Characterization of IgA Deposition in the Kidney of Patients with IgA Nephropathy and Minimal Change Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
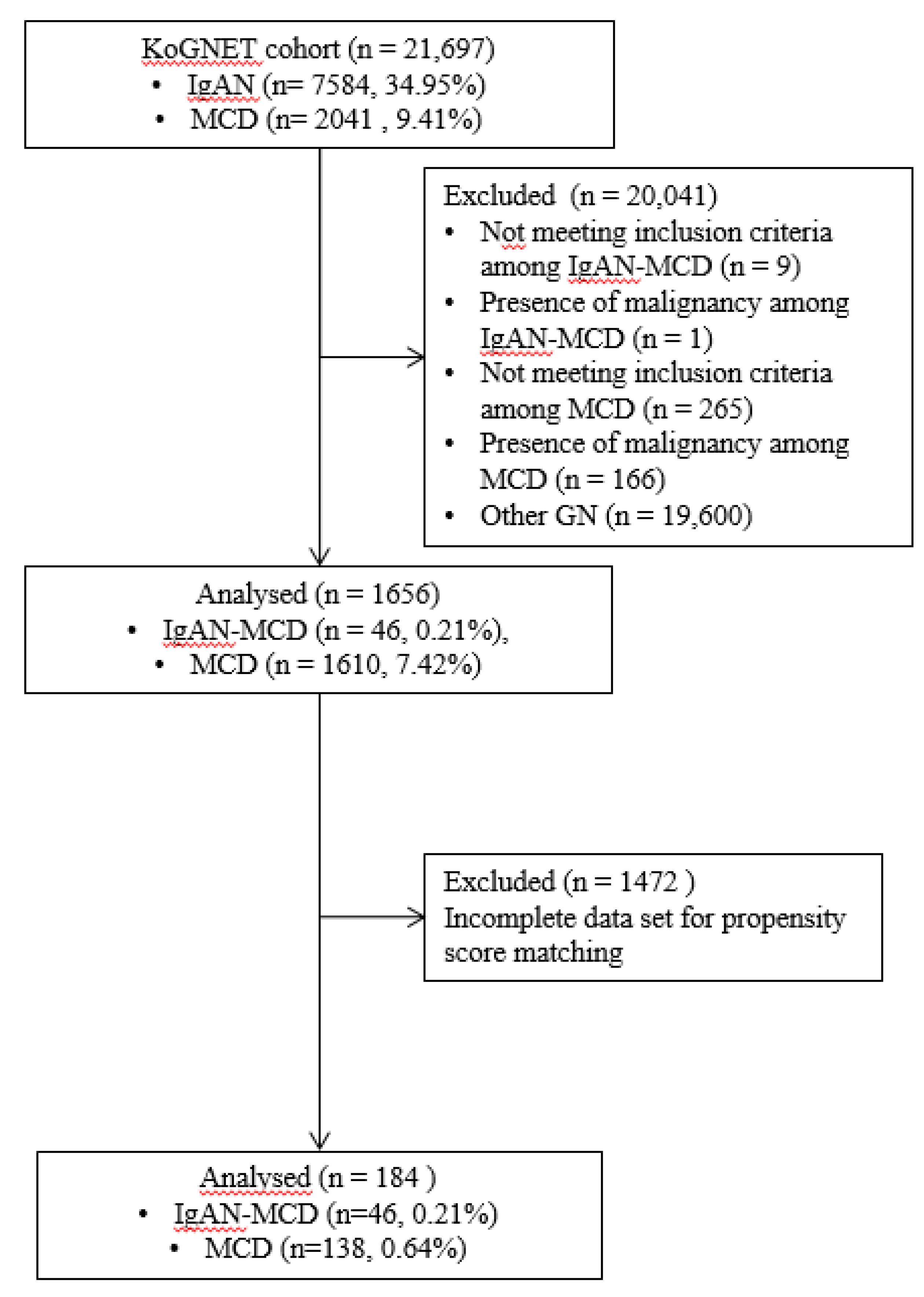
2.2. Preparation of Propensity Score-Matched Pairs
2.3. Evaluation of Treatment Efficacy
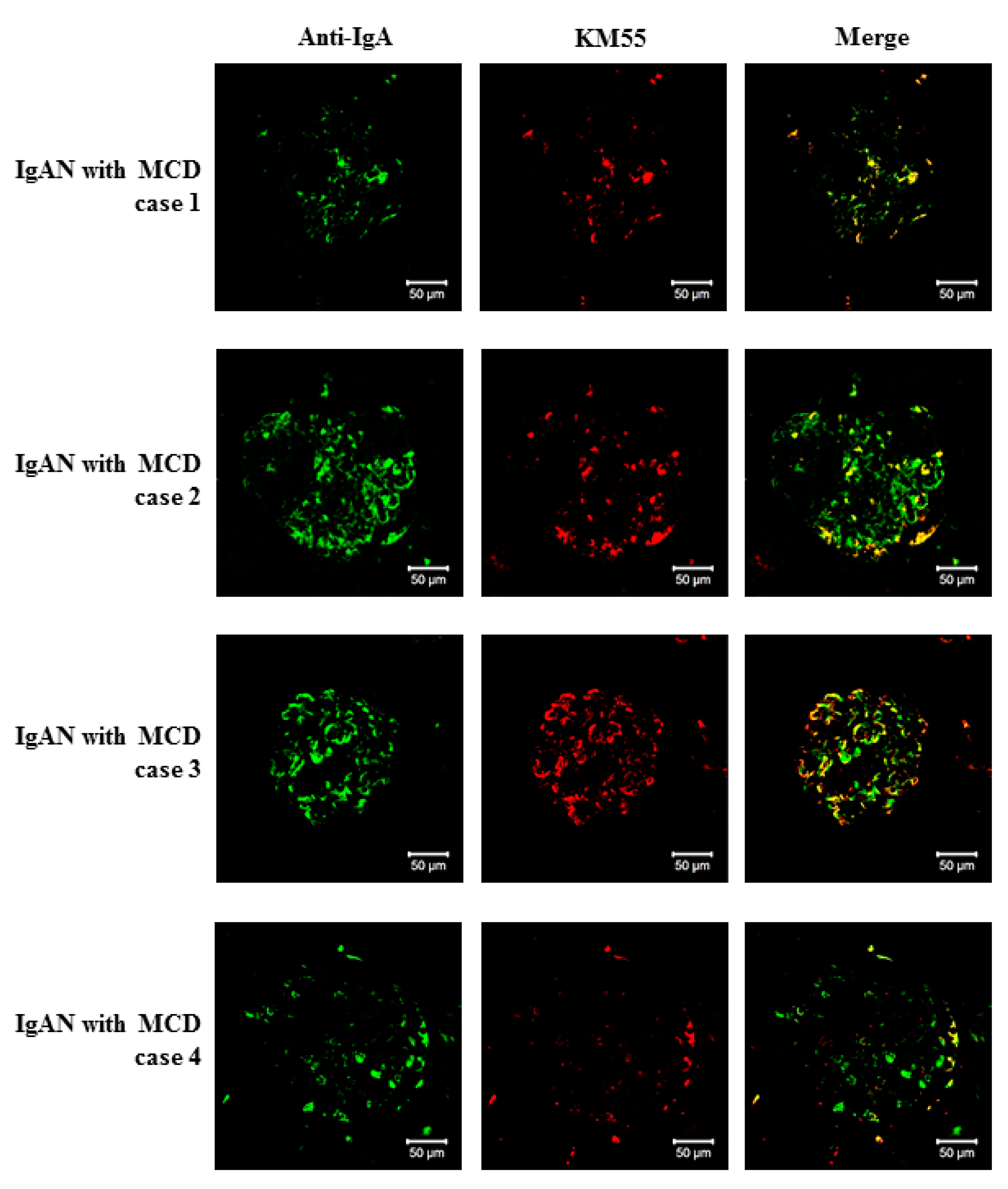
2.4. Double Immunofluorescence Staining of Kidney Biopsy Tissues
2.5. Statistical Analysis
3. Results
3.1. Incidence of IgAN with MCD
3.2. Clinical Feature of the Patients with IgAN-MCD
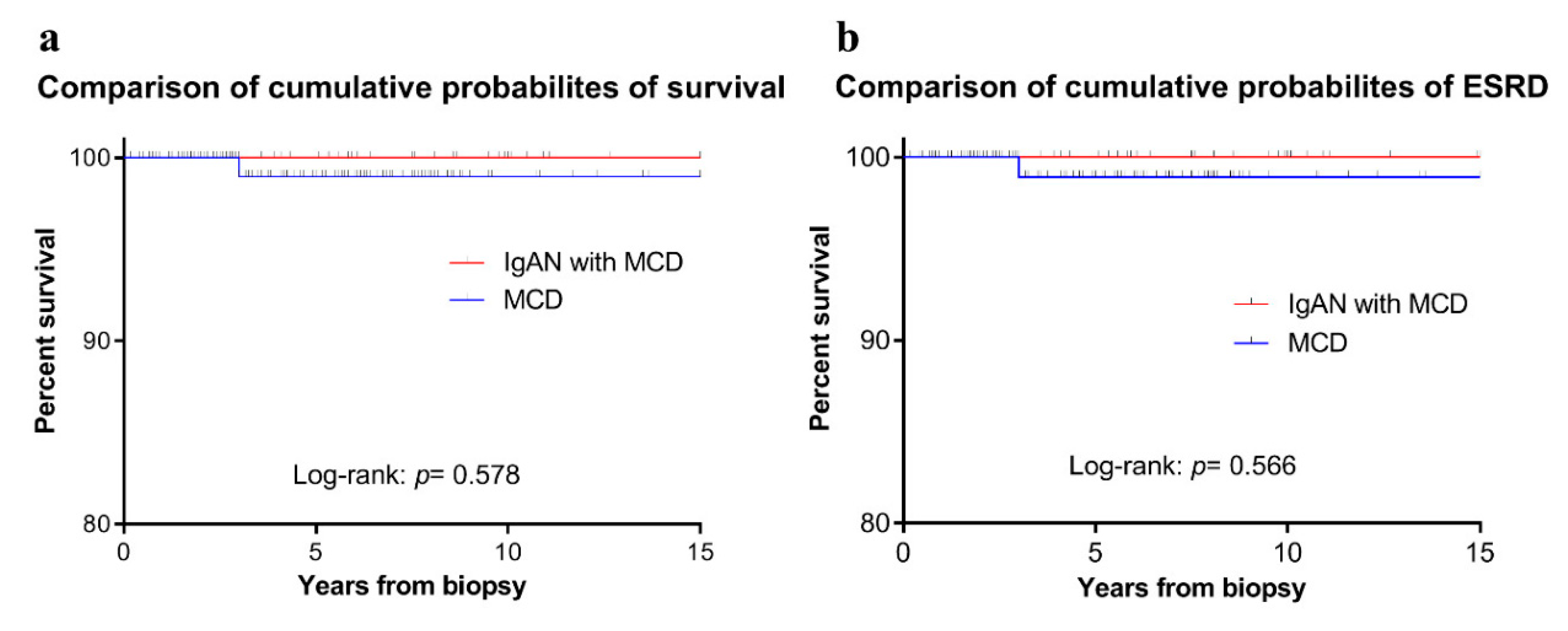
3.3. Prognosis of the Patients with IgAN-MCD
3.4. Characterization of IgA Deposition in Patients with IgAN-MCD
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Ethics Statements
Abbreviations
| BMI | Body mass index |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| DM | Diabetes mellitus |
| HTN | Hypertension |
| CHD | Coronary heart disease |
| CVD | Cerebrovascular disease |
| Cr | Creatinine |
| LDL | Low-density lipoprotein |
| UPCR | Urine protein to creatinine ratio |
| UACR | Urine albumin creatinine ratio |
References
- Donadio, J.V.; Grande, J.P. IgA nephropathy. N. Engl. J. Med. 2002, 347, 738–748. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. In Seminars in Nephrology; WB Saunders: Philadelphia, PA, USA, 2004; pp. 179–196. [Google Scholar]
- Donadio, J.V.; Bergstralh, E.J.; Grande, J.P.; Rademcher, D.M. Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol. Dial. Transplant. 2002, 17, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N.; Lai, F.M.-M.; Chan, K.W.; Ho, C.P.; Leung, A.C.; Vallance-Owen, J. An overlapping syndrome of IgA nephropathy and lipoid nephrosis. Am. J. Clin. Pathol. 1986, 86, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Sinnassamy, P.; O’Regan, S. Mesangial IgA deposits with steroid responsive nephrotic syndrome: Probable minimal lesion nephrosis. Am. J. Kidney Dis. 1985, 5, 267–269. [Google Scholar] [CrossRef]
