Early Valve Replacement for Severe Aortic Valve Disease: Effect on Mortality and Clinical Ramifications
Abstract
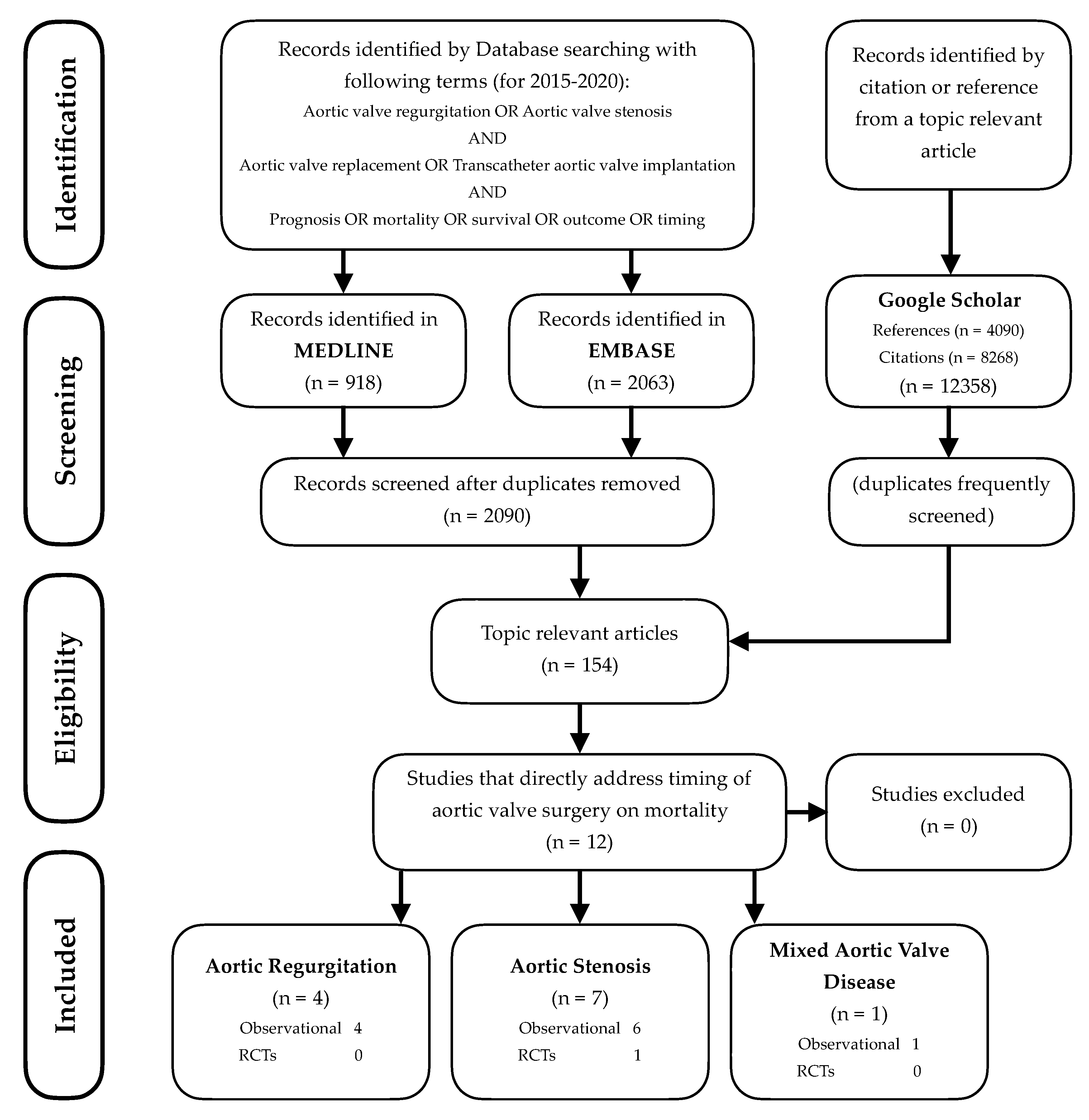
:1. Introduction
2. Literature Search and Information Sources
3. AHA/ACC 2014 Guidelines
4. Aortic Regurgitation: Studies Since 2015
5. Aortic Stenosis: Studies Since 2015
6. Perioperative Mortality
7. Relating Data Since 2015 to Previous Data
7.1. Chronic AR
7.1.1. Symptoms
7.1.2. LVEF
7.1.3. LVESD/LVESDi
7.1.4. LVEDD/LVEDDi
7.1.5. Chronic AR Summary Comparing New to Existing Data
7.2. Chronic AS
LVEF
8. Features in Common between AR and AS and Possible Mechanisms
9. Mixed Aortic Valve Disease
10. Ramifications of Earlier Surgery
10.1. Limited Durability of Bioprosthetic Valves or Risks from Anticoagulation
10.2. Infective Endocarditis
10.3. Effect of Age
10.4. Increased Reliance on Imaging for Decision Making
10.5. Heterogenous Nature of Aortic Valve Disease
11. Future Directions
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Horstkotte, D.; Loogen, F. The natural history of aortic valve stenosis. Eur. Heart J. 1988, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tillquist, M.N.; Maddox, T.M. Cardiac crossroads: Deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer. Adher. 2011, 5, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Chamandi, C.; Rodriguez-Gabella, T.; Rodes-Cabau, J. Future of transcatheter aortic valve implantation—Evolving clinical indications. Nat. Rev. Cardiol. 2018, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Strange, G.; Stewart, S.; Celermajer, D.; Prior, D.; Scalia, G.M.; Marwick, T.; Ilton, M.; Joseph, M.; Codde, J.; Playford, D.; et al. Poor Long-Term Survival in Patients With Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 74, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.; Persson, M.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Loss in Life Expectancy After Surgical Aortic Valve Replacement: SWEDEHEART Study. J. Am. Coll. Cardiol. 2019, 74, 26–33. [Google Scholar] [CrossRef]
- Lindblom, D.; Lindblom, U.; Qvist, J.; Lundstrom, H. Long-term relative survival rates after heart valve replacement. J. Am. Coll. Cardiol. 1990, 15, 566–573. [Google Scholar] [CrossRef] [Green Version]
- Kvidal, P.; Bergstrom, R.; Horte, L.G.; Stahle, E. Observed and relative survival after aortic valve replacement. J. Am. Coll. Cardiol. 2000, 35, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.H.; Park, S.J.; Lee, S.A.; Lee, S.; Kim, D.H.; Kim, H.K.; Yun, S.C.; Hong, G.R.; Song, J.M.; Chung, C.H.; et al. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N. Engl. J. Med. 2020, 382, 111–119. [Google Scholar] [CrossRef]
- Banovic, M.; Iung, B.; Bartunek, J.; Asanin, M.; Beleslin, B.; Biocina, B.; Casselman, F.; da Costa, M.; Deja, M.; Gasparovic, H.; et al. Rationale and design of the Aortic Valve replAcemenT versus conservative treatment in Asymptomatic seveRe aortic stenosis (AVATAR trial): A randomized multicenter controlled event-driven trial. Am. Heart J. 2016, 174, 147–153. [Google Scholar] [CrossRef]
- McCann, G.P.; Steadman, C.D.; Ray, S.G.; Newby, D.E. and on behalf of the British Heart Valve Society. Managing the asymptomatic patient with severe aortic stenosis: Randomised controlled trials of early surgery are overdue. Heart 2011, 97, 1119–1121. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, e521–e643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Feng, K.; Alashi, A.; Rodriguez, L.L.; Gillinov, A.M.; Johnston, D.R.; Sabik, J.F.; Svensson, L.G.; Grimm, R.A.; Griffin, B.P.; et al. Long-Term Outcomes in Patients With Aortic Regurgitation and Preserved Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2016, 68, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Murashita, T.; Schaff, H.V.; Suri, R.M.; Daly, R.C.; Li, Z.; Dearani, J.A.; Greason, K.L.; Nishimura, R.A. Impact of Left Ventricular Systolic Function on Outcome of Correction of Chronic Severe Aortic Valve Regurgitation: Implications for Timing of Surgical Intervention. Ann. Thorac. Surg. 2017, 103, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.T.; Michelena, H.I.; Scott, C.G.; Enriquez-Sarano, M.; Pislaru, S.V.; Schaff, H.V.; Pellikka, P.A. Outcomes in Chronic Hemodynamically Significant Aortic Regurgitation and Limitations of Current Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1741–1752. [Google Scholar] [CrossRef]
