Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Efficacy of Repeat Immunoadsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
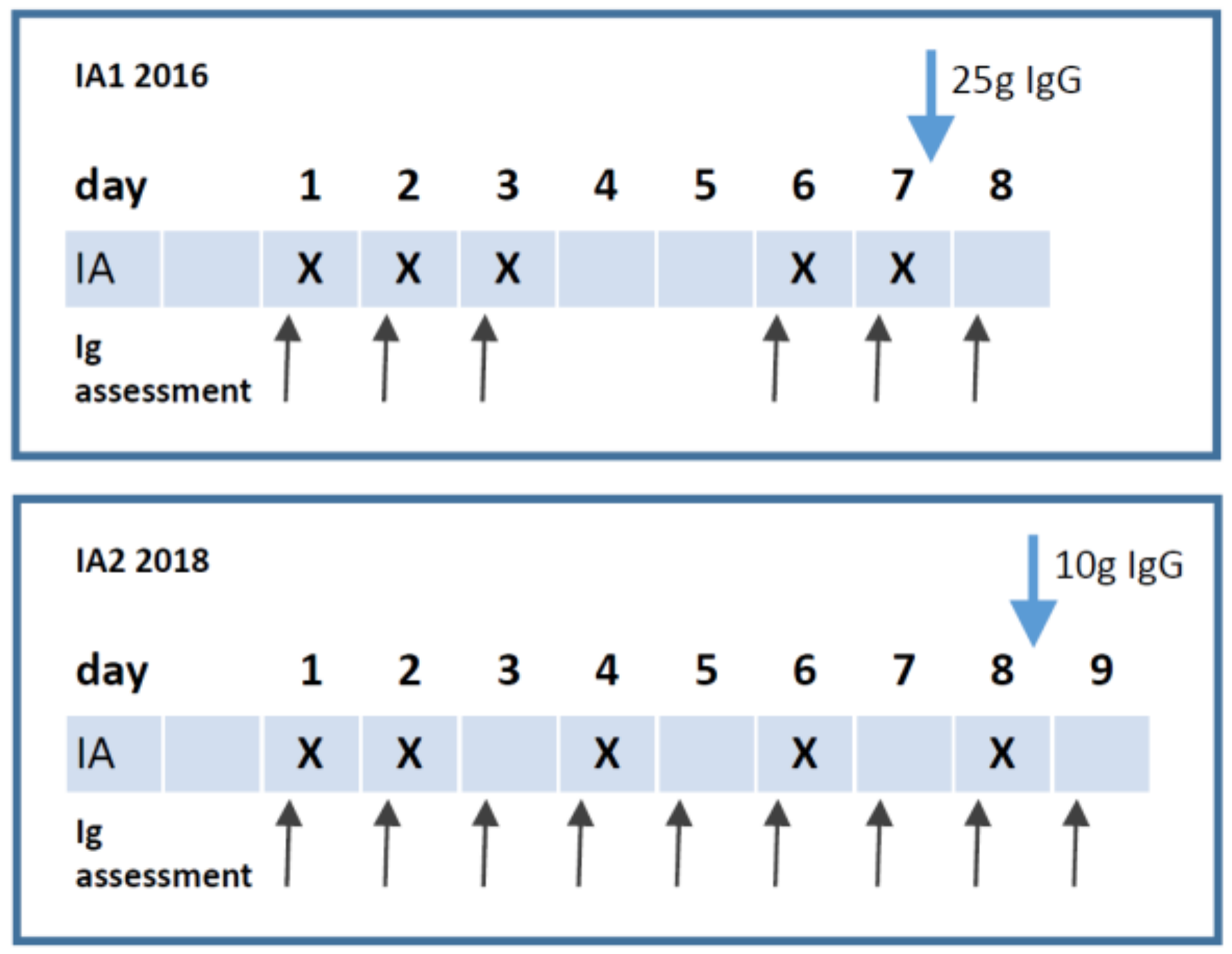
