0%) | 0.067 |
| For warfarin group | ||||
| - Time in therapeutic range (%) | 53.5 ± 26.4 | 53.0 ± 26.2 | 53.6 ± 26.4 | 0.722 |
| - Time under therapeutic range (%) | 32.1 ± 27.6 | 29.9 ± 26.2 | 32.4 ± 27.8 | 0.158 |
| - Time above therapeutic range (%) | 14.1 ± 17.5 | 17.0 ± 19.9 | 13.7 ± 17.1 | 0.010 |
| - Baseline INR | 2.2 ± 0.8 | 2.4 ± 0.9 | 2.2 ± 0.8 | <0.001 |
| All (n = 3367) | BW < 50 (n = 380) | BW ≥ 50 (n = 2987) | p-Value | |
|---|---|---|---|---|
| Ischemic stroke/TIA | 99 (2.9%) | 23 (6.1%) | 76 (2.5%) | <0.001 |
| Major bleeding | 148 (4.4%) | 27 (7.1%) | 121 (4.1%) | 0.006 |
| ICH | 48 (1.4%) | 11 (2.9%) | 37 (1.2%) | 0.010 |
| Death | 260 (7.7%) | 63 (16.6%) | 197 (6.6%) | <0.001 |
| Ischemic stroke/TIA or major bleeding or death | 414 (12.3%) | 90 (23.7%) | 324 (10.8%) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krittayaphong, R.; Chichareon, P.; Komoltri, C.; Kornbongkotmas, S.; Yindeengam, A.; Lip, G.Y.H., on behalf of the COOL-AF Investigators. Low Body Weight Increases the Risk of Ischemic Stroke and Major Bleeding in Atrial Fibrillation: The COOL-AF Registry. J. Clin. Med. 2020, 9, 2713. https://doi.org/10.3390/jcm9092713
Krittayaphong R, Chichareon P, Komoltri C, Kornbongkotmas S, Yindeengam A, Lip GYH on behalf of the COOL-AF Investigators. Low Body Weight Increases the Risk of Ischemic Stroke and Major Bleeding in Atrial Fibrillation: The COOL-AF Registry. Journal of Clinical Medicine. 2020; 9(9):2713. https://doi.org/10.3390/jcm9092713
Chicago/Turabian StyleKrittayaphong, Rungroj, Ply Chichareon, Chulalak Komoltri, Sakaorat Kornbongkotmas, Ahthit Yindeengam, and Gregory Y. H. Lip on behalf of the COOL-AF Investigators. 2020. "Low Body Weight Increases the Risk of Ischemic Stroke and Major Bleeding in Atrial Fibrillation: The COOL-AF Registry" Journal of Clinical Medicine 9, no. 9: 2713. https://doi.org/10.3390/jcm9092713
APA StyleKrittayaphong, R., Chichareon, P., Komoltri, C., Kornbongkotmas, S., Yindeengam, A., & Lip, G. Y. H., on behalf of the COOL-AF Investigators. (2020). Low Body Weight Increases the Risk of Ischemic Stroke and Major Bleeding in Atrial Fibrillation: The COOL-AF Registry. Journal of Clinical Medicine, 9(9), 2713. https://doi.org/10.3390/jcm9092713






