Molecular Mechanisms of AKI in the Elderly: From Animal Models to Therapeutic Intervention
Abstract
1. Introduction
2. Epidemiology
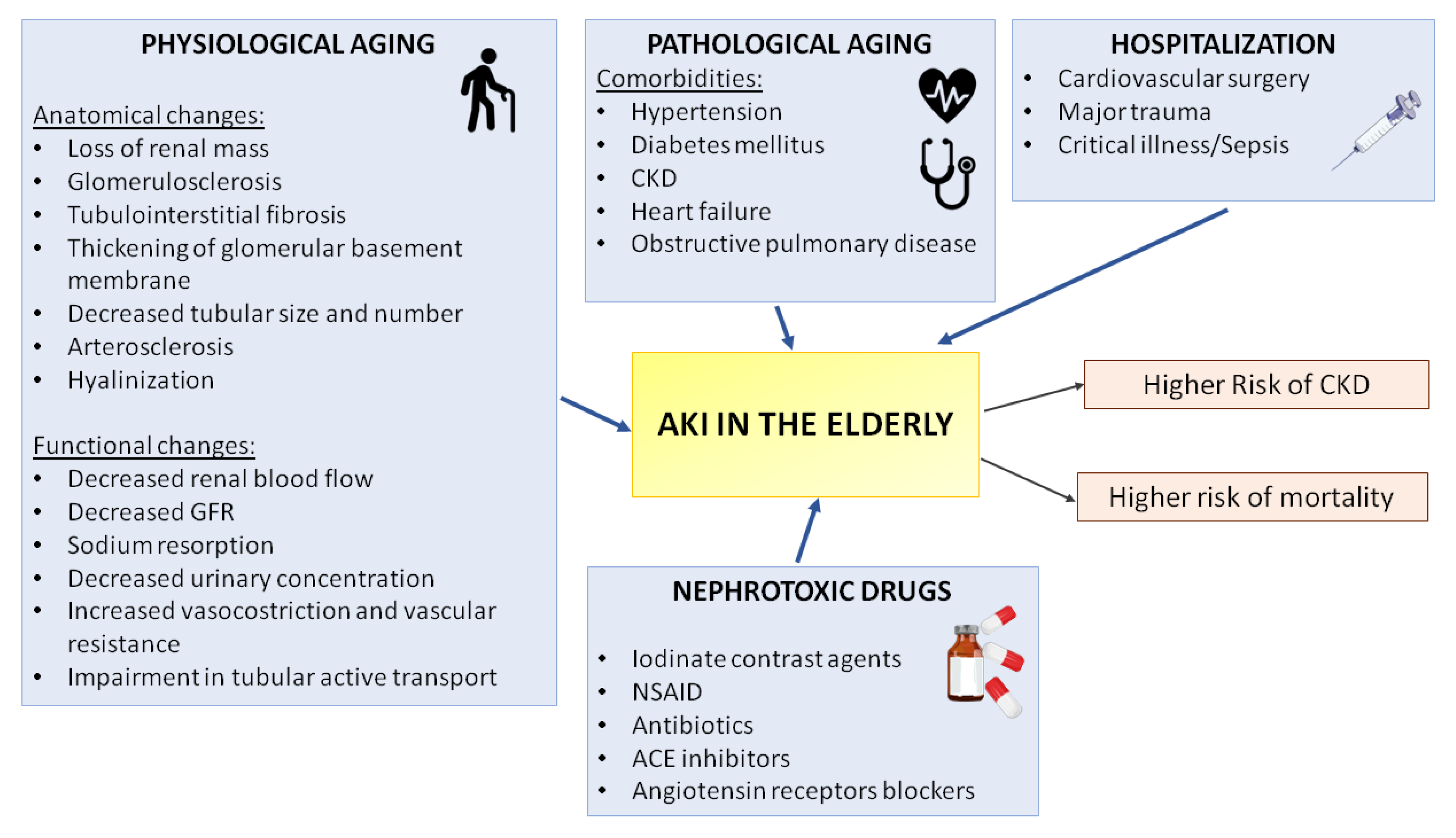
3. Pathophysiology of AKI in the Elderly
4. Risk Factors for AKI Development in the Elderly
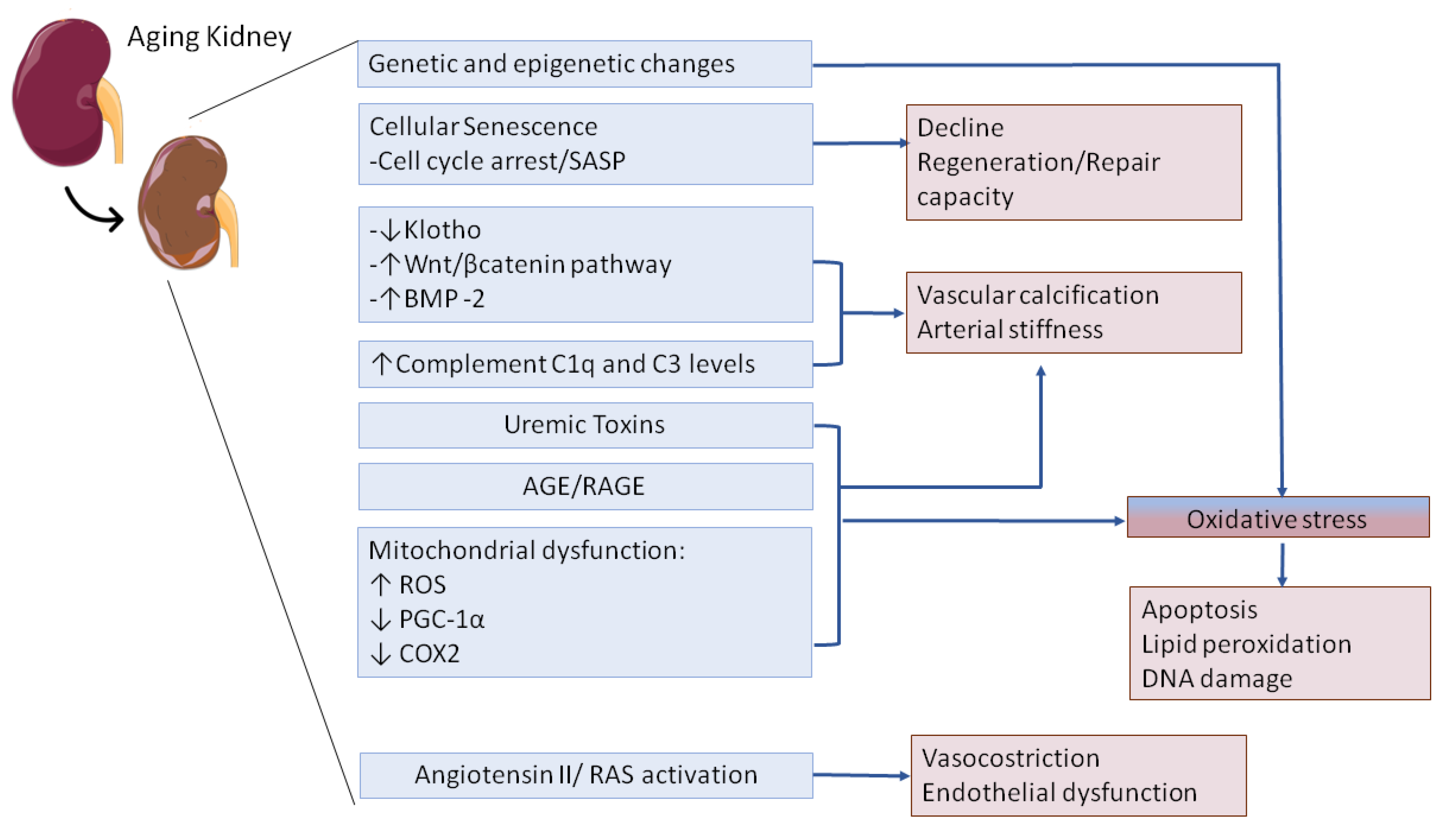
4.1. Kidney Aging
4.2. Comorbidities
4.3. Polypharmacy
4.4. Other Causes
5. Molecular Mechanisms of AKI in the Elderly: Lessons from Animal Models and Clinical Trial
5.1. Klotho Downregulation in the Elderly
5.2. Wnt/ β-Catenin Pathway Is Activated in the Elderly during AKI
5.3. Complement System in the Elderly
5.4. Oxidative Stress
6. The Diagnosis of AKI in Older Population
7. Prevention and Treatment of AKI in the Elderly
8. Outcome
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute Kidney Disease and Renal Recovery: Consensus Report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.; Hafeez, A.; Hou, S. Hospital-Acquired Renal Insufficiency. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2002, 39, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Chertow, G.M. Acute Renal Failure Definitions and Classification: Time for Change? J. Am. Soc. Nephrol. 2003, 14, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Feest, T.G.; Round, A.; Hamad, S. Incidence of Severe Acute Renal Failure in Adults: Results of a Community Based Study. BMJ 1993, 306, 481–483. [Google Scholar] [CrossRef]
- Lameire, N.; Van Biesen, W.; Vanholder, R. Acute Kidney Injury. Lancet 2008, 372, 1863–1865. [Google Scholar] [CrossRef]
- Lo, L.J.; Go, A.S.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordoñez, J.D.; Hsu, C. Dialysis-Requiring Acute Renal Failure Increases the Risk of Progressive Chronic Kidney Disease. Kidney Int. 2009, 76, 893–899. [Google Scholar] [CrossRef]
- Liangos, O.; Wald, R.; O’Bell, J.W.; Price, L.; Pereira, B.J.; Jaber, B.L. Epidemiology and Outcomes of Acute Renal Failure in Hospitalized Patients: A National Survey. Clin. J. Am. Soc. Nephrol. 2006, 1, 43–51. [Google Scholar] [CrossRef]
- Oweis, A.O.; Alshelleh, S.A. Incidence and Outcomes of Acute Kidney Injury in Octogenarians in Jordan. BMC Res. Notes 2018, 11, 279. [Google Scholar] [CrossRef]
- Yao, H.K.; Omer Binan, A.Y.; Konan, S.-D.; N’Da, K.J.; Diopoh, S.P. Mortality in the Elderly with Acute Kidney Injury in an Internal Medicine Department in Abidjan, Cote D’Ivoire. Saudi J. Kidney Dis. Transplant. 2018, 29, 414–421. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N. Diagnosis, Evaluation, and Management of Acute Kidney Injury: A KDIGO Summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
- Hsu, R.K.; McCulloch, C.E.; Dudley, R.A.; Lo, L.J.; Hsu, C. Temporal Changes in Incidence of Dialysis-Requiring AKI. J. Am. Soc. Nephrol. 2013, 24, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Koza, Y. Acute Kidney Injury: Current Concepts and New Insights. J. Inj. Violence Res. 2016, 8, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.-P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.G.; Mahanani, W.R.; et al. The World Report on Ageing and Health: A Policy Framework for Healthy Ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- Wolff, J.L.; Starfield, B.; Anderson, G. Prevalence, Expenditures, and Complications of Multiple Chronic Conditions in the Elderly. Arch. Intern. Med. 2002, 162, 2269–2276. [Google Scholar] [CrossRef]
- Selmi, Y.; Ariba, Y.B.; Labidi, J. Epidemiology, Diagnosis, and Etiology of Acute Kidney Injury in the Elderly: A Retrospective Analysis. Saudi J. Kidney Dis. Transplant. 2019, 30, 678–685. [Google Scholar] [CrossRef]
- Jiesisibieke, Z.L.; Tung, T.-H.; Xu, Q.-Y.; Chen, P.-E.; Hsu, S.-Y.; Liu, Y.; Chien, C.-W. Association of Acute Kidney Injury with Frailty in Elderly Population: A Systematic Review and Meta-Analysis. Ren. Fail. 2019, 41, 1021–1027. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, M.; Wang, X. AKI in the Very Elderly Patients without Preexisting Chronic Kidney Disease: A Comparison of 48-Hour Window and 7-Day Window for Diagnosing AKI Using the KDIGO Criteria. Clin. Interv. Aging 2018, 13, 1151–1160. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute Renal Failure in Critically Ill Patients: A Multinational, Multicenter Study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Pascual, J.; Liaño, F.; Ortuño, J. The Elderly Patient with Acute Renal Failure. J. Am. Soc. Nephrol. 1995, 6, 144–153. [Google Scholar]
- Rosner, M.H.; La Manna, G.; Ronco, C. Acute Kidney Injury in the Geriatric Population. Contrib. Nephrol. 2018, 193, 149–160. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Rosner, M.H.; Cruz, D.N.; Ronco, C. Acute Kidney Injury in Elderly Intensive Care Patients: A Review. Intensive Care Med. 2010, 36, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.M.M.; Cassiani, S.H.D.B. Adverse Drug Events in an Intensive Care Unit of a University Hospital. Eur. J. Clin. Pharmacol. 2011, 67, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.; Politano, P.; Marazzi, F.; Tamagnone, M.; Serra, I.; Marengo, M.; Falconi, D.; Gherzi, M.; Tattoli, F.; Bottaro, C.; et al. Acute Kidney Injury and Chronic Kidney Disease in the Elderly and Polypharmacy. Blood Purif. 2018, 46, 332–336. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef]
