Limited Mandibular Movements as a Consequence of Unilateral or Asymmetrical Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis Patients
Abstract
1. Introduction
1.1. Background
1.2. The Aim of the Study
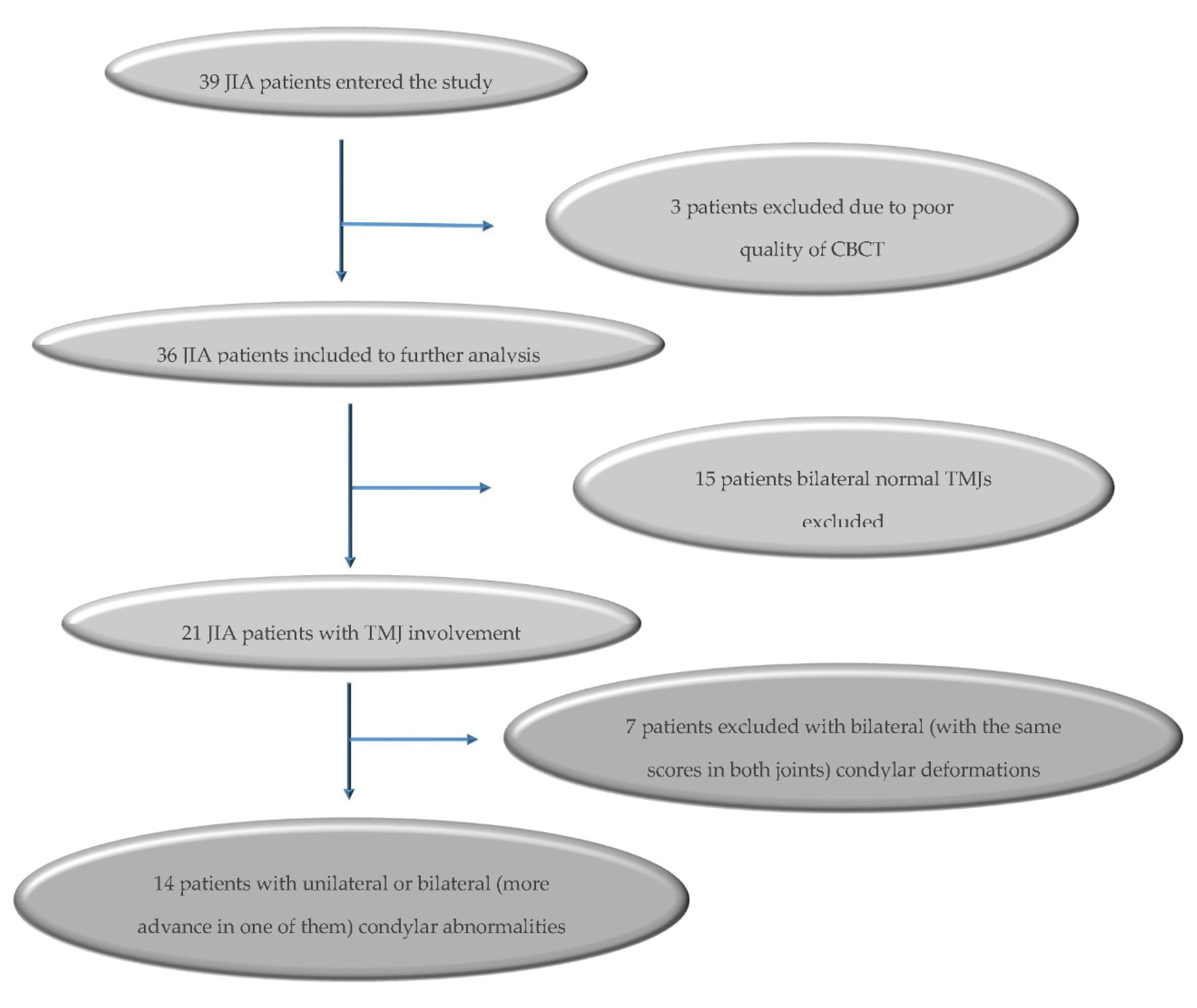
2. Material and Methods
2.1. Range of MMO
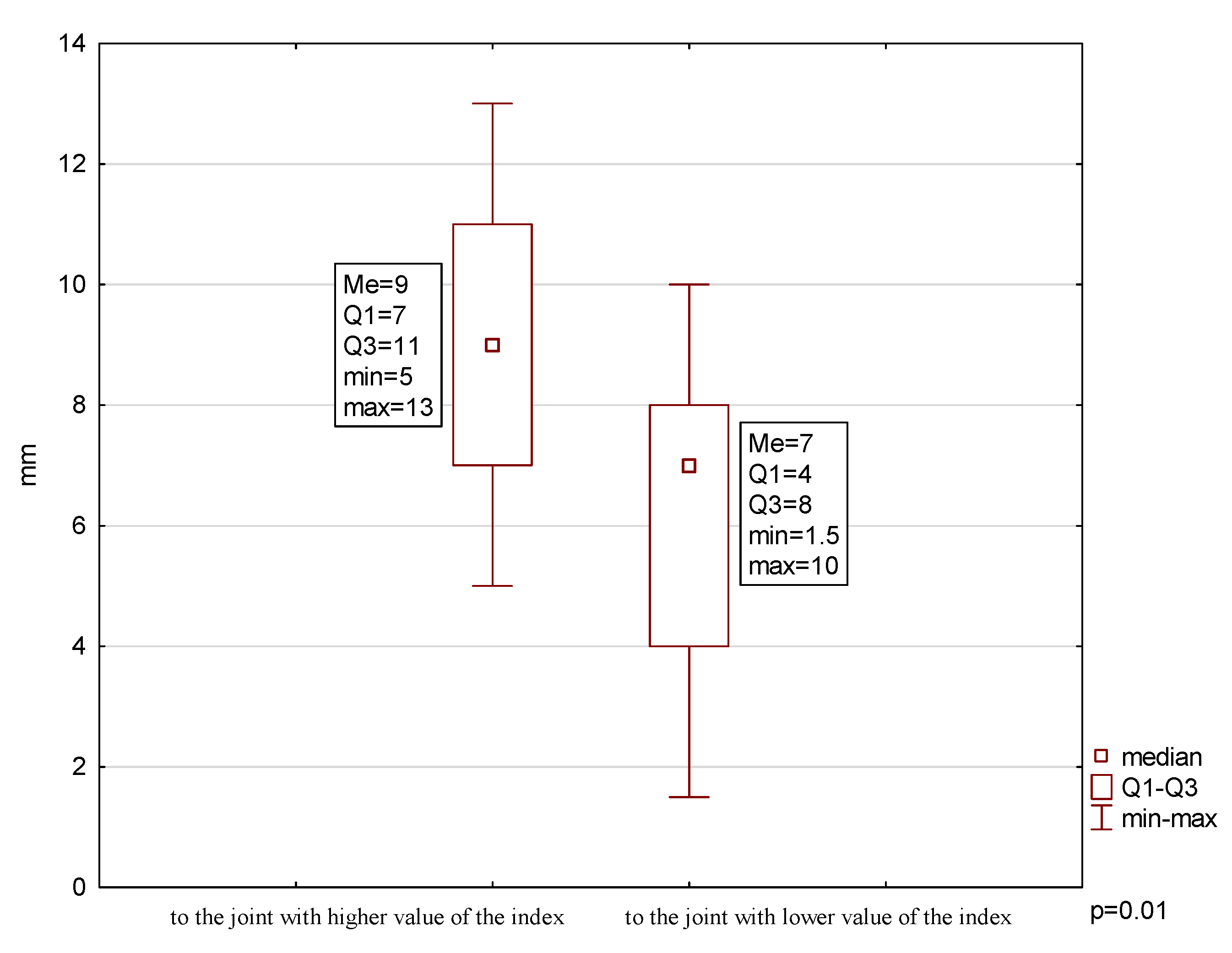
2.2. Mandibular Lateral Deviation During MMO
2.3. Range of Lateral Movements
2.4. Maximum Mandible Protrusion
2.5. Facial Asymmetry
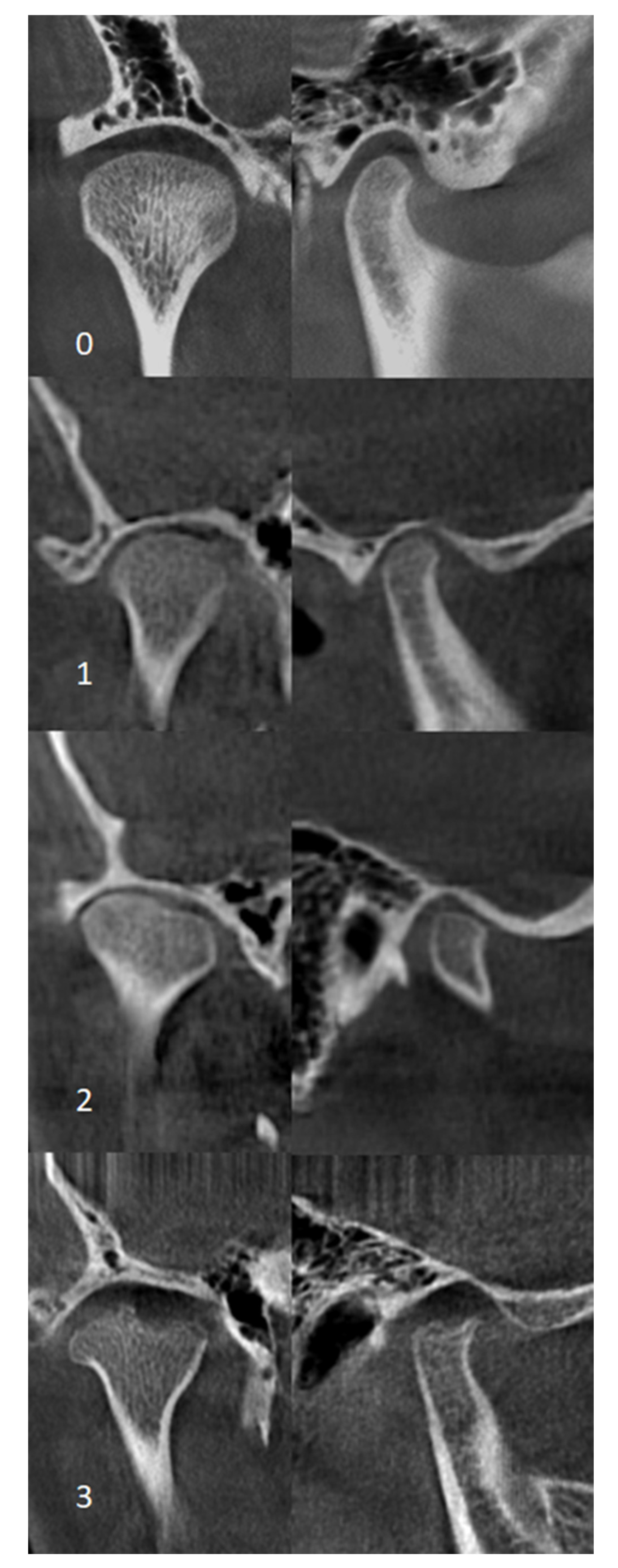
2.6. CBCT Examination
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- (1)
- In JIA children, unilateral or bilateral (with one of the two sides, TMJ is affected more severely concerning the other one) TMJ involvement, the asymmetry of the lower face, deviation of the mandible on maximum mouth opening, and reduced range of mandibular lateral movements deserve particular attention. These patients should be under control for dentofacial assessment development and TMJ function.
- (2)
- The obtained results do not show a relationship between the degree of condylar process deformity and the presence of asymmetry of the lower face and the presence and degree of mandibular motor dysfunction.
- (3)
- In JIA patients with severe condylar process destruction, there are no significant detectable clinically abnormalities; and facial asymmetry and/or impaired jaw movement may be noted with even slight damage to the condylar process.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.M.; et al. International League of Associations for Rheumatology classification of JIA second revision, Edmonton 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar] [PubMed]
- Martini, G.; Bacciliero, U.; Tregnaghi, A.; Montesco, M.C.; Zulian, F. Isolated temporomandibular synovitis as unique presentation of juvenile idiopathic arthritis. J. Rheumatol. 2001, 28, 1689–1692. [Google Scholar] [PubMed]
- Scolozzi, P.; Bosson, G.; Jaques, B. Severe isolated temporomandibular joint involvement in juvenile idiopathic arthritis. J. Oral Maxillofac. Surg. 2005, 63, 1368–1371. [Google Scholar] [CrossRef]