- Lai, K.N.; Lai, F.M.-M.; Li, P.K.; Chan, K.W.; Au, T.C.; Tong, K.L. The clinicopathological characteristics of IgA nephropathy in Hong Kong. Pathology 1988, 20, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Moon, K.C.; Oh, K.-H.; Joo, K.W.; Kim, Y.S.; Ahn, C.; Han, J.S.; Kim, S. Clinicopathologic characteristics of IgA nephropathy with steroid-responsive nephrotic syndrome. J. Korean Med. Sci. 2009, 24, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, J.; Pasternack, A.; Rantala, I. The nephrotic syndrome in IgA glomerulonephritis: Response to corticosteroid therapy. Clin. Nephrol. 1983, 20, 172–176. [Google Scholar] [PubMed]
- Wu, G.; Katz, A.; Cardella, C.; Oreopoulos, D.G. Spontaneous remission of nephrotic syndrome in IgA glomerular disease. Am. J. Kidney Dis. 1985, 6, 96–99. [Google Scholar] [CrossRef]
- Choi, I.J.; Jeong, H.J.; Lee, H.Y.; Kim, P.K.; Lee, J.S.; Han, D.S. Significance of mesangial IgA deposition in minimal change nephrotic syndrome: A study of 60 cases. Yonsei Med. J. 1990, 31, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, H.; Miya, K.; Arai, M.; Miyawaki, T.; Inaba, S. Minimal change variants with mesangial IgA deposits. Clin. Nephrol. 2007, 68, 337. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; Gharavi, A.G. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, J.; Suzuki, Y.; Suzuki, H.; Hiura, N.; Yanagawa, H.; Makita, Y.; Kaneko, E.; Tomino, Y. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol. Dial. Transplant. 