- Angus, D.C.; Barnato, A.E.; Linde-Zwirble, W.T.; Weissfeld, L.A.; Watson, R.S.; Rickert, T.; Rubenfeld, G.D. Use of Intensive Care at the End of Life in the United States: An Epidemiologic Study. Crit. Care Med. 2004, 32, 638–643. [Google Scholar] [CrossRef]
- Dos Santos, E.R. RIFLE: Association with Mortality and Length of Stay in Critically Ill Acute Kidney Injury Patients. Rev. Bras. Ter. Intensiva 2009, 21, 359–368. [Google Scholar]
- Joannidis, M.; Metnitz, B.; Bauer, P.; Schusterschitz, N.; Moreno, R.; Druml, W.; Metnitz, P.G.H. Acute Kidney Injury in Critically Ill Patients Classified by AKIN versus RIFLE Using the SAPS 3 Database. Intensive Care Med. 2009, 35, 1692–1702. [Google Scholar] [CrossRef]
- Groeneveld, A.B.; Tran, D.D.; van der Meulen, J.; Nauta, J.J.; Thijs, L.G. Acute Renal Failure in the Medical Intensive Care Unit: Predisposing, Complicating Factors and Outcome. Nephron 1991, 59, 602–610. [Google Scholar] [CrossRef]
- Ali, T.; Khan, I.; Simpson, W.; Prescott, G.; Townend, J.; Smith, W.; Macleod, A. Incidence and Outcomes in Acute Kidney Injury: A Comprehensive Population-Based Study. J. Am. Soc. Nephrol. 2007, 18, 1292–1298. [Google Scholar] [CrossRef]
- Rosner, M.H. The Pathogenesis of Susceptibility to Acute Kidney Injury in the Elderly. Curr. Aging Sci. 2009, 2, 158–164. [Google Scholar] [CrossRef]
- Long, D.A.; Mu, W.; Price, K.L.; Johnson, R.J. Blood Vessels and the Aging Kidney. Nephron. Exp. Nephrol. 2005, 101, e95–e99. [Google Scholar] [CrossRef] [PubMed]
- Zappitelli, M. Epidemiology and Diagnosis of Acute Kidney Injury. Semin. Nephrol. 2008, 28, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerda, J.; Chawla, L.S. Global Epidemiology and Outcomes of Acute Kidney Injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Yokota, L.G.; Sampaio, B.M.; Rocha, E.P.; Balbi, A.L.; Sousa Prado, I.R.; Ponce, D. Acute Kidney Injury in Elderly Patients: Narrative Review on Incidence, Risk Factors, and Mortality. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 217–224. [Google Scholar] [CrossRef]
- Raman, M.; Middleton, R.J.; Kalra, P.A.; Green, D. Estimating Renal Function in Old People: An in-Depth Review. Int. Urol. Nephrol. 2017, 49, 1979–1988. [Google Scholar] [CrossRef]
- Fliser, D.; Zeier, M.; Nowack, R.; Ritz, E. Renal Functional Reserve in Healthy Elderly Subjects. J. Am. Soc. Nephrol. 1993, 3, 1371–1377. [Google Scholar]
- Chronopoulos, A.; Cruz, D.N.; Ronco, C. Hospital-Acquired Acute Kidney Injury in the Elderly. Nat. Rev. Nephrol. 2010, 6, 141–149. [Google Scholar] [CrossRef]
- Funk, I.; Seibert, E.; Markau, S.; Girndt, M. Clinical Course of Acute Kidney Injury in Elderly Individuals Above 80 Years. Kidney Blood Press. Res. 2016, 41, 947–955. [Google Scholar] [CrossRef]
- Petronijevic, Z.; Selim, G.; Petkovska, L.; Georgievska-Ismail, L.; Spasovski, G.; Tozija, L. The Effect of Treatment on Short-Term Outcomes in Elderly Patients with Acute Kidney Injury. Open Access Maced. J. Med. Sci. 2017, 5, 635–640. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Bunn, D.; Copley, M.; Cowap, V.; Dickinson, A.; Gray, L.; Howe, A.; Killett, A.; Lee, J.; Li, F.; et al. Effectiveness of Interventions to Directly Support Food and Drink Intake in People with Dementia: Systematic Review and Meta-Analysis. BMC Geriatr. 2016, 16, 26. [Google Scholar] [CrossRef]
- Flynn, E.; Smith, C.H.; Walsh, C.D.; Walshe, M. Modifying the Consistency of Food and Fluids for Swallowing Difficulties in Dementia. Cochrane Database Syst. Rev. 2018, 9, CD011077. [Google Scholar] [CrossRef] [PubMed]
- da Silveira Santos, C.G.; Romani, R.F.; Benvenutti, R.; Ribas Zahdi, J.O.; Riella, M.C.; Mazza do Nascimento, M. Acute Kidney Injury in Elderly Population: A Prospective Observational Study. Nephron 2018, 138, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Aucella, F.; Netti, G.S.; Piemontese, M.; Cincione, I.R.; Infante, B.; Gesualdo, L. Proteinuria in the Prognosis of IgA Nephropathy. Minerva Urol. Nefrol. 2009, 61, 235–248. [Google Scholar] [PubMed]
- Mooradian, A.D. Evidence-Based Management of Diabetes in Older Adults. Drugs Aging 2018, 35, 1065–1078. [Google Scholar] [CrossRef]
- Shah, B.R.; Hux, J.E. Quantifying the Risk of Infectious Diseases for People with Diabetes. Diabetes Care 2003, 26, 510–513. [Google Scholar] [CrossRef]
- Mittalhenkle, A.; Stehman-Breen, C.O.; Shlipak, M.G.; Fried, L.F.; Katz, R.; Young, B.A.; Seliger, S.; Gillen, D.; Newman, A.B.; Psaty, B.M.; et al. Cardiovascular Risk Factors and Incident Acute Renal Failure in Older Adults: The Cardiovascular Health Study. Clin. J. Am. Soc. Nephrol. 2008, 3, 450–456. [Google Scholar] [CrossRef]
- Patschan, D.; Müller, G.A. Acute Kidney Injury in Diabetes Mellitus. Int. J. Nephrol. 2016, 2016, 6232909. [Google Scholar] [CrossRef]
- Davison, B.A.; Metra, M.; Cotter, G.; Massie, B.M.; Cleland, J.G.F.; Dittrich, H.C.; Edwards, C.; Filippatos, G.; Givertz, M.M.; Greenberg, B.; et al. Worsening Heart Failure Following Admission for Acute Heart Failure: A Pooled Analysis of the PROTECT and RELAX-AHF Studies. JACC Heart Fail. 2015, 3, 395–403. [Google Scholar] [CrossRef]