- Cedströmer, A.-L.; Andlin-Sobocki, A.; Berntson, L.; Hedenberg-Magnusson, B.; Dahlström, L. Temporomandibular signs, symptoms, joint alterations and disease activity in juvenile idiopathic arthritis—An observational study. Pediatr. Rheumatol. 2013, 11, 37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arabshahi, B.; Cron, R.Q. Temporomandibular joint arthritis in juvenile idiopathic arthritis: The forgotten joint. Curr. Opin. Rheumatol. 2006, 18, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Thilander, B.; Carlsson, G.E.; Ingervall, B. Postnatal development of the human temporomandibular joint I. A histological study. Acta Odontol. Scand. 1976, 34, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Stoustrup, P.; Resnick, C.M.; Pedersen, T.K.; Abramowicz, S.; Michelotti, A.; Küseler, A.; Verna, C.; Kellenberger, C.J.; Nordal, E.; Caserta, G.; et al. Standardizing terminology and assessment for orofacial conditions in juvenile idiopathic arthritis: International, multidisciplinary consensus-based recommendations. J. Rheumatol. 2019, 46, 518–522. [Google Scholar] [CrossRef]
- Twilt, M.; Schulten, A.J.M.; Nicolaas, P.; Dülger, A.; A Van Suijlekom-Smit, L.W. Facioskeletal changes in children with juvenile idiopathic arthritis. Ann. Rheum. Dis. 2006, 65, 823–825. [Google Scholar] [CrossRef]
- Stoll, M.L.; Kau, C.H.; Waite, P.D.; Cron, R. Temporomandibular joint arthritis in juvenile idiopathic arthritis, now what? Pediatr. Rheumatol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Keller, H.; Müller, L.M.; Markic, G.; Schraner, T.; Kellenberger, C.J.; Saurenmann, R.K. Is early TMJ involvement in children with juvenile idiopathic arthritis clinically detectable? Clinical examination of the TMJ in comparison with contrast enhanced MRI in patients with juvenile idiopathic arthritis. Pediatr. Rheumatol. 2015, 13, 1–10. [Google Scholar] [CrossRef][Green Version]
- Koos, B.; Twilt, M.; Kyank, U.; Fischer-Brandies, H.; Gaßling, V.; Tzaribachev, N. Reliability of clinical symptoms in diagnosing temporomandibular joint arthritis in juvenile idiopathic arthritis. J. Rheumatol. 2014, 41, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Billiau, A.D.; Verdonck, A.; Wouters, C.; Carels, C. Variation in dentofacial morphology and occlusion in juvenile idiopathic arthritis subjects: A case-control study. Eur. J. Orthod. 2009, 31, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ringold, S.; Thapa, M.; Shaw, E.A.; Wallace, C. Heterotopic ossification of the temporomandibular joint in juvenile idiopathic arthritis. J. Rheumatol. 