The Hypotension Period after Initiation of Appropriate Antimicrobial Administration Is Crucial for Survival of Bacteremia Patients Initially Experiencing Severe Sepsis and Septic Shock
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Microbiological Methods
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics of the Study Cohort
3.2. Causative Microorganisms and Susceptibilities
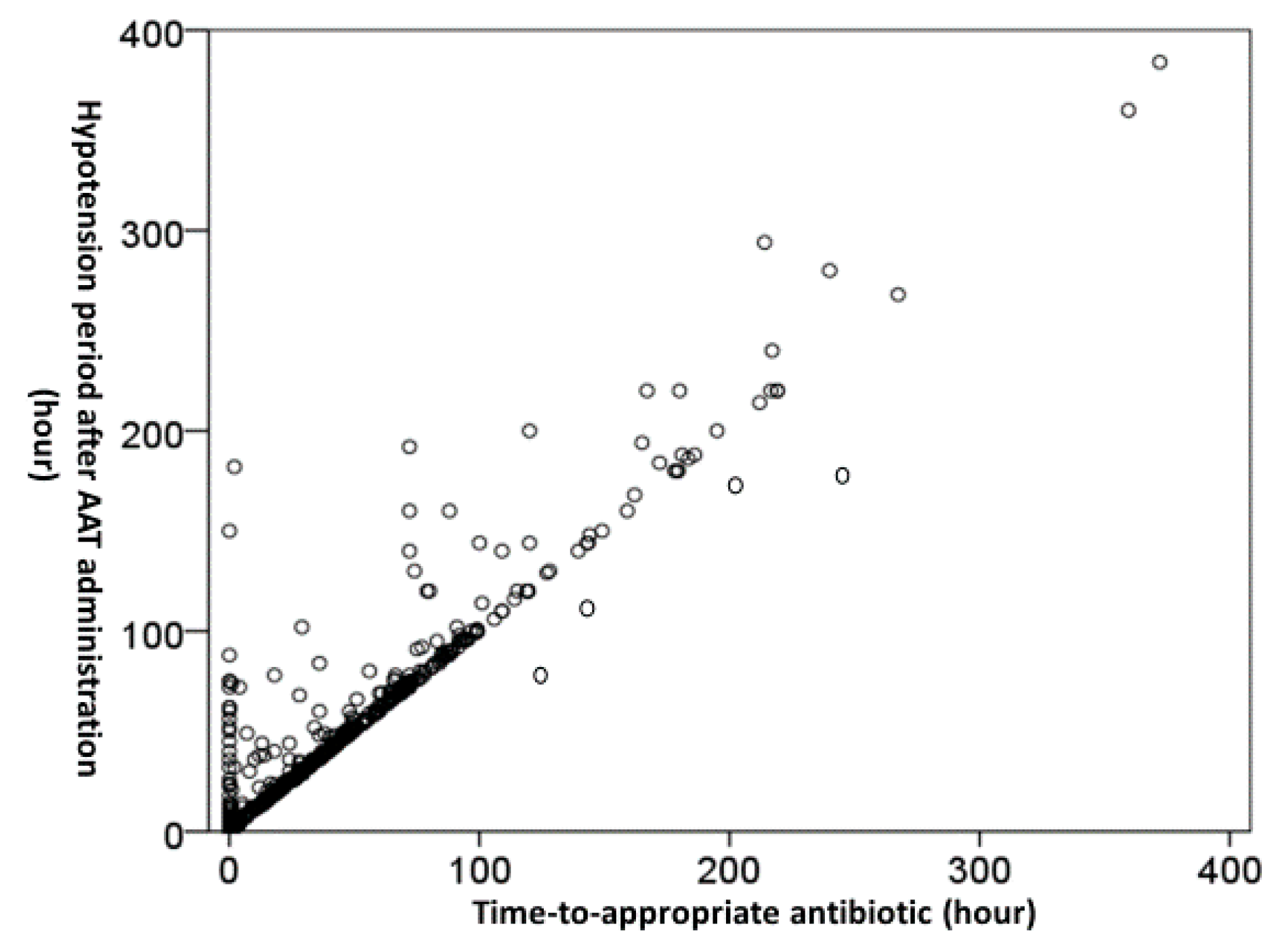
3.3. Association of the Time-to-Appropriate Antibiotic (TtAa) or Hypotension Periods and Mortality Rates
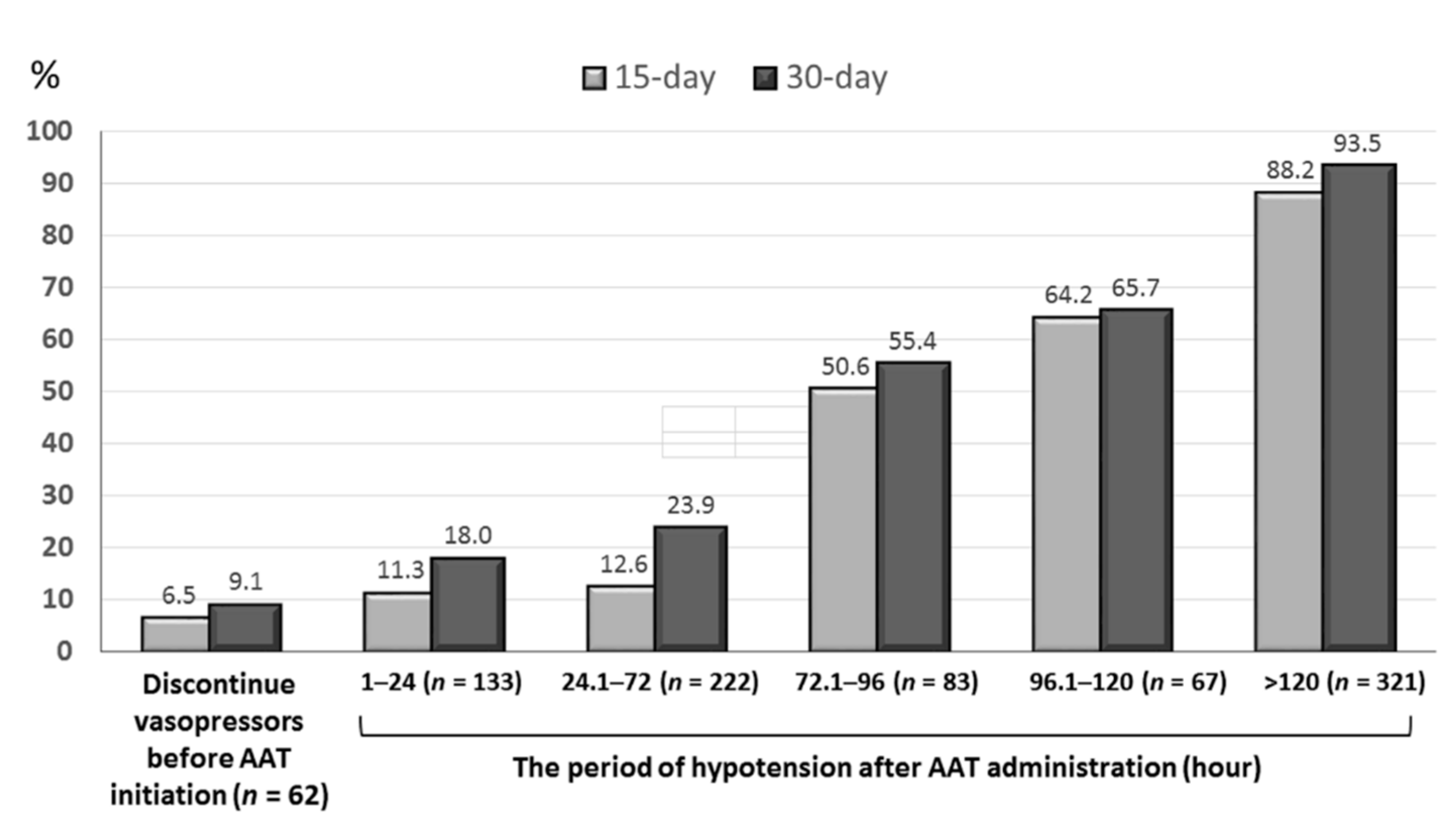
3.4. Independent Impacts of the TtAa and Hypotension Period on 30-Day Mortality
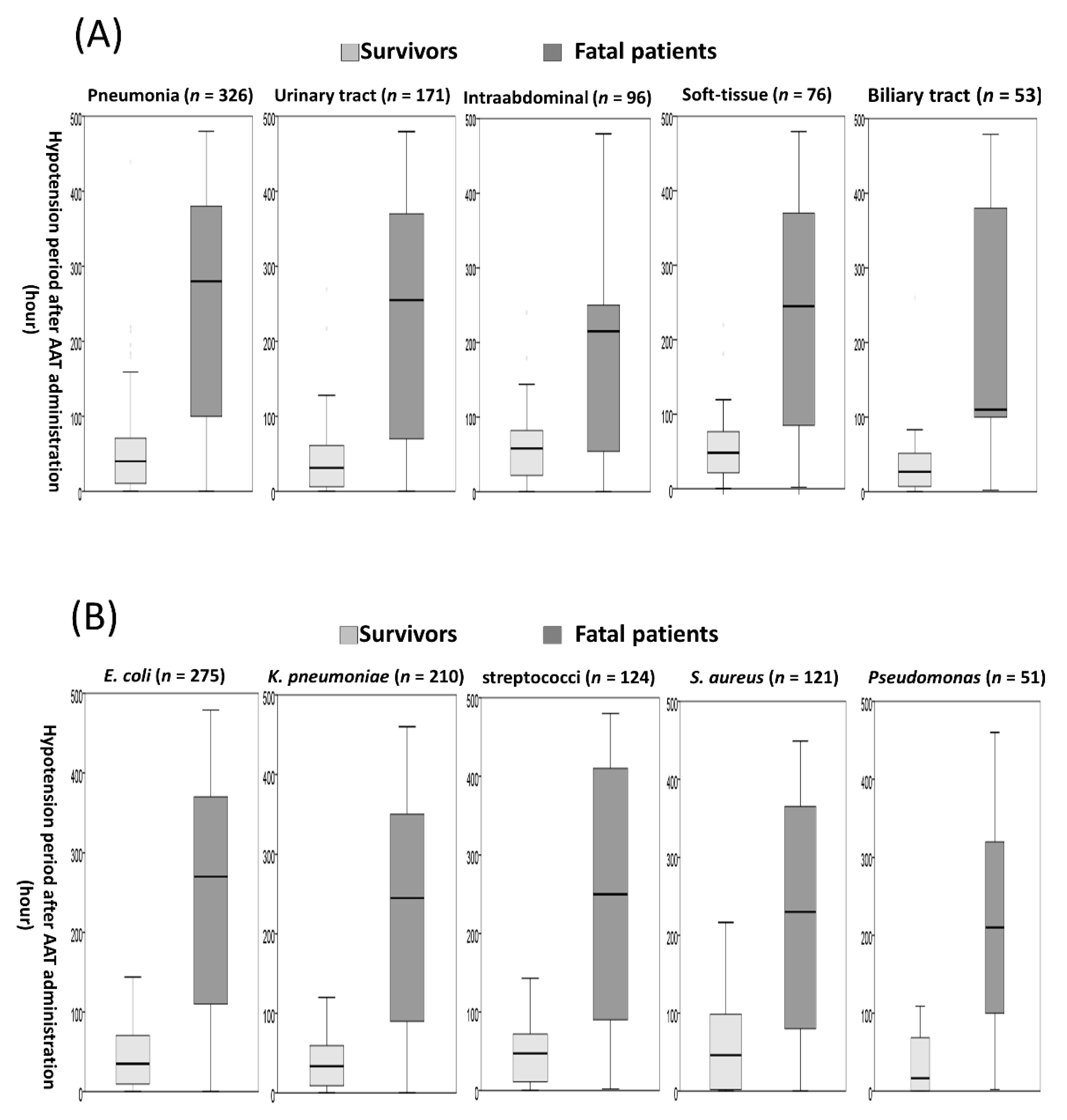
3.5. The Hypotension Period after Appropriate Antimicrobial Therapy (AAT) Initiation in Subgroup Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAT | appropriate antimicrobial therapy |
| ACLS | advanced cardiac life support |
| AHA | American Heart Association |
| CLSI | Clinical and Laboratory Standards Institute |
| ED | emergency department |
| IQR | interquartile range |
| SSC | Surviving Sepsis Campaign |
| SD | standard deviation |
| TtAa | time-to-appropriate antibiotic |
References
- Bates, D.W.; Pruess, K.E.; Lee, T.H. How bad are bacteremia and sepsis? Outcomes in a cohort with suspected bacteremia. Arch. Intern. Med. 1995, 155, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lee, C.H.; Yang, C.Y.; Hsieh, C.C.; Tang, H.J.; Ko, W.C. Beneficial effects of early empirical administration of appropriate antimicrobials on survival and defervescence in adults with community-onset bacteremia. Crit. Care 2019, 23, 363. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lee, C.H.; Hong, M.Y.; Tang, H.J.; Ko, W.C. Timing of appropriate empirical antimicrobial administration and outcome of adults with community-onset bacteremia. Crit. Care 2017, 21, 119. [Google Scholar] [CrossRef]