- de Meester, C.; Gerber, B.L.; Vancraeynest, D.; Pouleur, A.C.; Noirhomme, P.; Pasquet, A.; de Kerchove, L.; El Khoury, G.; Vanoverschelde, J.L. Do Guideline-Based Indications Result in an Outcome Penalty for Patients With Severe Aortic Regurgitation? JACC Cardiovasc. Imaging 2019, 12, 2126–2138. [Google Scholar] [CrossRef]
- Taniguchi, T.; Morimoto, T.; Shiomi, H.; Ando, K.; Kanamori, N.; Murata, K.; Kitai, T.; Kawase, Y.; Izumi, C.; Miyake, M.; et al. Initial Surgical Versus Conservative Strategies in Patients With Asymptomatic Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2015, 66, 2827–2838. [Google Scholar] [CrossRef] [Green Version]
- Genereux, P.; Stone, G.; O’Gara, P.; Gravel, G.M.; Redfors, B.; Giustino, G.; Pibarot, P.; Bax, J.; Bonow, R.; Leon, M. Early Aortic Valve Replacement Versus a Conservative Strategy for Asymptomatic Severe Aortic Stenosis: Meta-Analysis of Observational Studies. J. Am. Coll. Cardiol. 2016, 67, 2210. [Google Scholar] [CrossRef]
- Masri, A.; Goodman, A.L.; Barr, T.; Grimm, R.A.; Sabik, J.F.; Gillinov, A.M.; Rodriguez, L.L.; Svensson, L.G.; Griffin, B.P.; Desai, M.Y. Predictors of Long-Term Outcomes in Asymptomatic Patients With Severe Aortic Stenosis and Preserved Left Ventricular Systolic Function Undergoing Exercise Echocardiography. Circ. Cardiovasc. Imaging 2016, 9, e004689. [Google Scholar] [CrossRef] [Green Version]
- Campo, J.; Tsoris, A.; Kruse, J.; Karim, A.; Andrei, A.-C.; Li, Z.; Shi, H.; Bonow, R.O.; McCarthy, P.; Malaisrie, S.C. Prognosis of Severe Asymptomatic Aortic Stenosis with and without Surgery. Ann. Cardiothorac. Surg. 2019, 108, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Magne, J.; Dulgheru, R.; Clavel, M.A.; Donal, E.; Vannan, M.A.; Chambers, J.; Rosenhek, R.; Habib, G.; Lloyd, G.; et al. Outcomes of Patients With Asymptomatic Aortic Stenosis Followed Up in Heart Valve Clinics. JAMA Cardiol. 2018, 3, 1060–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Kim, J.B.; Kim, H.R.; Ju, M.H.; Kang, D.Y.; Lee, S.A.; Lee, S.; Ahn, J.M.; Kim, D.H.; Jung, S.H.; et al. Impact of Valve Replacement on Long-Term Survival in Asymptomatic Patients With Severe Aortic Stenosis. Am. J. Cardiol. 2019, 123, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Sondergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Edwards, F.H.; Peterson, E.D.; Coombs, L.P.; DeLong, E.R.; Jamieson, W.R.; Shroyer, A.L.W.; Grover, F.L. Prediction of operative mortality after valve replacement surgery. J. Am. Coll. Cardiol. 2001, 37, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Scott, W.C.; Miller, D.C.; Haverich, A.; Dawkins, K.; Mitchell, R.S.; Jamieson, S.W.; Oyer, P.E.; Stinson, E.B.; Baldwin, J.C.; Shumway, N.E. Determinants of operative mortality for patients undergoing aortic valve replacement. Discriminant analysis of 1,479 operations. J. Thorac. Cardiovasc. Surg. 1985, 89, 400–413. [Google Scholar] [CrossRef]
- Fernandez, F.G.; Shahian, D.M.; Kormos, R.; Jacobs, J.P.; D’Agostino, R.S.; Mayer, J.E., Jr.; Kozower, B.D.; Higgins, R.S.D.; Badhwar, V. The Society of Thoracic Surgeons National Database 2019 Annual Report. Ann. Thorac. Surg. 2019, 108, 1625–1632. [Google Scholar] [CrossRef]
- Goodwin, A.; Bradley, A.; Wang, J. National Adult Cardiac Surgery Audit 2019 Summary Report; NICOR: Naperville, IL, USA, 2019. [Google Scholar]
- Gilbert, S.; Williams-Spence, J.; Tran, L.; Solman, N.; McLaren, J.; Marrow, N.; Brennan, A.; Baker, R.; Newcomb, A.; Reid, C.; et al. The Australian and New Zealand Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database Program. In National Annual Report 2018; Monash University: Melbourne, Australia, 2019. [Google Scholar]
