Decreased Thymic Output Contributes to Immune Defects in Septic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Processing
2.3. CD4+ and CD8+ T Cell Isolation
2.4. Flow Cytometry and Staining Procedure
2.5. Protein Miniarrays
2.6. Determination of IL-7 and IL-15
2.7. Analysis of Telomerase Activity
2.8. Telomere Length Analysis
2.9. T-Cell Receptor Excision Circle (TREC) Analysis
2.10. RT-PCR of Telomerase- and Shelterin-Associated Genes
2.11. Statistics
3. Results
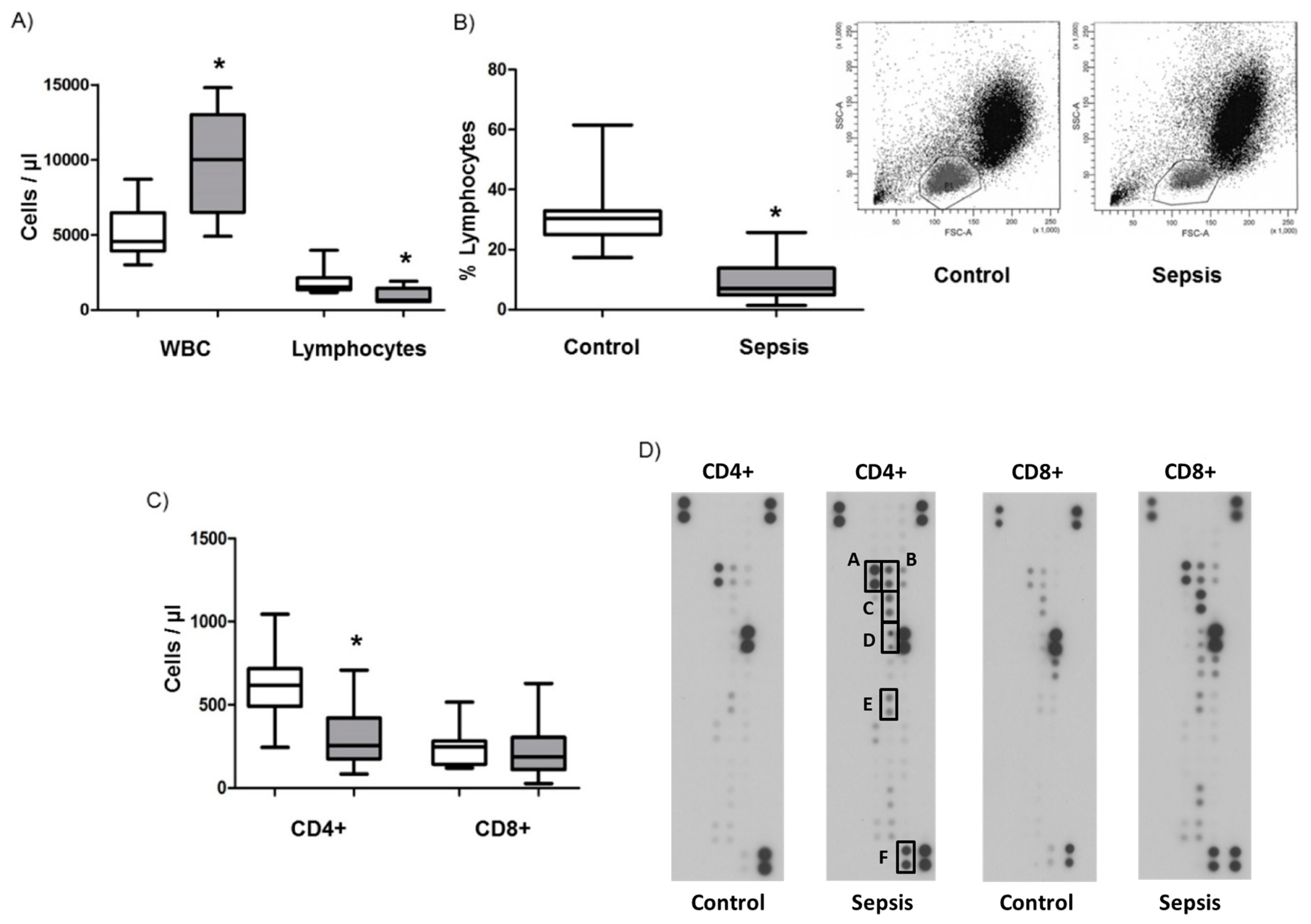
3.1. Lymphopenia, Apoptosis, and Proliferation in Patients with Septic Shock
3.2. Cytokine Levels in Septic Shock Patients
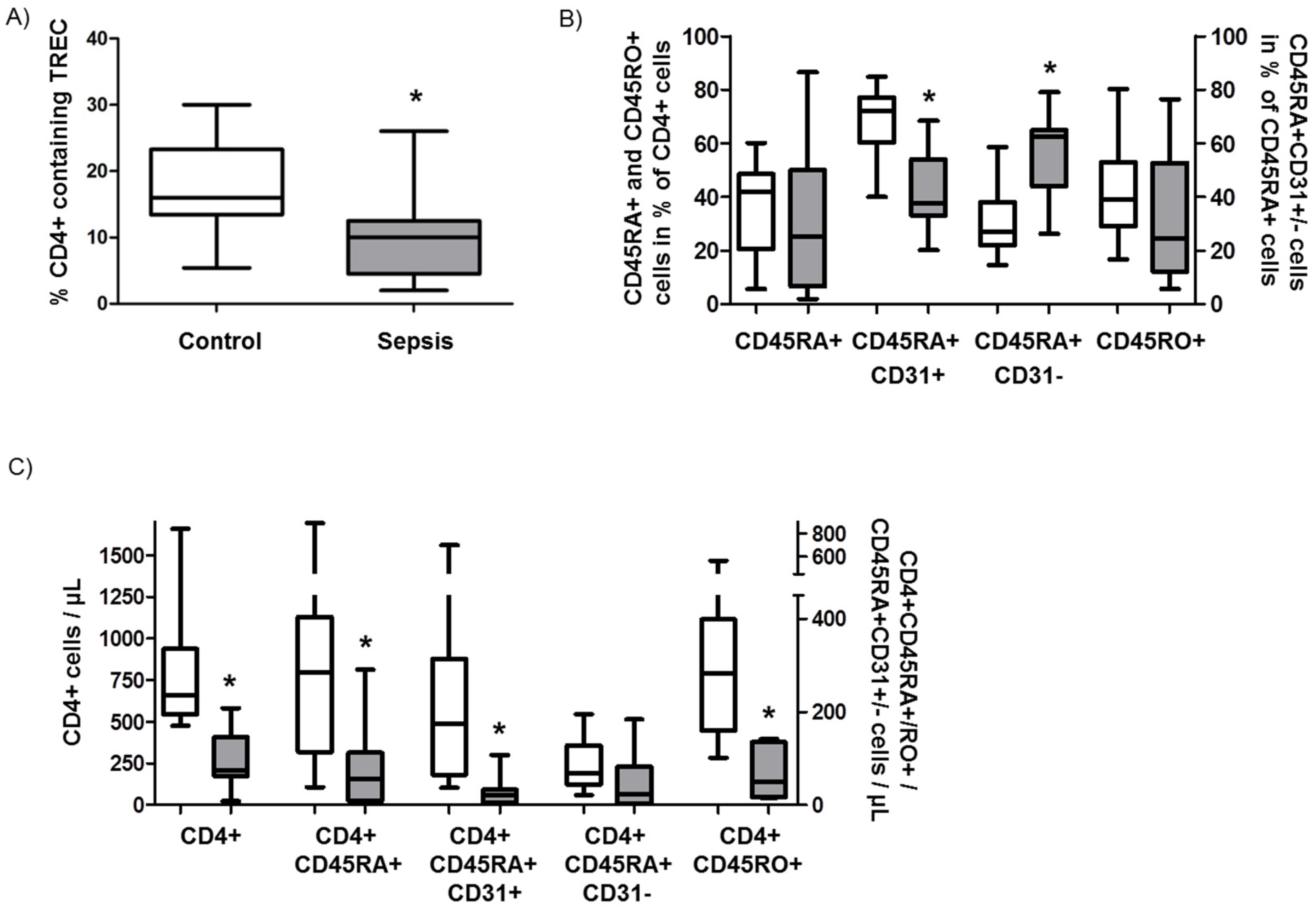
3.3. Role of Recent Thymic Emigrants and Thymic Dysfunction in Septic Lymphopenia
3.4. Analysis of Telomere Length, Telomerase Activity, and the Shelterin Complex
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the Incidence and Mortality of Severe Sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kempker, J.A.; Martin, G.S. The Changing Epidemiology and Definitions of Sepsis. Clin. Chest Med. 2016, 37, 165–179. [Google Scholar] [CrossRef]
- Cabrera-Perez, J.; Condotta, S.A.; Badovinac, V.P.; Griffith, T.S. Impact of sepsis on CD4 T cell immunity. J. Leukoc. Biol. 2014, 96, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Welch, K.; Siddiqui, J.; Remick, D.G. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 2006, 177, 1967–1974. [Google Scholar] [CrossRef]
- Xiao, W.; Mindrinos, M.N.; Seok, J.; Cuschieri, J.; Cuenca, A.G.; Gao, H.; Hayden, D.L.; Hennessy, L.; Moore, E.E.; Minei, J.P.; et al. A genomic storm in critically injured humans. J. Exp. Med. 2011, 208, 2581–2590. [Google Scholar] [CrossRef]
- Otto, G.P.; Sossdorf, M.; Claus, R.A.; Rödel, J.; Menge, K.; Reinhart, K.; Bauer, M.; Riedemann, N.C. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit. Care 2011, 15, R183. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.G.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230–1251. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef]