- Eliopoulos, G.M.; Paterson, D.L.; Rice, L.B. Empirical antibiotic choice for the seriously ill patient: Are minimization of selection of resistant organisms and maximization of individual outcome mutually exclusive? Clin. Infect. Dis. 2003, 36, 1006–1012. [Google Scholar]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, Approved Standard. CLSI Document M100-S30, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Lee, C.C.; Lin, W.J.; Shih, H.I.; Wu, C.J.; Chen, P.L.; Lee, H.C.; Lee, N.Y.; Chang, C.M.; Wang, L.R.; Ko, W.C. Clinical significance of potential contaminants in blood cultures among patients in a medical center. J. Microbiol. Immunol. Infect. 2007, 40, 438–444. [Google Scholar]
- Hsieh, C.C.; Chen, P.L.; Lee, C.H.; Yang, C.Y.; Lee, C.C.; Ko, W.C. Definitive Cefazolin Therapy for Stabilized Adults with Community-Onset Escherichia coli, Klebsiella Species, and Proteus mirabilis Bacteremia: MIC Matters. J. Clin. Med. 2020, 9, 157. [Google Scholar] [CrossRef]
- Laupland, K.B.; Church, D.L. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin. Microbiol. Rev. 2014, 27, 647–664. [Google Scholar] [CrossRef]
- David, N.; Gilbert, R.C.M., Jr.; George, M.; Eliopoulos, G.M.; Chambers, H.F.; Saag, M.S. Selected pharmacologic faetures of antimicrobial agents. Sanford Guide Antimicrob. Ther. 2016, 1, 78–82. [Google Scholar]
- Chotiprasitsakul, D.; Han, J.H.; Cosgrove, S.E.; Harris, A.D.; Lautenbach, E.; Conley, A.T.; Tolomeo, P.; Wise, J.; Tamma, P.D.; Antibacterial Resistance Leadership Group. Comparing the Outcomes of Adults with Enterobacteriaceae Bacteremia Receiving Short-Course Versus Prolonged-Course Antibiotic Therapy in a Multicenter, Propensity Score-Matched Cohort. Clin. Infect. Dis. 2018, 66, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, R.G.; Claridge, J.A.; Nathens, A.B.; Rotstein, O.D.; Duane, T.M.; Evans, H.L.; Cook, C.H.; O’Neill, P.J.; Mazuski, J.E.; Askari, R.; et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N. Engl. J. Med. 2015, 372, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Citerio, G.; Vascotto, E.; Villa, F.; Celotti, S.; Pesenti, A. Induced