- Henrich, W.L. Dialysis Considerations in the Elderly Patient. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1990, 16, 339–341. [Google Scholar] [CrossRef]
- Hu, W.; He, W.; Liu, W.; Fang, X.; Wu, Y.; Yu, F.; Hao, W. Risk Factors and Prognosis of Cardiorenal Syndrome Type 1 in Elderly Chinese Patients: A Retrospective Observational Cohort Study. Kidney Blood Press. Res. 2016, 41, 672–679. [Google Scholar] [CrossRef]
- Vandenberghe, W.; Gevaert, S.; Kellum, J.A.; Bagshaw, S.M.; Peperstraete, H.; Herck, I.; Decruyenaere, J.; Hoste, E.A.J. Acute Kidney Injury in Cardiorenal Syndrome Type 1 Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2016, 6, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Uchiyama, S.; Sawatani, T.; Asai, K.; Shimizu, W. Worsening Renal Failure in Patients with Acute Heart Failure: The Importance of Cardiac Biomarkers. ESC Heart Fail. 2019, 6, 416–427. [Google Scholar] [CrossRef]
- Aymanns, C.; Keller, F.; Maus, S.; Hartmann, B.; Czock, D. Review on Pharmacokinetics and Pharmacodynamics and the Aging Kidney. Clin. J. Am. Soc. Nephrol. 2010, 5, 314–327. [Google Scholar] [CrossRef]
- Pierson-Marchandise, M.; Gras, V.; Moragny, J.; Micallef, J.; Gaboriau, L.; Picard, S.; Choukroun, G.; Masmoudi, K.; Liabeuf, S. The Drugs That Mostly Frequently Induce Acute Kidney Injury: A Case - Noncase Study of a Pharmacovigilance Database. Br. J. Clin. Pharmacol. 2017, 83, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.R.; Yared, A.; Ray, W.A. Nonsteroidal Antiinflammatory Drugs and Acute Renal Failure in Elderly Persons. Am. J. Epidemiol. 2000, 151, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Tsai, H.-B.; Wu, C.-Y.; Lin, Y.-F.; Hsu, N.-C.; Chen, J.-S.; Hung, K.-Y. Cumulative Cardiovascular Polypharmacy Is Associated With the Risk of Acute Kidney Injury in Elderly Patients. Medicine 2015, 94, e1251. [Google Scholar] [CrossRef]
- Mallappallil, M.; Sabu, J.; Friedman, E.A.; Salifu, M. What Do We Know about Opioids and the Kidney? Int. J. Mol. Sci. 2017, 18, 223. [Google Scholar] [CrossRef]
- Chau, D.L.; Walker, V.; Pai, L.; Cho, L.M. Opiates and Elderly: Use and Side Effects. Clin. Interv. Aging 2008, 3, 273–278. [Google Scholar] [CrossRef]
- Solomon, R.; Dauerman, H.L. Contrast-Induced Acute Kidney Injury. Circulation 2010, 122, 2451–2455. [Google Scholar] [CrossRef]
- Heyman, S.N.; Rosenberger, C.; Rosen, S. Regional Alterations in Renal Haemodynamics and Oxygenation: A Role in Contrast Medium-Induced Nephropathy. Nephrol. Dial. Transplant. 2005, 20 (Suppl. S1), i6–i11. [Google Scholar] [CrossRef]
- Whitehouse, T.; Stotz, M.; Taylor, V.; Stidwill, R.; Singer, M. Tissue Oxygen and Hemodynamics in Renal Medulla, Cortex, and Corticomedullary Junction during Hemorrhage-Reperfusion. Am. J. Physiol. Renal Physiol. 2006, 291, F647–F653. [Google Scholar] [CrossRef]
- Haq, M.F.U.; Yip, C.S.; Arora, P. The Conundrum of Contrast-Induced Acute Kidney Injury. J. Thorac. Dis. 2020, 12, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-H.; Lee, Y.-J.; Chao, P.-W.; Kuo, S.-C.; Ou, S.-M.; Huang, H.-M.; Chen, Y.-T. Association between Influenza Vaccination and the Reduced Risk of Acute Kidney Injury among Older People: A Nested Case-Control Study. Eur. J. Intern. Med. 2018, 54, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, M.; Pesce, F.; Schena, A.; Simone, S.; Castellano, G.; Gesualdo, L. Updates on Urinary Tract Infections in Kidney Transplantation. J. Nephrol. 2019, 32, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Fani, F.; Regolisti, G.; Delsante, M.; Cantaluppi, V.; Castellano, G.; Gesualdo, L.; Villa, G.; Fiaccadori, E. Recent Advances in the Pathogenetic Mechanisms of Sepsis-Associated Acute Kidney Injury. J. Nephrol. 2018, 31, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute Kidney Injury from Sepsis: Current Concepts, Epidemiology, Pathophysiology, Prevention and Treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Netti, G.S.; Sangregorio, F.; Spadaccino, F.; Staffieri, F.; Crovace, A.; Infante, B.; Maiorano, A.; Godeas, G.; Castellano, G.; Di Palma, A.M.; et al. LPS Removal Reduces CD80-Mediated Albuminuria in Critically Ill Patients with Gram-Negative Sepsis. Am. J. Physiol. Renal. Physiol. 2019, 316, F723–F731. [Google Scholar] [CrossRef]
- Fanelli, V.; Fiorentino, M.; Cantaluppi, V.; Gesualdo, L.; Stallone, G.; Ronco, C.; Castellano, G. Acute Kidney Injury in SARS-CoV-2 Infected Patients. Crit. Care 2020, 24, 155. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic Acute Kidney Injury in Critically Ill Patients: Clinical Characteristics and Outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 431–439. [Google Scholar] [CrossRef]
- Castellano, G.; Franzin, R.; Sallustio, F.; Stasi, A.; Banelli, B.; Romani, M.; De Palma, G.; Lucarelli, G.; Divella, C.; Battaglia, M.; et al. Complement Component C5a Induces Aberrant Epigenetic Modifications in Renal Tubular Epithelial Cells Accelerating Senescence by Wnt4/Betacatenin Signaling after Ischemia/Reperfusion Injury. Aging 2019, 11, 4382–4406. [Google Scholar] [CrossRef]