- Drewry, A.M.; Samra, N.; Skrupky, L.P.; Fuller, B.M.; Compton, S.M.; Hotchkiss, R.S. Persistent Lymphopenia After Diagnosis of Sepsis Predicts Mortality. Shock 2014, 42, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Lugosi, M.; Sonneville, R.; Souweine, B.; Ruckly, S.; Cartier, J.-C.; Garrouste-Orgeas, M.; Schwebel, C.; Timsit, J.-F.; OUTCOMEREA Study Group. Persistent lymphopenia is a risk factor for ICU-acquired infections and for death in ICU patients with sustained hypotension at admission. Ann. Intensive Care 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef] [PubMed]
- Perl, M.; Chung, C.-S.; Swan, R.; Ayala, A. Role of Programmed Cell Death in the Immunopathogenesis of Sepsis. Drug Discov. Today Dis. Mech. 2007, 4, 223–230. [Google Scholar] [CrossRef]
- Hecker, M.; Sommer, N.; Foch, S.; Hecker, A.; Hackstein, H.; Witzenrath, M.; Weissmann, N.; Seeger, W.; Mayer, K. Resolvin E1 and its precursor 18 R -HEPE restore mitochondrial function in inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1016–1028. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Ponchel, F.; Toomes, C.; Bransfield, K.; Leong, F.T.; Douglas, S.H.; Field, S.L.; Bell, S.M.; Combaret, V.; Puisieux, A.; Mighell, A.J.; et al. Real-time PCR based on SYBR-Green I fluorescence: An alternative to the TaqMan assay for a relative quantification of gene rearrangements, gene amplifications and micro gene deletions. BMC Biotechnol. 2003, 3, 18. [Google Scholar] [CrossRef]
- Hecker, M.; Behnk, A.; Morty, R.E.; Sommer, N.; Vadász, I.; Herold, S.; Seeger, W.; Mayer, K. PPAR-α activation reduced LPS-induced inflammation in alveolar epithelial cells. Exp. Lung Res. 2015, 2148, 1–11. [Google Scholar] [CrossRef]
- Hecker, M.; Linder, T.; Ott, J.; Walmrath, H.-D.; Lohmeyer, J.; Vadász, I.; Marsh, L.M.; Herold, S.; Reichert, M.; Buchbinder, A.; et al. Immunomodulation by lipid emulsions in pulmonary inflammation: A randomized controlled trial. Crit. Care 2015, 19, 226. [Google Scholar] [CrossRef]