abdominal compartment syndrome increases intracranial pressure in neurotrauma patients: A prospective study. Crit. Care Med. 2001, 29, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Schellevis, F.G.; van der Velden, J.; van de Lisdonk, E.; van Eijk, J.T.; van Weel, C. Comorbidity of chronic diseases in general practice. J. Clin. Epidemiol. 1993, 46, 469–473. [Google Scholar] [CrossRef]
- McCabe, W.R. Gram-negative bacteremia. Adv. Intern. Med. 1974, 19, 135–158. [Google Scholar]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Paterson, D.L.; Ko, W.C.; Von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G.; Klugman, K.P.; Bonomo, R.A.; et al. International prospective study of Klebsiella pneumoniae bacteremia: Implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann. Intern. Med. 2004, 140, 26–32. [Google Scholar] [CrossRef]
- Brun-Buisson, C.; Doyon, F.; Carlet, J.; Dellamonica, P.; Gouin, F.; Lepoutre, A.; Mercier, J.-C.; Offenstadt, G.; Régnier, B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: A multicenter prospective study in intensive care units. JAMA 1995, 274, 968–974. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008, 34, 17–60. [Google Scholar] [CrossRef]
- Lin, M.Y.; Weinstein, R.A.; Hota, B. Delay of active antimicrobial therapy and mortality among patients with bacteremia: Impact of severe neutropenia. Antimicrob. Agents Chemother. 2008, 52, 3188–3194. [Google Scholar] [CrossRef]
- Corona, A.; Bertolini, G.; Lipman, J.; Wilson, A.P.; Singer, M. Antibiotic use and impact on outcome from bacteraemic critical illness: The BActeraemia Study in Intensive Care (BASIC). J. Antimicrob. Chemother. 2010, 65, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H.; Sherman, G.; Ward, S.; Fraser, V.J.; Kollef, M.H. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000, 118, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Lin, W.L.; Lin, C.C.; Hsieh, W.H.; Hsieh, C.H.; Wu, M.H.; Wu, J.Y.; Lee, C.C. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Leibovici, L.; Shraga, I.; Drucker, M.; Konigsberger, H.; Samra, Z.; Pitlik, S.D. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 1998, 244, 379–386. [Google Scholar] [CrossRef]