- Valentijn, F.A.; Falke, L.L.; Nguyen, T.Q.; Goldschmeding, R. Cellular Senescence in the Aging and Diseased Kidney. J. Cell Commun. Signal. 2018, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Maddens, B.; Vandendriessche, B.; Demon, D.; Vanholder, R.; Chiers, K.; Cauwels, A.; Meyer, E. Severity of Sepsis-Induced Acute Kidney Injury in a Novel Mouse Model Is Age Dependent. Crit. Care Med. 2012, 40, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.E.; Chaber, C.J.; Ledbetter, S.R.; Zuk, A. Increased Cellular Senescence and Vascular Rarefaction Exacerbate the Progression of Kidney Fibrosis in Aged Mice Following Transient Ischemic Injury. PLoS ONE 2013, 8, e70464. [Google Scholar] [CrossRef] [PubMed]
- Simone, S.; Rascio, F.; Castellano, G.; Divella, C.; Chieti, A.; Ditonno, P.; Battaglia, M.; Crovace, A.; Staffieri, F.; Oortwijn, B.; et al. Complement-Dependent NADPH Oxidase Enzyme Activation in Renal Ischemia/Reperfusion Injury. Free Radic. Biol. Med. 2014, 74, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. The Klotho Proteins in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho as a Regulator of Oxidative Stress and Senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef]
- Yang, H.; Fogo, A.B. Cell Senescence in the Aging Kidney. J. Am. Soc. Nephrol. 2010, 21, 1436–1439. [Google Scholar] [CrossRef]
- Dalton, G.D.; Xie, J.; An, S.-W.; Huang, C.-L. New Insights into the Mechanism of Action of Soluble Klotho. Front. Endocrinol. 2017, 8, 323. [Google Scholar] [CrossRef]
- Hu, M.-C.; Shi, M.; Zhang, J.; Quinones, H.; Kuro-o, M.; Moe, O.W. Klotho Deficiency Is an Early Biomarker of Renal Ischemia-Reperfusion Injury and Its Replacement Is Protective. Kidney Int. 2010, 78, 1240–1251. [Google Scholar] [CrossRef]
- Sugiura, H.; Yoshida, T.; Tsuchiya, K.; Mitobe, M.; Nishimura, S.; Shirota, S.; Akiba, T.; Nihei, H. Klotho Reduces Apoptosis in Experimental Ischaemic Acute Renal Failure. Nephrol. Dial. Transplant. 2005, 20, 2636–2645. [Google Scholar] [CrossRef]
- Castellano, G.; Intini, A.; Stasi, A.; Divella, C.; Gigante, M.; Pontrelli, P.; Franzin, R.; Accetturo, M.; Zito, A.; Fiorentino, M.; et al. Complement Modulation of Anti-Aging Factor Klotho in Ischemia/Reperfusion Injury and Delayed Graft Function. Am. J. Transplant 2016, 16, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Keles, N.; Caliskan, M.; Dogan, B.; Keles, N.N.; Kalcik, M.; Aksu, F.; Kostek, O.; Aung, S.M.; Isbilen, B.; Oguz, A. Low Serum Level of Klotho Is an Early Predictor of Atherosclerosis. Tohoku J. Exp. Med. 2015, 237, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, Z. Molecular Basis of Klotho: From Gene to Function in Aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.L.; Pastor, J.; Carranza, D.; Quinones, H.; Griffith, C.; Goetz, R.; Mohammadi, M.; Ye, J.; Zhang, J.; Hu, M.C.; et al. The Demonstration of AlphaKlotho Deficiency in Human Chronic Kidney Disease with a Novel Synthetic Antibody. Nephrol. Dial. Transplant. 2015, 30, 223–233. [Google Scholar] [CrossRef]
- Koh, N.; Fujimori, T.; Nishiguchi, S.; Tamori, A.; Shiomi, S.; Nakatani, T.; Sugimura, K.; Kishimoto, T.; Kinoshita, S.; Kuroki, T.; et al. Severely Reduced Production of Klotho in Human Chronic Renal Failure Kidney. Biochem. Biophys. Res. Commun. 2001, 280, 1015–1020. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho Deficiency Causes Vascular Calcification in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef]
- Wang, Q.; Su, W.; Shen, Z.; Wang, R. Correlation between Soluble α-Klotho and Renal Function in Patients with Chronic Kidney Disease: A Review and Meta-Analysis. Biomed Res. Int. 2018, 2018, 9481475. [Google Scholar] [CrossRef]
- Hage, V.; Pelletier, S.; Dubourg, L.; Drai, J.; Cuerq, C.; Lemoine, S.; Hadj-Aissa, A.; Laville, M.; Fouque, D. In Chronic Kidney Disease, Serum α-Klotho Is Related to Serum Bicarbonate and Proteinuria. J. Ren. Nutr. 2014, 24, 390–394. [Google Scholar] [CrossRef]
- Kim, H.R.; Nam, B.Y.; Kim, D.W.; Kang, M.W.; Han, J.-H.; Lee, M.J.; Shin, D.H.; Doh, F.M.; Koo, H.M.; Ko, K.I.; et al. Circulating α-Klotho Levels in CKD and Relationship to Progression. Am. J. Kidney Dis. 2013, 61, 899–909. [Google Scholar] [CrossRef]
- Scholze, A.; Liu, Y.; Pedersen, L.; Xia, S.; Roth, H.J.; Hocher, B.; Rasmussen, L.M.; Tepel, M. Soluble α-Klotho and Its Relation to Kidney Function and Fibroblast Growth Factor-23. J. Clin. Endocrinol. Metab. 2014, 99, E855–E861. [Google Scholar] [CrossRef][Green Version]
- Seiler, S.; Wen, M.; Roth, H.J.; Fehrenz, M.; Flügge, F.; Herath, E.; Weihrauch, A.; Fliser, D.; Heine, G.H. Plasma Klotho Is Not Related to Kidney Function and Does Not Predict Adverse Outcome in Patients with Chronic Kidney Disease. Kidney Int. 2013, 83, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Imura, A.; Urakawa, I.; Shimada, T.; Murakami, J.; Aono, Y.; Hasegawa, H.; Yamashita, T.; Nakatani, K.; Saito, Y.; et al. Establishment of Sandwich ELISA for Soluble Alpha-Klotho Measurement: Age-Dependent Change of Soluble Alpha-Klotho Levels in Healthy Subjects. Biochem. Biophys. Res. Commun. 2010, 398, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Neyra, J.A.; Hu, M.C. Potential Application of Klotho in Human Chronic Kidney Disease. Bone 2017, 100, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Kobori, H.; Inoue, T.; Miyazaki, T.; Suzuki, H.; Nishiyama, A.; Ishii, N.; Hayashi, M. Klotho Supplementation Ameliorates Blood Pressure and Renal Function in DBA/2-Pcy Mice, a Model of Polycystic Kidney Disease. Am. J. Physiol. Renal Physiol. 2020, 318, F557–F564. [Google Scholar] [CrossRef]