- White, M.; Mahon, V.; Grealy, R.; Doherty, D.G.; Stordeur, P.; Kelleher, D.P.; McManus, R.; Ryan, T. Post-operative infection and sepsis in humans is associated with deficient gene expression of γc cytokines and their apoptosis mediators. Crit. Care 2011, 15, R158. [Google Scholar] [CrossRef]
- Kimura, A.; Ono, S.; Hiraki, S.; Takahata, R.; Tsujimoto, H.; Miyazaki, H.; Kinoshita, M.; Hatsuse, K.; Saitoh, D.; Hase, K.; et al. The postoperative serum interleukin-15 concentration correlates with organ dysfunction and the prognosis of septic patients following emergency gastrointestinal surgery. J. Surg. Res. 2012, 175, e83–e88. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luan, L.; Patil, N.K.; Wang, J.; Bohannon, J.K.; Rabacal, W.; Fensterheim, B.A.; Hernandez, A.; Sherwood, E.R. IL-15 Enables Septic Shock by Maintaining NK Cell Integrity and Function. J. Immunol. 2017, 198, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.D. The complete blood count to diagnose septic shock. J. Thorac. Dis. 2020, 12, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Holub, M.; Klucková, Z.; Helcl, M.; Príhodov, J.; Rokyta, R.; Beran, O. Lymphocyte subset numbers depend on the bacterial origin of sepsis. Clin. Microbiol. Infect. 2003, 9, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Douek, D.C.; Vescio, R.A.; Betts, M.R.; Brenchley, J.M.; Hill, B.J.; Zhang, L.; Berenson, J.R.; Collins, R.H.; Koup, R.A. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 2000, 355, 1875–1881. [Google Scholar] [CrossRef]
- Hazenberg, M.D.; Borghans, J.A.M.; de Boer, R.J.; Miedema, F. Thymic output: A bad TREC record. Nat. Immunol. 2003, 4, 97–99. [Google Scholar] [CrossRef]
- Francois, B.; Jeannet, R.; Daix, T.; Walton, A.H.; Shotwell, M.S.; Unsinger, J.; Monneret, G.; Rimmelé, T.; Blood, T.; Morre, M.; et al. Interleukin-7 restores lymphocytes in septic shock: The IRIS-7 randomized clinical trial. JCI Insight 2018, 3, e98960. [Google Scholar] [CrossRef]