- Lee, C.-C.; Lee, C.-H.; Hong, M.-Y.; Hsieh, C.-C.; Tang, H.-J.; Ko, W.-C. Propensity-matched analysis of the impact of extended-spectrum β-lactamase production on adults with community-onset Escherichia coli, Klebsiella species, and Proteus mirabilis bacteremia. J. Microbiol. Immunol. Infect. 2018, 51, 519–526. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef]
- Yu, V.L.; Chiou, C.C.; Feldman, C.; Ortqvist, A.; Rello, J.; Morris, A.J.; Baddour, L.M.; Luna, C.M.; Snydman, D.R.; Ip, M.; et al. An international prospective study of pneumococcal bacteremia: Correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin. Infect. Dis. 2003, 37, 230–237. [Google Scholar] [CrossRef]
- Yeh, C.-F.; Chen, K.-F.; Ye, J.-J.; Huang, C.-T. Derivation of a clinical prediction rule for bloodstream infection mortality of patients visiting the emergency department based on predisposition, infection, response, and organ dysfunction concept. J. Microbiol. Immunol. Infect. 2014, 47, 469–477. [Google Scholar] [CrossRef][Green Version]
- Rac, H.; Gould, A.; Bookstaver, P.; Justo, J.; Kohn, J.; Al-Hasan, M. Evaluation of early clinical failure criteria for Gram-negative bloodstream infections. Clin. Microbiol. Infect. 2020, 26, 73–77. [Google Scholar] [CrossRef]
- Annane, D.; Ouanes-Besbes, L.; De Backer, D.; Bin, D.; Gordon, A.C.; Hernandez, G.; Olsen, K.M.; Osborn, T.M.; Peake, S.; Russell, J.A.; et al. A global perspective on vasoactive agents in shock. Intensive Care Med. 2018, 44, 833–846. [Google Scholar] [CrossRef]

| Variables | Patient Number (%) | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| Death, n = 472 | Survival, n = 416 | OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Patient demographics | ||||||