- Bhudia, S.K.; McCarthy, P.M.; Kumpati, G.S.; Helou, J.; Hoercher, K.J.; Rajeswaran, J.; Blackstone, E.H. Improved outcomes after aortic valve surgery for chronic aortic regurgitation with severe left ventricular dysfunction. J. Am. Coll. Cardiol. 2007, 49, 1465–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonow, R.O.; Lakatos, E.; Maron, B.J.; Epstein, S.E. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation 1991, 84, 1625–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonow, R.O.; Picone, A.L.; McIntosh, C.L.; Jones, M.; Rosing, D.R.; Maron, B.J.; Lakatos, E.; Clark, R.E.; Epstein, S.E. Survival and functional results after valve replacement for aortic regurgitation from 1976 to 1983: Impact of preoperative left ventricular function. Circulation 1985, 72, 1244–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaliki, H.P.; Mohty, D.; Avierinos, J.F.; Scott, C.G.; Schaff, H.V.; Tajik, A.J.; Enriquez-Sarano, M. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 2002, 106, 2687–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, W.G.; Hood, W.P., Jr.; Siart, A.; Hausmann, D.; Nellessen, U.; Oelert, H.; Lichtlen, P.R. Chronic aortic regurgitation: Reassessment of the prognostic value of preoperative left ventricular end-systolic dimension and fractional shortening. Circulation 1985, 71, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Detaint, D.; Messika-Zeitoun, D.; Maalouf, J.; Tribouilloy, C.; Mahoney, D.W.; Tajik, A.J.; Enriquez-Sarano, M. Quantitative echocardiographic determinants of clinical outcome in asymptomatic patients with aortic regurgitation: A prospective study. JACC Cardiovasc. Imaging 2008, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dujardin, K.S.; Enriquez-Sarano, M.; Schaff, H.V.; Bailey, K.R.; Seward, J.B.; Tajik, A.J. Mortality and morbidity of aortic regurgitation in clinical practice: A long-term follow-up study. Circulation 1999, 99, 1851–1857. [Google Scholar] [CrossRef] [Green Version]
- Fioretti, P.; Roelandt, J.; Bos, R.J.; Meltzer, R.S.; van Hoogenhuijze, D.; Serruys, P.W.; Nauta, J.; Hugenholtz, P.G. Echocardiography in chronic aortic insufficiency. Is valve replacement too late when left ventricular end-systolic dimension reaches 55 mm? Circulation 1983, 67, 216–221. [Google Scholar] [CrossRef] [Green Version]
- Forman, R.; Firth, B.G.; Barnard, M.S. Prognostic significance of preoperative left ventricular ejection fraction and valve lesion in patients with aortic valve replacement. Am. J. Cardiol. 1980, 45, 1120–1125. [Google Scholar] [CrossRef]
- Greves, J.; Rahimtoola, S.H.; McAnulty, J.H.; DeMots, H.; Clark, D.G.; Greenberg, B.; Starr, A. Preoperative criteria predictive of late survival following valve replacement for severe aortic regurgitation. Am. Heart J. 1981, 101, 300–308. [Google Scholar] [CrossRef]
- Henry, W.L.; Bonow, R.O.; Borer, J.S.; Ware, J.H.; Kent, K.M.; Redwood, D.R.; McIntosh, C.L.; Morrow, A.G.; Epstein, S.E. Observations on the optimum time for operative intervention for aortic regurgitation. I. Evaluation of the results of aortic valve replacement in symptomatic patients. Circulation 1980, 61, 471–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klodas, E.; Enriquez-Sarano, M.; Tajik, A.J.; Mullany, C.J.; Bailey, K.R.; Seward, J.B. Optimizing timing of surgical correction in patients with severe aortic regurgitation: Role of symptoms. J. Am. Coll. Cardiol. 1997, 30, 746–752. [Google Scholar] [CrossRef]
- Pizarro, R.; Bazzino, O.O.; Oberti, P.F.; Falconi, M.L.; Arias, A.M.; Krauss, J.G.; Cagide, A.M. Prospective validation of the prognostic usefulness of B-type natriuretic peptide in asymptomatic patients with chronic severe aortic regurgitation. J. Am. Coll. Cardiol. 2011, 58, 1705–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tornos, P.; Sambola, A.; Permanyer-Miralda, G.; Evangelista, A.; Gomez, Z.; Soler-Soler, J. Long-term outcome of surgically treated aortic regurgitation: Influence of guideline adherence toward early surgery. J. Am. Coll. Cardiol. 2006, 47, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Kelly, T.A.; Rothbart, R.M.; Cooper, C.M.; Kaiser, D.L.; Smucker, M.L.; Gibson, R.S. Comparison of outcome of asymptomatic to symptomatic patients older than 20 years of age with valvular aortic stenosis. Am. J. Cardiol. 1988, 61, 123–130. [Google Scholar] [CrossRef]