- Somech, R. T-cell receptor excision circles in primary immunodeficiencies and other T-cell immune disorders. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 517–524. [Google Scholar] [CrossRef]
- Ammer-Herrmenau, C.; Kulkarni, U.; Andreas, N.; Ungelenk, M.; Ravens, S.; Hübner, C.; Kather, A.; Kurth, I.; Bauer, M.; Kamradt, T. Sepsis induces long-lasting impairments in CD4+ T-cell responses despite rapid numerical recovery of T-lymphocyte populations. PLoS ONE 2019, 14, e0211716. [Google Scholar] [CrossRef]
- Tomino, A.; Tsuda, M.; Aoki, R.; Kajita, Y.; Hashiba, M.; Terajima, T.; Kano, H.; Takeyama, N. Increased PD-1 Expression and Altered T Cell Repertoire Diversity Predict Mortality in Patients with Septic Shock: A Preliminary Study. PLoS ONE 2017, 12, e0169653. [Google Scholar] [CrossRef]
- Venet, F.; Filipe-Santos, O.; Lepape, A.; Malcus, C.; Poitevin-Later, F.; Grives, A.; Plantier, N.; Pasqual, N.; Monneret, G. Decreased T-cell repertoire diversity in sepsis: A preliminary study. Crit. Care Med. 2013, 41, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Den Braber, I.; Mugwagwa, T.; Vrisekoop, N.; Westera, L.; Mögling, R.; de Boer, A.B.; Willems, N.; Schrijver, E.H.R.; Spierenburg, G.; Gaiser, K.; et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012, 36, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Jensen, I.J.; Sjaastad, F.V.; Griffith, T.S.; Badovinac, V.P. Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J. Immunol. 2018, 200, 1543–1553. [Google Scholar] [PubMed]
- Hiramatsu, M.; Hotchkiss, R.S.; Karl, I.E.; Buchmann, T.G. Cecal Ligation and Puncture (CLP) Induces Apoptosis in Thymus, Spleen, Lung, and Gut by an Endotoxin and TNF-independent Pathway. Shock 1997, 7, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Unsinger, J.; Kazama, H.; McDonough, J.S.; Hotchkiss, R.S.; Ferguson, T.A. Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury. J. Leukoc. Biol. 2009, 85, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhang, J.; Chen, W. Thymic output: Influence factors and molecular mechanism. Cell. Mol. Immunol. 2006, 3, 341–350. [Google Scholar]