- Bian, A.; Neyra, J.A.; Zhan, M.; Hu, M.C. Klotho, Stem Cells, and Aging. Clin. Interv. Aging 2015, 10, 1233–1243. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.J.; Liu, Y. Wnt Signaling in Kidney Development and Disease. Prog. Mol. Biol. Transl. Sci. 2018, 153, 181–207. [Google Scholar] [CrossRef]
- Rong, S.; Zhao, X.; Jin, X.; Zhang, Z.; Chen, L.; Zhu, Y.; Yuan, W. Vascular Calcification in Chronic Kidney Disease Is Induced by Bone Morphogenetic Protein-2 via a Mechanism Involving the Wnt/Beta-Catenin Pathway. Cell. Physiol. Biochem. 2014, 34, 2049–2060. [Google Scholar] [CrossRef]
- Tan, R.J.; Zhou, D.; Zhou, L.; Liu, Y. Wnt/Beta-Catenin Signaling and Kidney Fibrosis. Kidney Int. Suppl. 2014, 4, 84–90. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, D.; Tan, R.J.; Fu, H.; Zhou, L.; Hou, F.F.; Liu, Y. Sustained Activation of Wnt/Beta-Catenin Signaling Drives AKI to CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1727–1740. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Teng, S.; Fu, X.; Zha, Y.; Liu, H.; Wang, L.; Tian, J.; Zhang, X.; Liu, Y.; et al. Urinary Matrix Metalloproteinase-7 Predicts Severe AKI and Poor Outcomes after Cardiac Surgery. J. Am. Soc. Nephrol. 2017, 28, 3373–3382. [Google Scholar] [CrossRef]
- Kamo, T.; Akazawa, H.; Komuro, I. Pleiotropic Effects of Angiotensin II Receptor Signaling in Cardiovascular Homeostasis and Aging. Int. Heart J. 2015, 56, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Kamo, T.; Akazawa, H.; Suzuki, J.-I.; Komuro, I. Roles of Renin-Angiotensin System and Wnt Pathway in Aging-Related Phenotypes. Inflamm. Regen. 2016, 36, 12. [Google Scholar] [CrossRef] [PubMed]
- Yabumoto, C.; Akazawa, H.; Yamamoto, R.; Yano, M.; Kudo-Sakamoto, Y.; Sumida, T.; Kamo, T.; Yagi, H.; Shimizu, Y.; Saga-Kamo, A.; et al. Angiotensin II Receptor Blockade Promotes Repair of Skeletal Muscle through Down-Regulation of Aging-Promoting C1q Expression. Sci. Rep. 2015, 5, 14453. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Liu, J.; Niu, J.; Zhang, Y.; Shen, W.; Luo, C.; Liu, Y.; Li, C.; Li, H.; Yang, P.; et al. Wnt/Beta-Catenin/RAS Signaling Mediates Age-Related Renal Fibrosis and Is Associated with Mitochondrial Dysfunction. Aging Cell 2019, 18, e13004. [Google Scholar] [CrossRef]
- Chaumont, M.; Pourcelet, A.; van Nuffelen, M.; Racapé, J.; Leeman, M.; Hougardy, J.-M. Acute Kidney Injury in Elderly Patients With Chronic Kidney Disease: Do Angiotensin-Converting Enzyme Inhibitors Carry a Risk? J. Clin. Hypertens. 2016, 18, 514–521. [Google Scholar] [CrossRef]
- Gravesen, E.; Nordholm, A.; Mace, M.; Morevati, M.; Høgdall, E.; Nielsen, C.; Kjær, A.; Olgaard, K.; Lewin, E. Effect of Inhibition of CBP-Coactivated β-Catenin-Mediated Wnt Signalling in Uremic Rats with Vascular Calcifications. PLoS ONE 2018, 13, e0201936. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Lambris, J.D. Complement in Disease: A Defence System Turning Offensive. Nat. Rev. Nephrol. 2016, 12, 383–401. [Google Scholar] [CrossRef]
- Franzin, R.; Stasi, A.; Fiorentino, M.; Stallone, G.; Cantaluppi, V.; Gesualdo, L.; Castellano, G. Inflammaging and Complement System: A Link Between Acute Kidney Injury and Chronic Graft Damage. Front. Immunol. 2020, 11, 734. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A Key System for Immune Surveillance and Homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Morgan, B.P. Complement in the Pathogenesis of Alzheimer’s Disease. Semin. Immunopathol. 2018, 40, 113–124. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Agodi, A. Complement System and Age-Related Macular Degeneration: Implications of Gene-Environment Interaction for Preventive and Personalized Medicine. Biomed Res. Int. 2018, 2018, 7532507. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.T.; Sumida, T.; Nomura, S.; Liu, M.-L.; Higo, T.; Nakagawa, A.; Okada, K.; Sakai, T.; Hashimoto, A.; Hara, Y.; et al. Complement C1q Activates Canonical Wnt Signaling and Promotes Aging-Related Phenotypes. Cell 2012, 149, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Yonemasu, K.; Kitajima, H.; Tanabe, S.; Ochi, T.; Shinkai, H. Effect of Age on C1q and C3 Levels in Human Serum and Their Presence in Colostrum. Immunology 1978, 35, 523–530. [Google Scholar]
- Gaya da Costa, M.; Poppelaars, F.; Berger, S.P.; Daha, M.R.; Seelen, M.A. The Lectin Pathway in Renal Disease: Old Concept and New Insights. Nephrol. Dial. Transplant. 2018, 33, 2073–2079. [Google Scholar] [CrossRef]