| The elderly, ≥65 years | 312 (66.1) | 250 (60.1) | 1.30 (0.99–1.70) | 0.06 | NS | NS |
| Gender, male | 272 (57.6) | 246 (59.1) | 0.94 (0.72–1.23) | 0.65 | – | – |
| Nursing-home residents | 66 (14.0) | 34 (8.2) | 1.83 (1.18–2.83) | 0.006 | NS | NS |
| Time-to-appropriate antibiotic, hours | – | – | – | – | 1.008 (1.006–1.010) | <0.001 |
| Hypotension period after AAT initiation, hours | – | – | – | – | 1.011 (1.010–1.013) | <0.001 |
| Inadequate source control | 26 (5.5) | 10 (2.4) | 2.37 (1.13–4.97) | 0.02 | NS | NS |
| Pitt bacteremia score ≥ 4 at onset | 427 (90.5) | 245 (58.9) | 6.63 (4.60–9.53) | <0.001 | 3.45 (2.01–5.93) | <0.001 |
| Major bacteremia sources | ||||||
| Pneumonia | 234 (49.6) | 92 (22.1) | 3.46 (2.58–4.64) | <0.001 | NS | NS |
| Intra-abdominal | 46 (9.7) | 50 (12.0) | 0.79 (0.52–1.21) | 0.28 | – | – |
| Urinary tracts | 42 (8.9) | 129 (31.0) | 0.22 (0.15–0.32) | <0.001 | 0.34 (0.18–0.62) | <0.001 |
| Soft-tissue | 40 (8.5) | 36 (8.7) | 0.98 (0.61–1.57) | 0.92 | – | – |
| Biliary tracts | 17 (3.6) | 36 (8.7) | 0.39 (0.22–0.71) | 0.002 | 0.20 (0.07–0.58) | 0.003 |
| Polymicrobial bacteremia | 90 (19.1) | 62 (14.9) | 1.35 (0.94–1.92) | 0.10 | – | – |
| Major causative microorganisms | ||||||
| Escherichia coli | 117 (24.8) | 158 (38.0) | 0.54 (0.40–0.72) | <0.001 | NS | NS |
| Klebsiella pneumoniae | 112 (23.7) | 98 (23.6) | 1.01 (0.74–1.38) | 0.95 | – | – |
| Streptococcus species | 79 (16.7) | 45 (10.8) | 1.66 (1.12–2.45) | 0.01 | NS | NS |
| Staphylococcus aureus | 75 (15.9) | 46 (11.1) | 1.52 (1.03–2.25) | 0.04 | NS | NS |
| Pseudomonas species | 33 (7.0) | 18 (4.3) | 1.66 (0.92–3.00) | 0.09 | NS | NS |
| Enterococcus species | 16 (3.4) | 19 (4.6) | 0.73 (0.37–1.45) | 0.37 | – | – |
| Fatal comorbidities (McCabe classification) | 201 (42.6) | 111 (26.7) | 2.04 (1.54–2.71) | <0.001 | 2.03 (1.27–3.23) | 0.003 |
| Major comorbidities | ||||||
| Hypertension | 207 (43.9) | 184 (44.2) | 0.99 (0.76–1.28) | 0.91 | – | – |