2011, 38, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Weiss, P.F.; Arabshahi, B.; Johnson, A.; Bilaniuk, L.T.; Zarnow, D.; Cahill, A.M.; Feudtner, C.; Cron, R. High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis, as detected by magnetic resonance imaging but not by ultrasound. Arthritis Rheum. 2008, 58, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Huntjens, E.; Kiss, G.; Wouters, C.; Carels, C.E.L. Condylar asymmetry in children with juvenile idiopathic arthritis assessed by cone-beam computed tomography. Eur. J. Orthod. 2008, 30, 545–551. [Google Scholar] [CrossRef]
- Abramowicz, S.; Susarla, H.K.; Kim, S.; Kaban, L.B. Physical findings associated with active temporomandibular joint inflammation in children with juvenile idiopathic arthritis. J. Oral Maxillofac. Surg. 2013, 71, 1683–1687. [Google Scholar] [CrossRef]
- Arvidsson, L.Z.; Flatø, B.; Larheim, T.A. Radiographic TMJ abnormalities in patients with juvenile idiopathic arthritis followed for 27 years. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 114–123. [Google Scholar] [CrossRef]
- Pedersen, T.K.; Jensen, J.J.; Melsen, B.; Herlin, T. Resorption of the temporomandibular condylar bone according to subtypes of juvenile chronic arthritis. J. Rheumatol. 2001, 28, 2109–2115. [Google Scholar]
- Pawlaczyk-Kamieńska, T.; Pawlaczyk-Wróblewska, E.; Borysewicz-Lewicka, M. Early diagnosis of temporomandibular joint arthritis in children with juvenile idiopathic arthritis—Systematic review. Eur. J. Paediatr. Dent 2020, 21. accepted for publication. [Google Scholar]
- Stoustrup, P.; Pedersen, T.K.; Nørholt, S.E.; Resnick, C.M.; Abramowicz, S. Interdisciplinary management of dentofacial deformity in juvenile idiopathic arthritis. Oral Maxillofac. Surg. Clin. N. Am. 2020, 32, 117–134. [Google Scholar] [CrossRef]
- Billiau, A.D.; Hu, Y.; Verdonck, A.; Carels, C.E.L.; Wouters, C. Temporomandibular joint arthritis in juvenile idiopathic arthritis: Prevalence, clinical and radiological signs, and relation to dentofacial morphology. J. Rheumatol. 2007, 34, 1925–1933. [Google Scholar] [PubMed]
- Farkas, L.G.; Posnick, J.C.; Hreczko, T. Anthropometry of the head and face in 95 Down syndrome patients. Prog. Clin. Boil. Res. 1991, 373, 53–97. [Google Scholar]