- Chou, J.P.; Effros, R.B. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar]
- Oliveira, N.M.; Rios, E.C.S.; de Lima, T.M.; Victorino, V.J.; Barbeiro, H.; Pinheiro da Silva, F.; Szabo, C.; Soriano, F.G. Sepsis induces telomere shortening: A potential mechanism responsible for delayed pathophysiological events in sepsis survivors? Mol. Med. 2017, 22, 886–891. [Google Scholar] [CrossRef]
- Barsov, E.V. Telomerase and primary T cells: Biology and immortalization for adoptive immunotherapy. Immunotherapy 2011, 3, 407–421. [Google Scholar] [CrossRef]
- Wallace, D.L.; Bérard, M.; Soares, M.V.D.; Oldham, J.; Cook, J.E.; Akbar, A.N.; Tough, D.F.; Beverley, P.C.L. Prolonged exposure of naïve CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length. Immunology 2006, 119, 243–253. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, N.; Noack, S.; Hecker, A.; Hackstein, H.; Bein, G.; Weissmann, N.; Seeger, W.; Mayer, K.; Hecker, M. Decreased Thymic Output Contributes to Immune Defects in Septic Patients. J. Clin. Med. 2020, 9, 2695. https://doi.org/10.3390/jcm9092695
Sommer N, Noack S, Hecker A, Hackstein H, Bein G, Weissmann N, Seeger W, Mayer K, Hecker M. Decreased Thymic Output Contributes to Immune Defects in Septic Patients. Journal of Clinical Medicine. 2020; 9(9):2695. https://doi.org/10.3390/jcm9092695
Chicago/Turabian StyleSommer, Natascha, Steffen Noack, Andreas Hecker, Holger Hackstein, Gregor Bein, Norbert Weissmann, Werner Seeger, Konstantin Mayer, and Matthias Hecker. 2020. "Decreased Thymic Output Contributes to Immune Defects in Septic Patients" Journal of Clinical Medicine 9, no. 9: 2695. https://doi.org/10.3390/jcm9092695
APA StyleSommer, N., Noack, S., Hecker, A., Hackstein, H., Bein, G., Weissmann, N., Seeger, W., Mayer, K., & Hecker, M. (2020). Decreased Thymic Output Contributes to Immune Defects in Septic Patients. Journal of Clinical Medicine, 9(9), 2695. https://doi.org/10.3390/jcm9092695