- Gaya da Costa, M.; Poppelaars, F.; van Kooten, C.; Mollnes, T.E.; Tedesco, F.; Würzner, R.; Trouw, L.A.; Truedsson, L.; Daha, M.R.; Roos, A.; et al. Age and Sex-Associated Changes of Complement Activity and Complement Levels in a Healthy Caucasian Population. Front. Immunol. 2018, 9, 2664. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Orandi, B.J.; Racusen, L.; Jackson, A.M.; Garonzik-Wang, J.M.; Shah, T.; Woodle, E.S.; Sommerer, C.; Fitts, D.; Rockich, K.; et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody-Mediated Rejection Following Kidney Transplantation: Results of a Randomized Double-Blind Placebo-Controlled Pilot Study. Am. J. Transplant. 2016, 16, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Tatapudi, V.S.; Montgomery, R.A. Pharmacologic Complement Inhibition in Clinical Transplantation. Curr. Transplant. Rep. 2017, 4, 91–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jordan, S.C.; Choi, J.; Aubert, O.; Haas, M.; Loupy, A.; Huang, E.; Peng, A.; Kim, I.; Louie, S.; Ammerman, N.; et al. A Phase I/II, Double-Blind, Placebo-Controlled Study Assessing Safety and Efficacy of C1 Esterase Inhibitor for Prevention of Delayed Graft Function in Deceased Donor Kidney Transplant Recipients. Am. J. Transplant. 2018, 18, 2955–2964. [Google Scholar] [CrossRef]
- Grenda, R.; Durlik, M. Eculizumab in Renal Transplantation: A 2017 Update. Ann. Transplant. 2017, 22, 550–554. [Google Scholar] [CrossRef]
- Vo, A.A.; Zeevi, A.; Choi, J.; Cisneros, K.; Toyoda, M.; Kahwaji, J.; Peng, A.; Villicana, R.; Puliyanda, D.; Reinsmoen, N.; et al. A Phase I/II Placebo-Controlled Trial of C1-Inhibitor for Prevention of Antibody-Mediated Rejection in HLA Sensitized Patients. Transplantation 2015, 99, 299–308. [Google Scholar] [CrossRef]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms-Biomarkers-Interventions, and Future Perspectives. Oxid. Med. Cell. Longev. 2017, 2017, 6193694. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Torreggiani, M.; Post, J.B.; Zheng, F.; Uribarri, J.; Striker, G.E. Role of Oxidants/Inflammation in Declining Renal Function in Chronic Kidney Disease and Normal Aging. Kidney Int. Suppl. 2009, 114, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Bennett, N.C.; Roy, S.; Gabrielli, B.G.; Johnson, D.W.; Gobe, G.C. Oxidative Stress and Cell Senescence Combine to Cause Maximal Renal Tubular Epithelial Cell Dysfunction and Loss in an in Vitro Model of Kidney Disease. Nephron. Exp. Nephrol. 2012, 122, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ralto, K.M.; Parikh, S.M. Mitochondria in Acute Kidney Injury. Semin. Nephrol. 2016, 36, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative Stress and Autophagy: Crucial Modulators of Kidney Injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial Energetics in the Kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Simone, S.; Loverre, A.; Cariello, M.; Divella, C.; Castellano, G.; Gesualdo, L.; Pertosa, G.; Grandaliano, G. Arteriovenous Fistula Stenosis in Hemodialysis Patients Is Characterized by an Increased Adventitial Fibrosis. J. Nephrol. 2014, 27, 555–562. [Google Scholar] [CrossRef]
- Barja, G. Updating the Mitochondrial Free Radical Theory of Aging: An Integrated View, Key Aspects, and Confounding Concepts. Antioxid. Redox Signal. 2013, 19, 1420–1445. [Google Scholar] [CrossRef]
- Dorn, G.W.; Vega, R.B.; Kelly, D.P. Mitochondrial Biogenesis and Dynamics in the Developing and Diseased Heart. Genes Dev. 2015, 29, 1981–1991. [Google Scholar] [CrossRef]
- Martin, R.; Fitzl, G.; Mozet, C.; Martin, H.; Welt, K.; Wieland, E. Effect of Age and Hypoxia/Reoxygenation on MRNA Expression of Antioxidative Enzymes in Rat Liver and Kidneys. Exp. Gerontol. 2002, 37, 1481–1487. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced Glycation End Products in the Pathogenesis of Chronic Kidney Disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Díaz, A.G.; Pazarín-Villaseñor, L.; Yanowsky-Escatell, F.G.; Andrade-Sierra, J. Oxidative Stress in Diabetic Nephropathy with Early Chronic Kidney Disease. J. Diabetes Res. 2016, 2016, 7047238. [Google Scholar] [CrossRef] [PubMed]
- Stinghen, A.E.M.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J.; Ferrucci, L.; Cai, W.; Torreggiani, M.; Post, J.B.; Zheng, F.; Striker, G.E. Identifying Advanced Glycation End Products as a Major Source of Oxidants in Aging: Implications for the Management and/or Prevention of Reduced Renal Function in Elderly Persons. Semin. Nephrol. 2009, 29, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Jao, T.-M.; Inagi, R. Dietary Metabolites and Chronic Kidney Disease. Nutrients 2017, 9, 358. [Google Scholar] [CrossRef]
- Clarke, R.E.; Dordevic, A.L.; Tan, S.M.; Ryan, L.; Coughlan, M.T. Dietary Advanced Glycation End Products and Risk Factors for Chronic Disease: A Systematic Review of Randomised Controlled Trials. Nutrients 2016, 8, 125. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression. Am. J. Nephrol. 2017, 45, 473–483. [Google Scholar] [CrossRef]
- De Zeeuw, D.; Akizawa, T.; Agarwal, R.; Audhya, P.; Bakris, G.L.; Chin, M.; Krauth, M.; Lambers Heerspink, H.J.; Meyer, C.J.; McMurray, J.J.; et al. Rationale and Trial Design of Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes: The Occurrence of Renal Events (BEACON). Am. J. Nephrol. 2013, 37, 212–222. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Yang, Y.-X.; Zhe, H.; He, Z.-X.; Zhou, S.-F. Bardoxolone Methyl (CDDO-Me) as a Therapeutic Agent: An Update on Its Pharmacokinetic and Pharmacodynamic Properties. Drug Des. Devel. Ther. 2014, 8, 2075–2088. [Google Scholar] [CrossRef]