- Müller, L.; Van Waes, H.; Langerweger, C.; Molinari, L.; Saurenmann, R.K. Maximal mouth opening capacity: Percentiles for healthy children 4–17 years of age. Pediatr. Rheumatol. 2013, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Helkimo, M. Studies on function and dysfunction of the masticatory system. II. Index for anamnestic and clinical dysfunction and occlusal state. Sven. Tandlak. Tidskr. Swed. Dent. J. 1974, 67, 101–121. [Google Scholar]
- Stoustrup, P.; Ahlefeldt-Laurvig-Lehn, N.; Kristensen, K.D.; Arvidsson, L.Z.; Twilt, M.; Cattaneo, P.M.; Küseler, A.; Christensen, A.E.; Herlin, T.; Pedersen, T.K. No association between types of unilateral mandibular condylar abnormalities and facial asymmetry in orthopedic-treated patients with juvenile idiopathic arthritis. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Twilt, M.; Schulten, A.J.M.; Verschure, F.; Wisse, L.; Prahl-Andersen, B.; Van Suijlekom-Smit, L.W.A. Long-term followup of temporomandibular joint involvement in juvenile idiopathic arthritis. Arthritis Rheum. 2008, 59, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Maspero, C.; Farronato, M.; Bellincioni, F.; Cavagnetto, D.; Abate, A. Assessing mandibular body changes in growing subjects: A comparison of CBCT and reconstructed lateral cephalogram measurements. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Kuseler, A.; Pedersen, T.K.; Gelineck, J.; Herlin, T. A 2 year follow up study of enhanced magnetic resonance imaging and clinical examination of the temporomadibular joint in children with juvenile idiopathic arthritis. J. Reumatol. 2005, 32, 162–169. [Google Scholar]
- Portelli, M.; Gatto, E.; Matarese, G.; Militi, A.; Catalfamo, L.; Gherlone, E.; Lucchese, A. Unilateral condylar hyperplasia: Diagnosis, clinical aspects and operative treatment. A case report. Eur. J. Paediatr. Dent. 2015, 16, 99–102. [Google Scholar]
- Meyers, A.B.; Laor, T. Magnetic resonance imaging of the temporomandibular joint in children with juvenile idiopathic arthritis. Pediatr. Radiol. 2013, 43, 1632–1641. [Google Scholar] [CrossRef]
- Fjeld, M.G.; Arvidsson, L.Z.; Stabrun, A.E.; Birkeland, K.; Larheim, T.A.; Øgaard, B. Average craniofacial development from 6 to 35 years of age in a mixed group of patients with juvenile idiopathic arthritis. Acta Odontol. Scand. 2009, 67, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Liukkonen, M.; Sillanmäki, L.; Peltomäki, T. Mandibular asymmetry in healthy children. Acta Odontol. Scand. 