2015, 30, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Furuse, A.; Hiramatsu, M.; Adachi, N.; Karashima, S.; Hattori, S.; Matsuda, I. Dramatic response to corticosteroid therapy of nephrotic syndrome associated with IgA nephropathy. Int. J. Pediatr. Nephrol. 1985, 6, 205–208. [Google Scholar] [PubMed]
- Jiang, L.; Dong, B.; Yan, Y.; Zheng, S.; Hu, Y.; Zuo, L.; Shi, H. Clinicopathological analysis of IgA nephropathy combined with other glomerular diseases. Medicine 2019, 98, e17388. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Cheng, S.-Q.; Liang, S.-S.; Le, W.-B.; Zeng, C.-H.; Wang, J.-Q.; Liu, Z.-H. Comparison between patients with IgA nephropathy with minimal change disease and patients with minimal change disease. Clin. Nephrol. 2016, 85, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Kim, J.H.; Lee, S.C.; Kang, E.W.; Chang, T.I.; Moon, S.J.; Yoon, S.Y.; Yoo, T.-H.; Kang, S.-W.; Choi, K.H. Clinical features and outcomes of IgA nephropathy with nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 2012, 7, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yasutake, J.; Makita, Y.; Tanbo, Y.; Yamasaki, K.; Sofue, T.; Kano, T.; Suzuki, Y. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018, 93, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Cassol, C.A.; Bott, C.; Nadasdy, G.M.; Alberton, V.; Malvar, A.; Nagaraja, H.N.; Nadasdy, T.; Rovin, B.H.; Satoskar, A.A. Immunostaining for galactose-deficient immunoglobulin A is not specific for primary immunoglobulin A nephropathy. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Lingaiah, R.; Mani, K.; Barwad, A.; Singh, G.; Balooni, V.; Bhowmik, D.; Agarwal, S.K. Significance of serum galactose deficient IgA1 as a potential biomarker for IgA nephropathy: A case control study. PLoS ONE 2019, 14, e0214256. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Suzuki, H.; Yasutake, J.; Yamazaki, Y.; Suzuki, Y. Galactose-Deficient IgA1-Specific Antibody Recognizes GalNAc-Modified Unique Epitope on Hinge Region of IgA1. Monoclon. Antib. Immunodiagn. Immunother. 2018, 37, 252–256. [Google Scholar] [CrossRef] [PubMed]

| Before Matching | After Nearest Neighbor 1:3 Matching | |||||
|---|---|---|---|---|---|---|
| IgAN with MCD | MCD | p Value | IgAN with MCD | MCD | p Value | |
| (n = 46) | (n = 1574) | (n = 46) | (n = 138) | |||
| Age (years) | 46.24 ± 18.26 | 50.02 ± 18.36 | 0.140 | 46.24 ± 18.26 | 46.38 ± 17.94 | 0.930 |
| Male gender, n (%) | 29 (63.0) | 974 (61.9) | 1.000 | 29 (63.0) | 87 (63.0) | 1.000 |
| BMI (kg/m2) | 25.85 ± 3.59 | 24.12 ± 4.09 | 0.007 | 25.85 ± 3.59 | 24.79 ± 4.59 | 0.065 |
| SBP (mmHg) | 122.96 ± 17.10 | 123.72 ± 16.59 | 0.828 | 122.96 ± 17.10 | 125.11 ± 18.68 | 0.665 |
| DBP (mmHg) | 76.62 ± 12.42 | 77.61 ± 13.14 | 0.919 | 76.62 ± 12.42 | 77.78 ± 13.98 | 0.906 |
| DM, n (%) | 6 (13.0) | 82 (5.9) | 0.056 | 6 (13.0) | 18 (13.0) | 1.000 |
| HTN, n (%) | 11 (23.9) | 309 (22.1) | 0.765 | 11 (23.9) | 36 (26.1) | 0.770 |
| CHD, n (%) | 1 (2.2) | 29 (2.5) | 1.000 | 1 (2.2) | 5 (4.6) | 0.671 |
| CVD, n (%) | 1 (2.2) | 19 (1.6) | 0.534 | 1 (2.2) | 0 (0.0) | 0.294 |
| Serum Albumin (g/dL) | 2.22 ± 0.81 | 2.74 ± 1.16 | 0.003 | 2.23 ± 0.81 | 2.54 ± 1.02 | 0.051 |
| Serum Cr (mg/dL) | 0.86 ± 0.27 | 0.96 ± 0.36 | 0.156 | 0.86 ± 0.27 | 0.91 ± 0.34 | 0.544 |
| Total cholesterol (mg/dL) | 362.33 ± 131.34 | 336.76 ± 153.55 | 0.135 | 362.33 ± 131.34 | 342.01 ± 149.83 | 0.298 |
| LDL cholesterol (mg/dL) | 214.68 ± 133.55 | 217.08 ± 125.82 | 0.808 | 214.68 ± 133.55 | 211.76 ± 114.86 | 0.920 |