- Lancellotti, P.; Donal, E.; Magne, J.; Moonen, M.; O’Connor, K.; Daubert, J.C.; Pierard, L.A. Risk stratification in asymptomatic moderate to severe aortic stenosis: The importance of the valvular, arterial and ventricular interplay. Heart 2010, 96, 1364–1371. [Google Scholar] [CrossRef]
- Otto, C.M.; Burwash, I.G.; Legget, M.E.; Munt, B.I.; Fujioka, M.; Healy, N.L.; Kraft, C.D.; Miyake-Hull, C.Y.; Schwaegler, R.G. Prospective study of asymptomatic valvular aortic stenosis: Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997, 95, 2262–2270. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Nishimura, R.A.; Bailey, K.R.; Tajik, A.J. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J. Am. Coll. Cardiol. 1990, 15, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef] [Green Version]
- Rosenhek, R.; Binder, T.; Porenta, G.; Lang, I.; Christ, G.; Schemper, M.; Maurer, G.; Baumgartner, H. Predictors of outcome in severe, asymptomatic aortic stenosis. N. Engl. J. Med. 2000, 343, 611–617. [Google Scholar] [CrossRef]
- Rosenhek, R.; Zilberszac, R.; Schemper, M.; Czerny, M.; Mundigler, G.; Graf, S.; Bergler-Klein, J.; Grimm, M.; Gabriel, H.; Maurer, G. Natural history of very severe aortic stenosis. Circulation 2010, 121, 151–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Muro, T.; Takeda, H.; Hyodo, E.; Ehara, S.; Nakamura, Y.; Hanatani, A.; Shimada, K.; Yoshiyama, M. Prognostic value of aortic valve area index in asymptomatic patients with severe aortic stenosis. Am. J. Cardiol. 2012, 110, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Turina, J.; Hess, O.; Sepulcri, F.; Krayenbuehl, H.P. Spontaneous course of aortic valve disease. Eur. Heart J. 1987, 8, 471–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, K.D.; Nishimura, R.A.; Holmes, D.R., Jr.; Bailey, K.R. Natural history of moderate aortic stenosis. J. Am. Coll. Cardiol. 1991, 17, 313–319. [Google Scholar] [CrossRef]
- Hein, S.; Arnon, E.; Kostin, S.; Schonburg, M.; Elsasser, A.; Polyakova, V.; Bauer, E.P.; Klovekorn, W.-P.; Schaper, J. Progression From Compensated Hypertrophy to Failure in the Pressure-Overloaded Human Heart. Circulation 2003, 107, 984–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, C.W.L.; Everett, R.J.; Kwiecinski, J.; Vesey, A.T.; Yeung, E.; Esson, G.; Jenkins, W.; Koo, M.; Mirsadraee, S.; White, A.C.; et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC Cardiovasc. Imaging 2017, 10, 1320–1333. [Google Scholar] [CrossRef]
- Lund, O. Preoperative risk evaluation and stratification of long-term survival after valve replacement for aortic stenosis. Reasons for earlier operative intervention. Circulation 1990, 82, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Egbe, A.C.; Connolly, H.M.; Poterucha, J.T.; Warnes, C.A. Bicuspid and Unicuspid Aortic Valve: Fate of Moderate/Severe Mixed Aortic Valve Disease. Congenit. Heart Dis. 2017, 12, 24–31. [Google Scholar] [CrossRef]
- Egbe, A.C.; Luis, S.A.; Padang, R.; Warnes, C.A. Outcomes in Moderate Mixed Aortic Valve Disease: Is it Time for a Paradigm Shift? J. Am. Coll. Cardiol. 2016, 67, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P. Mixed Aortic Valve Disease: A Double Trouble. Struct. Heart 2018, 2, 405–407. [Google Scholar] [CrossRef]
- Sorajja, P.; Nelson, P.; Bae, R.; Sandoval, Y.; Lesser, J.; Pedersen, W.; Tindell, L.; Harris, K.; Farivar, R. Adverse impact of aortic regurgitation on the long-term survival of patients with native aortic valve stenosis. J. Am. Coll. Cardiol. 2016, 67, 244. [Google Scholar] [CrossRef]
- Sorajja, P.; Nelson, P.; Garberich, R.; Bradley, S.M.; Athappan, G.; Bae, R.; Harris, K.; Lesser, J.; Tindell, L.; Farivar, R.S.; et al. Clinical Impact of Chronic Aortic Regurgitation in Asymptomatic Patients with Native Aortic Valve Stenosis. Struct. Heart 2018, 2, 398–404. [Google Scholar] [CrossRef]
- Unger, P.; Pibarot, P.; Tribouilloy, C.; Lancellotti, P.; Maisano, F.; Iung, B.; Pierard, L.; European Society of Cardiology Council on Valvular Heart Diseases. Multiple and Mixed Valvular Heart Diseases. Circ. Cardiovasc. Imaging 2018, 11, e007862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilberszac, R.; Gabriel, H.; Schemper, M.; Zahler, D.; Czerny, M.; Maurer, G.; Rosenhek, R. Outcome of combined stenotic and regurgitant aortic valve disease. J. Am. Coll. Cardiol. 2013, 61, 1489–1495. [Google Scholar] [CrossRef] [Green Version]