- Fliser, D. Ren Sanus in Corpore Sano: The Myth of the Inexorable Decline of Renal Function with Senescence. Nephrol. Dial. Transplant. 2005, 20, 482–485. [Google Scholar] [CrossRef][Green Version]
- Duan, C.; Cao, Y.; Liu, Y.; Zhou, L.; Ping, K.; Tan, M.T.; Tan, N.; Chen, J.; Chen, P. A New Preprocedure Risk Score for Predicting Contrast-Induced Acute Kidney Injury. Can. J. Cardiol. 2017, 33, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Fu, N.-K.; Xu, J.; Yang, S.-C.; Li, S.; Liu, Y.-Y.; Cong, H.-L. A Simple Preprocedural Score for Risk of Contrast-Induced Acute Kidney Injury after Percutaneous Coronary Intervention. Catheter. Cardiovasc. Interv. 2014, 83, E8–E16. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.S.; Cruz, D.; Bobek, I.; Chionh, C.Y.; Nalesso, F.; Lentini, P.; de Cal, M.; Corradi, V.; Virzi, G.; Ronco, C. NGAL: A Biomarker of Acute Kidney Injury and Other Systemic Conditions. Int. Urol. Nephrol. 2010, 42, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Sarnak, M.J.; Katz, R.; Fried, L.F.; Seliger, S.L.; Newman, A.B.; Siscovick, D.S.; Stehman-Breen, C. Cystatin C and the Risk of Death and Cardiovascular Events among Elderly Persons. N. Engl. J. Med. 2005, 352, 2049–2060. [Google Scholar] [CrossRef]
- Parikh, C.R.; Abraham, E.; Ancukiewicz, M.; Edelstein, C.L. Urine IL-18 Is an Early Diagnostic Marker for Acute Kidney Injury and Predicts Mortality in the Intensive Care Unit. J. Am. Soc. Nephrol. 2005, 16, 3046–3052. [Google Scholar] [CrossRef]
- Siew, E.D.; Ware, L.B.; Gebretsadik, T.; Shintani, A.; Moons, K.G.M.; Wickersham, N.; Bossert, F.; Ikizler, T.A. Urine Neutrophil Gelatinase-Associated Lipocalin Moderately Predicts Acute Kidney Injury in Critically Ill Adults. J. Am. Soc. Nephrol. 2009, 20, 1823–1832. [Google Scholar] [CrossRef]
- Han, W.K.; Wagener, G.; Zhu, Y.; Wang, S.; Lee, H.T. Urinary Biomarkers in the Early Detection of Acute Kidney Injury after Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2009, 4, 873–882. [Google Scholar] [CrossRef]
- Lopes, M.B.; Araújo, L.Q.; Passos, M.T.; Nishida, S.K.; Kirsztajn, G.M.; Cendoroglo, M.S.; Sesso, R.C. Estimation of Glomerular Filtration Rate from Serum Creatinine and Cystatin C in Octogenarians and Nonagenarians. BMC Nephrol. 2013, 14, 265. [Google Scholar] [CrossRef]
- Odden, M.C.; Tager, I.B.; Gansevoort, R.T.; Bakker, S.J.L.; Katz, R.; Fried, L.F.; Newman, A.B.; Canada, R.B.; Harris, T.; Sarnak, M.J.; et al. Age and Cystatin C in Healthy Adults: A Collaborative Study. Nephrol. Dial. Transplant. 2010, 25, 463–469. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A. Accuracy of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Diagnosis and Prognosis in Acute Kidney Injury: A Systematic Review and Meta-Analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, J.W.; Lee, K.R.; Hong, D.Y.; Park, S.O.; Kim, S.Y.; Kim, J.Y.; Han, S.K. Urinary Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Acute Kidney Injury in Sepsis Patients in the Emergency Department. Clin. Chim. Acta 2019, 495, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhu, B.; Yuan, H.; Zhao, W. Evaluation of Serum Neutrophil Gelatinase-Associated Lipocalin in Older Patients with Chronic Kidney Disease. Aging Med. 2020, 3, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.R.; Murray, P.T.; Fitzgibbon, M.C. Establishment of a Reference Interval for Urinary Neutrophil Gelatinase-Associated Lipocalin. Ann. Clin. Biochem. 2012, 49, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Ronco, C.; Rosner, M.H. Computerized Decision Support Systems: Improving Patient Safety in Nephrology. Nat. Rev. Nephrol. 2011, 7, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Thompson, C.A. Contrast-Induced Acute Kidney Injury: The at-Risk Patient and Protective Measures. Curr. Cardiol. Rep. 2010, 12, 440–445. [Google Scholar] [CrossRef]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef]
- Kellum, J.A.; Prowle, J.R. Paradigms of Acute Kidney Injury in the Intensive Care Setting. Nat. Rev. Nephrol. 2018, 14, 217–230. [Google Scholar] [CrossRef]
- Tonkin-Crine, S.; Okamoto, I.; Leydon, G.M.; Murtagh, F.E.M.; Farrington, K.; Caskey, F.; Rayner, H.; Roderick, P. Understanding by Older Patients of Dialysis and Conservative Management for Chronic Kidney Failure. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2015, 65, 443–450. [Google Scholar] [CrossRef]
- Farrington, K.; Covic, A.; Aucella, F.; Clyne, N.; de Vos, L.; Findlay, A.; Fouque, D.; Grodzicki, T.; Iyasere, O.; Jager, K.J.; et al. Clinical Practice Guideline on Management of Older Patients with Chronic Kidney Disease Stage 3b or Higher (EGFR <45 ML/Min/1.73 M2). Nephrol. Dial. Transplant. 2016, 31 (Suppl. S2), ii1–ii66. [Google Scholar] [CrossRef]
- Berger, J.R.; Hedayati, S.S. Renal Replacement Therapy in the Elderly Population. Clin. J. Am. Soc. Nephrol. 2012, 7, 1039–1046. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Fiaccadori, E.; Regolisti, G.; Cademartiri, C.; Cabassi, A.; Picetti, E.; Barbagallo, M.; Gherli, T.; Castellano, G.; Morabito, S.; Maggiore, U. Efficacy and Safety of a Citrate-Based Protocol for Sustained Low-Efficiency Dialysis in AKI Using Standard Dialysis Equipment. Clin. J. Am. Soc. Nephrol. 2013, 8, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.K.; Siew, E.D. The Growth of AKI: Half Empty or Half Full, It’s the Size of the Glass That Matters. Kidney Int. 2017, 92, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, Q.-L.; Zhang, X.-Y.; Ma, Q.; Liu, Y.-L.; Pan, R.; Cai, X.-Y. Application of Continuous Renal Replacement Therapy for Acute Kidney Injury in Elderly Patients. Int. J. Clin. Exp. Med. 2015, 8, 9973–9978. [Google Scholar] [PubMed]