| Serum uric acid (mg/dL) | 6.23 ± 1.81 | 6.15 ± 1.82 | 0.735 | 6.21 ± 1.62 | 6.48 ± 1.64 | 0.370 |
| UPCR g/g | 7.45 ± 5.05 | 6.04 ± 5.33 | 0.032 | 7.45 ± 5.05 | 7.60 ± 6.00 | 0.923 |
| UACR g/g | 5.09 ± 3.32 | 4.18 ± 4.17 | <0.001 | 4.63 ± 3.40 | 4.92 ± 4.66 | 0.962 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, W.-H.; Park, S.-H.; Choi, S.-K.; Jung, S.W.; Jeong, K.H.; Kim, Y.-G.; Moon, J.-Y.; Lim, S.-J.; Sung, J.-Y.; Jhee, J.H.; et al. Characterization of IgA Deposition in the Kidney of Patients with IgA Nephropathy and Minimal Change Disease. J. Clin. Med. 2020, 9, 2619. https://doi.org/10.3390/jcm9082619
Cho W-H, Park S-H, Choi S-K, Jung SW, Jeong KH, Kim Y-G, Moon J-Y, Lim S-J, Sung J-Y, Jhee JH, et al. Characterization of IgA Deposition in the Kidney of Patients with IgA Nephropathy and Minimal Change Disease. Journal of Clinical Medicine. 2020; 9(8):2619. https://doi.org/10.3390/jcm9082619
Chicago/Turabian StyleCho, Won-Hee, Seon-Hwa Park, Seul-Ki Choi, Su Woong Jung, Kyung Hwan Jeong, Yang-Gyun Kim, Ju-Young Moon, Sung-Jig Lim, Ji-Youn Sung, Jong Hyun Jhee, and et al. 2020. "Characterization of IgA Deposition in the Kidney of Patients with IgA Nephropathy and Minimal Change Disease" Journal of Clinical Medicine 9, no. 8: 2619. https://doi.org/10.3390/jcm9082619
APA StyleCho, W.-H., Park, S.-H., Choi, S.-K., Jung, S. W., Jeong, K. H., Kim, Y.-G., Moon, J.-Y., Lim, S.-J., Sung, J.-Y., Jhee, J. H., Chin, H. J., Choi, B. S., & Lee, S.-H. (2020). Characterization of IgA Deposition in the Kidney of Patients with IgA Nephropathy and Minimal Change Disease. Journal of Clinical Medicine, 9(8), 2619. https://doi.org/10.3390/jcm9082619