| Malignancies | 188 (39.8) | 110 (26.4) | 1.84 (1.38–2.45) | <0.001 | NS | NS |
| Neurological diseases | 169 (35.8) | 124 (29.8) | 1.31 (0.99–1.74) | 0.06 | NS | NS |
| Diabetes mellitus | 162 (34.3) | 167 (40.1) | 0.78 (0.59–1.02) | 0.07 | 0.63 (0.39–1.01) | 0.06 |
| Chronic kidney diseases | 88 (18.6) | 93 (22.4) | 0.80 (0.57–1.10) | 0.17 | – | – |
| Liver cirrhosis | 70 (14.8) | 60 (14.4) | 1.03 (0.71–1.50) | 0.86 | – | – |
| Coronary artery diseases | 53 (11.2) | 40 (9.6) | 1.19 (0.77–1.83) | 0.43 | – | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; Yang, C.-Y.; Su, B.-A.; Hsieh, C.-C.; Hong, M.-Y.; Lee, C.-H.; Ko, W.-C. The Hypotension Period after Initiation of Appropriate Antimicrobial Administration Is Crucial for Survival of Bacteremia Patients Initially Experiencing Severe Sepsis and Septic Shock. J. Clin. Med. 2020, 9, 2617. https://doi.org/10.3390/jcm9082617
Lee C-C, Yang C-Y, Su B-A, Hsieh C-C, Hong M-Y, Lee C-H, Ko W-C. The Hypotension Period after Initiation of Appropriate Antimicrobial Administration Is Crucial for Survival of Bacteremia Patients Initially Experiencing Severe Sepsis and Septic Shock. Journal of Clinical Medicine. 2020; 9(8):2617. https://doi.org/10.3390/jcm9082617
Chicago/Turabian StyleLee, Ching-Chi, Chao-Yung Yang, Bo-An Su, Chih-Chia Hsieh, Ming-Yuan Hong, Chung-Hsun Lee, and Wen-Chien Ko. 2020. "The Hypotension Period after Initiation of Appropriate Antimicrobial Administration Is Crucial for Survival of Bacteremia Patients Initially Experiencing Severe Sepsis and Septic Shock" Journal of Clinical Medicine 9, no. 8: 2617. https://doi.org/10.3390/jcm9082617
APA StyleLee, C.-C., Yang, C.-Y., Su, B.-A., Hsieh, C.-C., Hong, M.-Y., Lee, C.-H., & Ko, W.-C. (2020). The Hypotension Period after Initiation of Appropriate Antimicrobial Administration Is Crucial for Survival of Bacteremia Patients Initially Experiencing Severe Sepsis and Septic Shock. Journal of Clinical Medicine, 9(8), 2617. https://doi.org/10.3390/jcm9082617