- Ricci, Z.; Cruz, D.; Ronco, C. The RIFLE Criteria and Mortality in Acute Kidney Injury: A Systematic Review. Kidney Int. 2008, 73, 538–546. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Laupland, K.B.; Doig, C.J.; Mortis, G.; Fick, G.H.; Mucenski, M.; Godinez-Luna, T.; Svenson, L.W.; Rosenal, T. Prognosis for Long-Term Survival and Renal Recovery in Critically Ill Patients with Severe Acute Renal Failure: A Population-Based Study. Crit. Care 2005, 9, R700–R709. [Google Scholar] [CrossRef]
- Stasi, A.; Intini, A.; Divella, C.; Franzin, R.; Montemurno, E.; Grandaliano, G.; Ronco, C.; Fiaccadori, E.; Pertosa, G.B.; Gesualdo, L.; et al. Emerging Role of Lipopolysaccharide Binding Protein in Sepsis-Induced Acute Kidney Injury. Nephrol. Dial. Transplant. 2017, 32, 24–31. [Google Scholar] [CrossRef]
- Regolisti, G.; Maggiore, U.; Cademartiri, C.; Belli, L.; Gherli, T.; Cabassi, A.; Morabito, S.; Castellano, G.; Gesualdo, L.; Fiaccadori, E. Renal Resistive Index by Transesophageal and Transparietal Echo-Doppler Imaging for the Prediction of Acute Kidney Injury in Patients Undergoing Major Heart Surgery. J. Nephrol. 2017, 30, 243–253. [Google Scholar] [CrossRef]
- James, M.T.; Ghali, W.A.; Tonelli, M.; Faris, P.; Knudtson, M.L.; Pannu, N.; Klarenbach, S.W.; Manns, B.J.; Hemmelgarn, B.R. Acute Kidney Injury Following Coronary Angiography Is Associated with a Long-Term Decline in Kidney Function. Kidney Int. 2010, 78, 803–809. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, M.; Du, J.; Wang, X. Outcomes of Renal Function in Elderly Patients with Acute Kidney Injury. Clin. Interv. Aging 2017, 12, 153–160. [Google Scholar] [CrossRef]
- Coca, S.G.; Cho, K.C.; Hsu, C. Acute Kidney Injury in the Elderly: Predisposition to Chronic Kidney Disease and Vice Versa. Nephron. Clin. Pract. 2011, 119 (Suppl. S1) (Suppl. S1), c19–c24. [Google Scholar] [CrossRef]
- Kumar, S.; Liu, J.; McMahon, A.P. Defining the Acute Kidney Injury and Repair Transcriptome. Semin. Nephrol. 2014, 34, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Stasi, A.; Franzin, R.; Sallustio, F.; Divella, C.; Spinelli, A.; Netti, G.S.; Fiaccadori, E.; Cantaluppi, V.; Crovace, A.; et al. LPS-Binding Protein Modulates Acute Renal Fibrosis by Inducing Pericyte-to-Myofibroblast Trans-Differentiation through TLR-4 Signaling. Int. J. Mol. Sci. 2019, 20, 3682. [Google Scholar] [CrossRef] [PubMed]
| Stage | Serum Creatinine (SCr) | Urine Output (UO) |
|---|---|---|
| 1 | Baseline increase of 1.5 to 2 times in 7 days | <0.5 mL/kg/h for 6–12 h |
| 2 | Baseline increase of 2 to 3 times | <0.5 mL/kg/h for ≥12 h |
| 3 | ≥4 mg/dL or a baseline increase >3 times or initiation of renal replacement therapy | <0.3 mL/kg/h for ≥24 h or anuria for ≥12 h |
| Changes in the Aging Kidney |
|---|
| Decrease in total renal mass |
| Glomerulosclerosis |
| Thickening of glomerular basement membrane |
| Thickening of large vessel walls |
| Decrease in amount and length of tubules |
| Mesangial expansion |
| Decrease in renal blood flow (10% for decade from age of 40 years) |
| Decrease in GFR (1 mL/min/years at age of 45 years) |
| Blunted nitric oxide production and decreased vasodilatory response |
| Decreased osmolality |
| Decreased in renal growth factors production (EGF, IGF-1, VEGF) |
| Increased susceptibility to cell’s apoptosis |
| Increased oxidative stress |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infante, B.; Franzin, R.; Madio, D.; Calvaruso, M.; Maiorano, A.; Sangregorio, F.; Netti, G.S.; Ranieri, E.; Gesualdo, L.; Castellano, G.; et al. Molecular Mechanisms of AKI in the Elderly: From Animal Models to Therapeutic Intervention. J. Clin. Med. 2020, 9, 2574. https://doi.org/10.3390/jcm9082574
Infante B, Franzin R, Madio D, Calvaruso M, Maiorano A, Sangregorio F, Netti GS, Ranieri E, Gesualdo L, Castellano G, et al. Molecular Mechanisms of AKI in the Elderly: From Animal Models to Therapeutic Intervention. Journal of Clinical Medicine. 2020; 9(8):2574. https://doi.org/10.3390/jcm9082574
Chicago/Turabian StyleInfante, Barbara, Rossana Franzin, Desirèe Madio, Martina Calvaruso, Annamaria Maiorano, Fabio Sangregorio, Giuseppe Stefano Netti, Elena Ranieri, Loreto Gesualdo, Giuseppe Castellano, and et al. 2020. "Molecular Mechanisms of AKI in the Elderly: From Animal Models to Therapeutic Intervention" Journal of Clinical Medicine 9, no. 8: 2574. https://doi.org/10.3390/jcm9082574
APA StyleInfante, B., Franzin, R., Madio, D., Calvaruso, M., Maiorano, A., Sangregorio, F., Netti, G. S., Ranieri, E., Gesualdo, L., Castellano, G., & Stallone, G. (2020). Molecular Mechanisms of AKI in the Elderly: From Animal Models to Therapeutic Intervention. Journal of Clinical Medicine, 9(8), 2574. https://doi.org/10.3390/jcm9082574