2005, 63, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.L.; Sharpe, T.; Beukelman, T.; Good, J.; Young, D.; Cron, R.Q. Risk factors for temporomandibular joint arthritis in children with juvenile idiopathic arthritis. J. Rheumatol. 2012, 39, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.; John, M.T.; Lautenschläger, C.; List, T. Mandibular jaw movement capacity in 10–17 yr-old children and adolescents: Normative values and the influence of gender, age, and temporomandibular disorders. Eur. J. Oral Sci. 2006, 114, 465–470. [Google Scholar] [CrossRef]
- Steinmassl, O.; Steinmassl, P.-A.; Schwarz, A.; Crismani, A. Dental age is more appropriate than chronological age for evaluating the mandibular movement range in children. J. Cranio Maxillofac. Surg. 2017, 45, 1201–1204. [Google Scholar] [CrossRef]
- Müller, L.; Kellenberger, C.J.; Cannizzaro, E.; Ettlin, D.; Schraner, T.; Bolt, I.B.; Peltomäki, T.; Saurenmann, R.K. Early diagnosis of temporomandibular joint involvement in juvenile idiopathic arthritis: A pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology 2009, 48, 680–685. [Google Scholar] [CrossRef]
- Stoustrup, P.; Twilt, M.; Spiegel, L.; Kristensen, K.D.; Koos, B.; Pedersen, T.K.; Küseler, A.; Cron, R.Q.; Abramowicz, S.; Verna, C.; et al. Clinical orofacial examination in juvenile idiopathic arthritis: International consensus-based recommendations for monitoring patients in clinical practice and research studies. J. Rheumatol. 2017, 44, 326–333. [Google Scholar] [CrossRef]
- Ciancaglini, R.; Gherlone, E.F.; Radaelli, G. Association between loss of occlusal support and symptoms of functional disturbances of the masticatory system. J. Oral Rehabil. 1999, 26, 248–253. [Google Scholar] [CrossRef]
| Landmark | Description |
|---|---|
| Trichion | The point on the hairline in the midline of the forehead. |
| Ophyron | The point at the mid-plane of a line tangent to the upper limits of the eyebrows. |
| Pronasale | The most protruded point of the apex nose identified in a lateral view of the rest position of the head. |
| Subnasale | The point at which the nasal septum merges, in the mid-sagittal plane, with the upper lip. |
| Stomion | The median point of the mouth when the mouth is closed. |
| Gnathion | The lowest median landmark on the lower border of the mandible. |
| Incision superius | The incisal tip of the most labially placed maxillary incisor. |