- Unger, P.; Rosenhek, R.; Dedobbeleer, C.; Berrebi, A.; Lancellotti, P. Management of multiple valve disease. Heart 2011, 97, 272–277. [Google Scholar] [CrossRef]
- Isaza, N.; Desai, M.Y.; Kapadia, S.R.; Krishnaswamy, A.; Rodriguez, L.L.; Grimm, R.A.; Conic, J.Z.; Saijo, Y.; Roselli, E.E.; Gillinov, A.M.; et al. Long-Term Outcomes in Patients With Mixed Aortic Valve Disease and Preserved Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2020, 9, e014591. [Google Scholar] [CrossRef]
- Une, D.; Ruel, M.; David, T.E. Twenty-year durability of the aortic Hancock II bioprosthesis in young patients: Is it durable enough? Eur. J. Cardiothorac. Surg. 2014, 46, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Shemin, R.J.; Guadiani, V.A.; Conkle, D.M.; Morrow, A.G. Prosthetic aortic valves: Indications for and results of reoperation. Arch. Surg. 1979, 114, 63–65. [Google Scholar] [CrossRef]
- Ferrari, E.; Stortecky, S.; Heg, D.; Muller, O.; Nietlispach, F.; Tueller, D.; Toggweiler, S.; Noble, S.; Maisano, F.; Roffi, M.; et al. The hospital results and 1-year outcomes of transcatheter aortic valve-in-valve procedures and transcatheter aortic valve implantations in the native valves: The results from the Swiss-TAVI Registry. Eur. J. Cardiothorac. Surg. 2019, 56, 55–63. [Google Scholar] [CrossRef]
- Gozdek, M.; Raffa, G.M.; Suwalski, P.; Kolodziejczak, M.; Anisimowicz, L.; Kubica, J.; Navarese, E.P.; Kowalewski, M. Comparative performance of transcatheter aortic valve-in-valve implantation versus conventional surgical redo aortic valve replacement in patients with degenerated aortic valve bioprostheses: Systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2018, 53, 495–504. [Google Scholar] [CrossRef]
- Vrachatis, D.A.; Vavuranakis, M.; Tsoukala, S.; Giotaki, S.; Papaioannou, T.G.; Siasos, G.; Deftereos, G.; Giannopoulos, G.; Raisakis, K.; Tousoulis, D.; et al. TAVI: Valve in valve. A new field for structuralists? Literature review. Hell. J. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, C.J.; Garcia-Alamino, J.M.; Spencer, E.A.; Ward, A.M.; Perera, R.; Bankhead, C.; Alonso-Coello, P.; Fitzmaurice, D.; Mahtani, K.R.; Onakpoya, I.J. Self-monitoring and self-management of oral anticoagulation. Cochrane Database Syst. Rev. 2016, 7, CD003839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyama, N.; Laakso, T.; Biancari, F.; Raivio, P.; Jalava, M.P.; Jaakkola, J.; Dahlbacka, S.; Kinnunen, E.M.; Juvonen, T.; Husso, A.; et al. Prosthetic valve endocarditis after transcatheter or surgical aortic valve replacement with a bioprosthesis: Results from the FinnValve Registry. EuroIntervention 2019, 15, e500–e507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butt, J.H.; Ihlemann, N.; De Backer, O.; Sondergaard, L.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Kober, L.; Fosbol, E.L. Long-Term Risk of Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 1646–1655. [Google Scholar] [CrossRef]
- Moore, B.; Cao, J.; Kotchetkova, I.; Celermajer, D.S. Incidence, predictors and outcomes of infective endocarditis in a contemporary adult congenital heart disease population. Int. J. Cardiol. 2017, 249, 161–165. [Google Scholar] [CrossRef]
- Siu, S.C.; Silversides, C.K. Bicuspid aortic valve disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef] [Green Version]
- Phan, K.; Wong, S.; Phan, S.; Ha, H.; Qian, P.; Yan, T.D. Transcatheter Aortic Valve Implantation (TAVI) in Patients With Bicuspid Aortic Valve Stenosis--Systematic Review and Meta-Analysis. Heart Lung Circ. 2015, 24, 649–659. [Google Scholar] [CrossRef]
- O’Sullivan, C.J.; Stortecky, S.; Buellesfeld, L.; Wenaweser, P.; Windecker, S. Preinterventional screening of the TAVI patient: How to choose the suitable patient and the best procedure. Clin. Res. Cardiol. 2014, 103, 259–274. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Cawley, P.J.; Hamilton-Craig, C.; Owens, D.S.; Krieger, E.V.; Strugnell, W.E.; Mitsumori, L.; D’Jang, C.L.; Schwaegler, R.G.; Nguyen, K.Q.; Nguyen, B.; et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ. Cardiovasc. Imaging 2013, 6, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everett, R.J.; Newby, D.E.; Jabbour, A.; Fayad, Z.A.; Dweck, M.R. The Role of Imaging in Aortic Valve Disease. Curr. Cardiovasc. Imaging Rep. 2016, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smedsrud, M.K.; Pettersen, E.; Gjesdal, O.; Svennevig, J.L.; Andersen, K.; Ihlen, H.; Edvardsen, T. Detection of left ventricular dysfunction by global longitudinal systolic strain in patients with chronic aortic regurgitation. J. Am. Soc. Echocardiogr. 2011, 24, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.; Khullar, T.; Mentias, A.; Gillinov, A.M.; Roselli, E.E.; Svensson, L.G.; Popovic, Z.B.; Griffin, B.P.; Desai, M.Y. Long-Term Outcomes After Aortic Valve Surgery in Patients With Asymptomatic Chronic Aortic Regurgitation and Preserved LVEF: Impact of Baseline and Follow-Up Global Longitudinal Strain. JACC Cardiovasc. Imaging 2020, 13, 12–21. [Google Scholar] [CrossRef]
- Park, S.H.; Yang, Y.A.; Kim, K.Y.; Park, S.M.; Kim, H.N.; Kim, J.H.; Jang, S.Y.; Bae, M.H.; Lee, J.H.; Yang, D.H. Left Ventricular Strain as Predictor of Chronic Aortic Regurgitation. J. Cardiovasc. Ultrasound 2015, 23, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Kusunose, K.; Goodman, A.; Parikh, R.; Barr, T.; Agarwal, S.; Popovic, Z.B.; Grimm, R.A.; Griffin, B.P.; Desai, M.Y. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ. Cardiovasc. Imaging 2014, 7, 938–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollema, E.M.; Sugimoto, T.; Shen, M.; Tastet, L.; Ng, A.C.T.; Abou, R.; Marsan, N.A.; Mertens, B.; Dulgheru, R.; Lancellotti, P.; et al. Association of Left Ventricular Global Longitudinal Strain With Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiol. 2018, 3, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attias, D.; Macron, L.; Dreyfus, J.; Monin, J.L.; Brochet, E.; Lepage, L.; Hekimian, G.; Iung, B.; Vahanian, A.; Messika-Zeitoun, D. Relationship between longitudinal strain and symptomatic status in aortic stenosis. J. Am. Soc. Echocardiogr. 2013, 26, 868–874. [Google Scholar] [CrossRef]
- Lancellotti, P.; Vannan, M.A. Timing of Intervention in Aortic Stenosis. N. Engl. J. Med. 2019, 382, 191–193. [Google Scholar] [CrossRef]
| Parameter | Details | COR | LOE |
|---|---|---|---|
| Symptomatic | AVR is indicated for symptomatic patients with severe AR regardless of LV systolic function (stage D) | I | B |
| LVEF < 50% | AVR is indicated for asymptomatic patients with chronic severe AR and LV systolic dysfunction (LVEF < 50%) (stage C2) | I | B |
| Other cardiac surgery, severe AR | AVR is indicated for patients with severe AR (stage C or D) while undergoing cardiac surgery for other indications | I | C |
| LVESD > 50 mm (LVESDi > 25 mm/m2) | AVR is reasonable for asymptomatic patients with severe AR with normal LV systolic function (LVEF ≥ 50%) but with severe LV dilation (LVESD > 50 mm, stage C2) | IIa | B |
| Other cardiac surgery, moderate AR | AVR is reasonable in patients with moderate AR (stage B) who are undergoing other cardiac surgery | IIa | C |
| LVEDD > 65 mm | AVR may be considered for asymptomatic patients with severe AR and normal LV systolic function (LVEF ≥ 50%, stage C1) but with progressive severe LV dilation (LVEDD > 65 mm) if surgical risk is low | IIb | C |
| Parameter | Details | COR | LOE |
|---|---|---|---|
| Symptomatic | AVR is recommended for symptomatic patients with severe high-gradient AS who have symptoms by history or on exercise testing (stage D1) | I | B |
| LVEF < 50% | AVR is recommended for asymptomatic patients with severe AS (stage C2) and LVEF < 50% | I | B |
| Other cardiac surgery, severe AS | AVR is indicated for patients with severe AS (stage C or D) when undergoing other cardiac surgery | I | B |
| Aortic velocity ≥ 5.0 m/s | AVR is reasonable for asymptomatic patients with very severe AS (stage C1, aortic velocity ≥ 5.0 m/s) and low surgical risk | IIa | B |