| Incision inferius | The incisal tip of the most labially placed mandibular incisor. |
| Number of Patients | Mean ± SD (years) | Mix (years) | Max (years) | |||
|---|---|---|---|---|---|---|
| Age at Examination | 11.92 ± 4.01 | 7 | 17 | |||
| Age at Onset of the Disease | 8.31 ± 3.9 | 3 | 15 | |||
| JIA Duration at Time of Study Entry | 3.62 ± 1.76 | 1 | 8 | |||
| JIA Subcategories | polyarticular | 9 | ||||
| oligoarticular | 5 | |||||
| Medication | NSAIDs | 6 | ||||
| DMARDs | 5 | |||||
| biologic | 3 | |||||
| Facial Asymmetry | Range of Mandibular Movement | Mandibular Lateral Deviation on MMO | ||||
|---|---|---|---|---|---|---|
| MMO | Laterotrusion to the Joint with the Higher Value of the Index | Laterotrusion to the Joint with the Lower Value of the Index | Protrusion | |||
| % patients | 23.08 | ↓54.85 | ↓23.08 | ↓38.46 | ↓23.08 | 46.15 |
| Mean ± SD (mm) | - | 37 ± 9.1 | 9.00 ± 2.67 | 6.08 ± 2.71 | 4.00 ± 2.03 | - |
| Min (mm) | - | 20 | 5 | 1.5 | 1 | - |
| Max (mm) | - | 48 | 13 | 10 | 8 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlaczyk-Kamieńska, T.; Kulczyk, T.; Pawlaczyk-Wróblewska, E.; Borysewicz-Lewicka, M.; Niedziela, M. Limited Mandibular Movements as a Consequence of Unilateral or Asymmetrical Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis Patients. J. Clin. Med. 2020, 9, 2576. https://doi.org/10.3390/jcm9082576
Pawlaczyk-Kamieńska T, Kulczyk T, Pawlaczyk-Wróblewska E, Borysewicz-Lewicka M, Niedziela M. Limited Mandibular Movements as a Consequence of Unilateral or Asymmetrical Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis Patients. Journal of Clinical Medicine. 2020; 9(8):2576. https://doi.org/10.3390/jcm9082576
Chicago/Turabian StylePawlaczyk-Kamieńska, Tamara, Tomasz Kulczyk, Elżbieta Pawlaczyk-Wróblewska, Maria Borysewicz-Lewicka, and Marek Niedziela. 2020. "Limited Mandibular Movements as a Consequence of Unilateral or Asymmetrical Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis Patients" Journal of Clinical Medicine 9, no. 8: 2576. https://doi.org/10.3390/jcm9082576
APA StylePawlaczyk-Kamieńska, T., Kulczyk, T., Pawlaczyk-Wróblewska, E., Borysewicz-Lewicka, M., & Niedziela, M. (2020). Limited Mandibular Movements as a Consequence of Unilateral or Asymmetrical Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis Patients. Journal of Clinical Medicine, 9(8), 2576. https://doi.org/10.3390/jcm9082576