| Decreased exercise tolerance or exercise fall in BP | AVR is reasonable in asymptomatic patients (stage C1) with severe AS and decreased exercise tolerance or an exercise fall in BP | IIa | B |
| Dobutamine stress test, low-flow/low gradient severe AS | AVR is reasonable in symptomatic patients with low-flow/low-gradient severe AS with reduced LVEF (stage D2) with a low-dose dobutamine stress study that shows an aortic velocity ≥4.0 m/s (or mean pressure gradient ≥40 mm Hg) with a valve area ≤1.0 cm2 at any dobutamine dose | IIa | B |
| Low-flow/low gradient severe AS | AVR is reasonable in symptomatic patients who have low-flow/low-gradient severe AS (stage D3) who are normotensive and have an LVEF ≥50% if clinical, hemodynamic, and anatomic data support valve obstruction as the most likely cause of symptoms | IIa | C |
| Other cardiac surgery, moderate AS | AVR is reasonable for patients with moderate AS (stage B) (aortic velocity 3.0–3.9 m/s) who are undergoing other cardiac surgery | IIa | C |
| Rapid progression, severe AS | AVR may be considered for asymptomatic patients with severe AS (stage C1) and rapid disease progression and low surgical risk | IIb | C |
| Author, Year, Location | Size | Type | Sample Details | Factor | Result | p-Value | |
|---|---|---|---|---|---|---|---|
| Mentias et al. 2016 [14] Cleveland, Ohio, US | 1417 | Retrospective, observational | Age 54 ± 16 years; 75% male. Severe AR with LVEF ≥50%. 933 (66%) underwent AVR | AVR surgery during follow up | 10 year survival: | <0.001 | |
| Yes | 87% | ||||||
| No | 71% | ||||||
| Symptomatic | 10 year mortality: | <0.001 | |||||
| HR 2.06 (1.76–2.49) (compared to symptomatic) | |||||||
| Murashita et al. 2017 [15] Rochester, Minnesota, US | 530 | Retrospective, observational | Age 57 ± 17 years; 80% male; 37% BAV All underwent AVR for severe AR | Symptomatic | 10 year survival (CI): | <0.01 | |
| Yes | 77.8% (59.7–99.9%) | ||||||
| No | 91.1% (85.7–96.6%) | ||||||
| LVEF | 10 year survival (CI): | 0.04 | |||||
| ≥60% | 85.4% (81.7–89.2%) | ||||||
| <60% | 69.5% (61.3–78.3%) | ||||||
| LVESD | Risk of left ventricular dysfunction at 1 year postoperatively, defined as LVEF below 60%: | <0.01 | |||||
| >40 mm | odds ratio 5.39 | ||||||
| Yang et al. 2019 [16] Rochester, Minnesota, US | 748 | Retrospective, observational | Severe AR Age 58 ± 17 years; 82% male; 39% BAV 361 (48%) underwent AVR | Time-dependent AVR (within 6-months of initial echocardiogram) | Multivariate hazard ratio (CI) for all-cause mortality at median 4.9 years: | 0.02 | |
| 0.36 (0.25–0.86) | |||||||
| Symptoms | Multivariate hazard ratio (CI) for all-cause mortality at median 4.9 years: | <0.0001 | |||||
| 3.16 (2.10–4.75) | |||||||
| LVESDi | Multivariate hazard ratio (CI) for all-cause mortality: | 0.04 0.003 | |||||
| <20 mm/m2 | Reference | ||||||
| 20–25 mm/m2 | 1.53 (1.01–2.31) | ||||||
| ≥25 mm/m2 | 2.23 (1.32–3.77) | ||||||
| de Meester et al. 2019 [17] Brussels, Belgium | 356 | Retrospective, observational | Age, 51 ± 15 years; 83% male; 42% BAV All underwent AVR for severe AR | Symptoms | 10 year survival: | 0.013 0.001 | |
| NYHA class I | 86 ± 4% | ||||||
| NYHA class II | 73 ± 7% | ||||||
| NYHA class III/IV | 65 ± 7% | ||||||
| LVEF | 10 year survival: | 0.011 | |||||
| ≥50% | 80 ± 3% | ||||||
| <50% | 69 ± 6% | ||||||
| Spline function analysis hazard ratio (CI) for cardiovascular events: | 0.002 | ||||||
| ≥55% | reference | ||||||
| <55% | 4.13 (1.65 to 10.33) | ||||||
| per 1% decrease in LVEF | |||||||
| LVESDi | 10 year survival: | <0.001 | |||||
| <25 mm/m2 | 84 ± 3% | ||||||
| ≥25 mm/m2 | 62 ± 6% | ||||||
| LVEDD | 10 year survival: | ||||||
| No difference between LVEDD <65 mm and LVEDD ≥65 mm | |||||||
| No difference between LVEDD <70 mm and LVEDD ≥70 mm | |||||||
| Author, Year, Location | Size | Type | Sample Details | Factor | Result | p-Value | |
|---|---|---|---|---|---|---|---|
| Taniguchi et al. 2015 [18] Kyoto, Japan | 1808 | Multicenter, retrospective, observational | Asymptomatic, severe AS; age 77 ± 9 years, 40% male Propensity score-matched cohort of 582 patients | Initial AVR or watchful waiting | 5-year survival: | 0.009 | |
| Initial AVR | 84.6% | ||||||
| Watchful waiting | 73.6% | ||||||
| 5-year rate of hospitalization for heart failure: | <0.001 | ||||||
| Initial AVR | 3.8% | ||||||
| Watchful waiting | 19.9% | ||||||
| Genereux et al. 2016 [19] New York, US | 4 trials 2486 patients | Meta-analysis | Asymptomatic, severe AS | Early AVR or watchful waiting | All-cause mortality (CI): | 0.01 | |
| Early AVR | reference | ||||||
| Watchful waiting | 3.7 (1.3–11.1) | ||||||
| fold higher | |||||||
| Masri et al. 2016 [20] Cleveland, Ohio, US | 533 | Retrospective, observational | Asymptomatic, severe AS, LVEF ≥50%; age, 66 ± 13 years, 78% men, 31% with coronary artery disease | AVR or no AVR | Multivariable Cox proportional hazard survival analysis for 6.9 ± 3 years all-cause mortality (CI): | <0.001 | |
| AVR | 0.26 (0.16–0.41) | ||||||
| No AVR | reference | ||||||
| Exercise stress echocardiography % age-gender predicted METs | Long-term (6.9 ± 3.3 years) survival: | <0.001 | |||||
| ≥85% | 85.0% | ||||||
| <85% | 67.6% | ||||||
| Lancellotti et al. 2018 [22] Liège, Belgium | 543 | Multicenter, retrospective, observational | Asymptomatic, severe AS; LVEF ≥ 50%; age 71 ± 13%; 61% male; all underwent AVR. | Peak aortic velocity | Survival at 2, 4, 6 years following AVR: | 0.03 | |
| <5.0 m/s | 84 ± 2%, 78 ± 4%, 70 ± 6% | ||||||
| ≥5.0 m/s | 73 ± 8%, 65 ± 10%, 54 ± 13% | ||||||
| LVEF | Survival at 2, 4, 6 years following AVR: | 0.02 | |||||
| ≥60% | 87 ± 5%, 78 ± 4%, 69 ± 7% | ||||||
| <60% | 67 ± 7%, 63 ± 8%, 63 ± 8% | ||||||
| Campo et al. 2019 [21] Chicago, Illinois, US | 265 | Retrospective, observational | Asymptomatic severe AS. | Early AVR or watchful waiting | Survival at 2, 4 years: | 0.033 | |
| Early AVR | 92.5%, 78.9% | ||||||
| Watching waiting | 83.9%, 91.0% | ||||||
| Kim et al. 2019 [23] Seoul, South Korea | 468 | Retrospective, observational | Asymptomatic, severe AS, LVEF ≥50%; age 64 years; 50% male. Early AVR was performed in 351 patients | AVR or medical treatment | All-cause mortality (median 60.9 months): | 0.036 | |
| AVR | 9.1% per year | ||||||
| Medical treatment | 2.4% per year | ||||||
| Hazard ratio 0.62 (0.40–0.97) | |||||||
| Kang et al. 2019 [8] Seoul, South Korea (RECOVERY trial) | 145 | Prospective, single center, RCT | Asymptomatic, severe AS, LVEF ≥50%; age 64 ± 9 years; 36% male | Early surgery or watchful waiting (randomized) | 5 year death from cardiovascular or surgery causes: | 0.003 | |
| Early surgery | 1% | ||||||
| Watchful waiting | 15% | ||||||
| 5 year death from any cause: | |||||||
| Early surgery | 7% | ||||||
| Watchful waiting | 21% | ||||||
| Year | Mortality | Author | Size | Location |
|---|---|---|---|---|
| 1985 | 7% | Scott et al. [29] | 1479 | Stanford, California |
| 2000 | 5.6% | Kvidal et al. [7] | 2359 | Uppsala, Sweden |
| 2001 | 4.0% | Edwards et al. [28] | 16,105 | Jacksonville, Florida |
| 2016 | 0.6% | Mentias et al. [14] | 1417 | Cleveland, Ohio |
| 2018 | 0.9% | Lancellotti et al. [22] | 1375 | Liège, Belgium |
| 2019 | 0.9% | Kim et al. [23] | 468 | Seoul, South Korea |
| 2019 | 0.3% | Yang et al. [16] | 748 | Rochester, Minnesota |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koerber, J.P.; Bennetts, J.S.; Psaltis, P.J. Early Valve Replacement for Severe Aortic Valve Disease: Effect on Mortality and Clinical Ramifications. J. Clin. Med. 2020, 9, 2694. https://doi.org/10.3390/jcm9092694
Koerber JP, Bennetts JS, Psaltis PJ. Early Valve Replacement for Severe Aortic Valve Disease: Effect on Mortality and Clinical Ramifications. Journal of Clinical Medicine. 2020; 9(9):2694. https://doi.org/10.3390/jcm9092694
Chicago/Turabian StyleKoerber, Jason P., Jayme S. Bennetts, and Peter J. Psaltis. 2020. "Early Valve Replacement for Severe Aortic Valve Disease: Effect on Mortality and Clinical Ramifications" Journal of Clinical Medicine 9, no. 9: 2694. https://doi.org/10.3390/jcm9092694
APA StyleKoerber, J. P., Bennetts, J. S., & Psaltis, P. J. (2020). Early Valve Replacement for Severe Aortic Valve Disease: Effect on Mortality and Clinical Ramifications. Journal of Clinical Medicine, 9(9), 2694. https://doi.org/10.3390/jcm9092694