2.2. Study Protocol
2.3. Assessment of Autoantibodies, Ig, Albumin and Fibrinogen
2.4. Symptom Assessment by Scores
2.5. Statistical Analysis
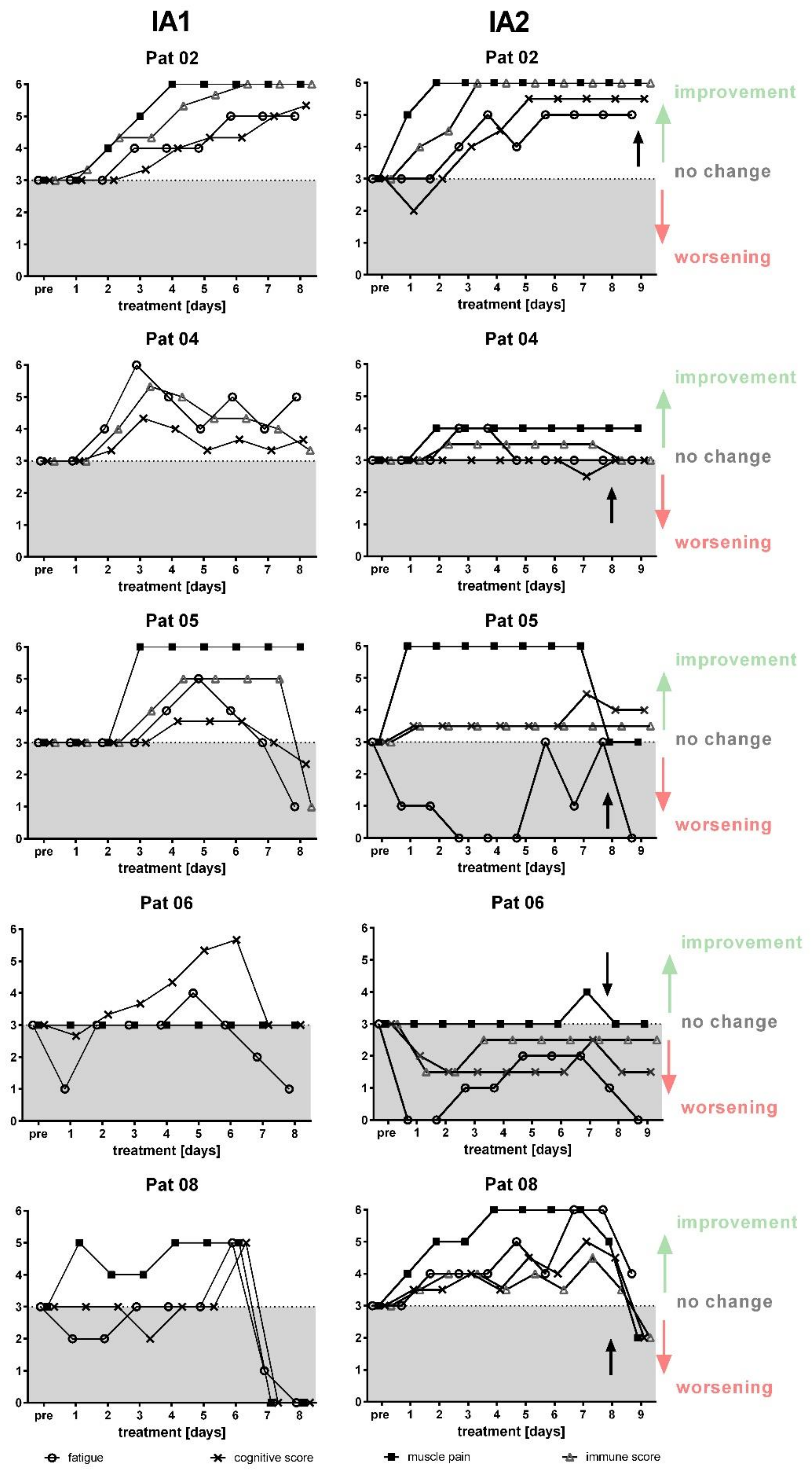
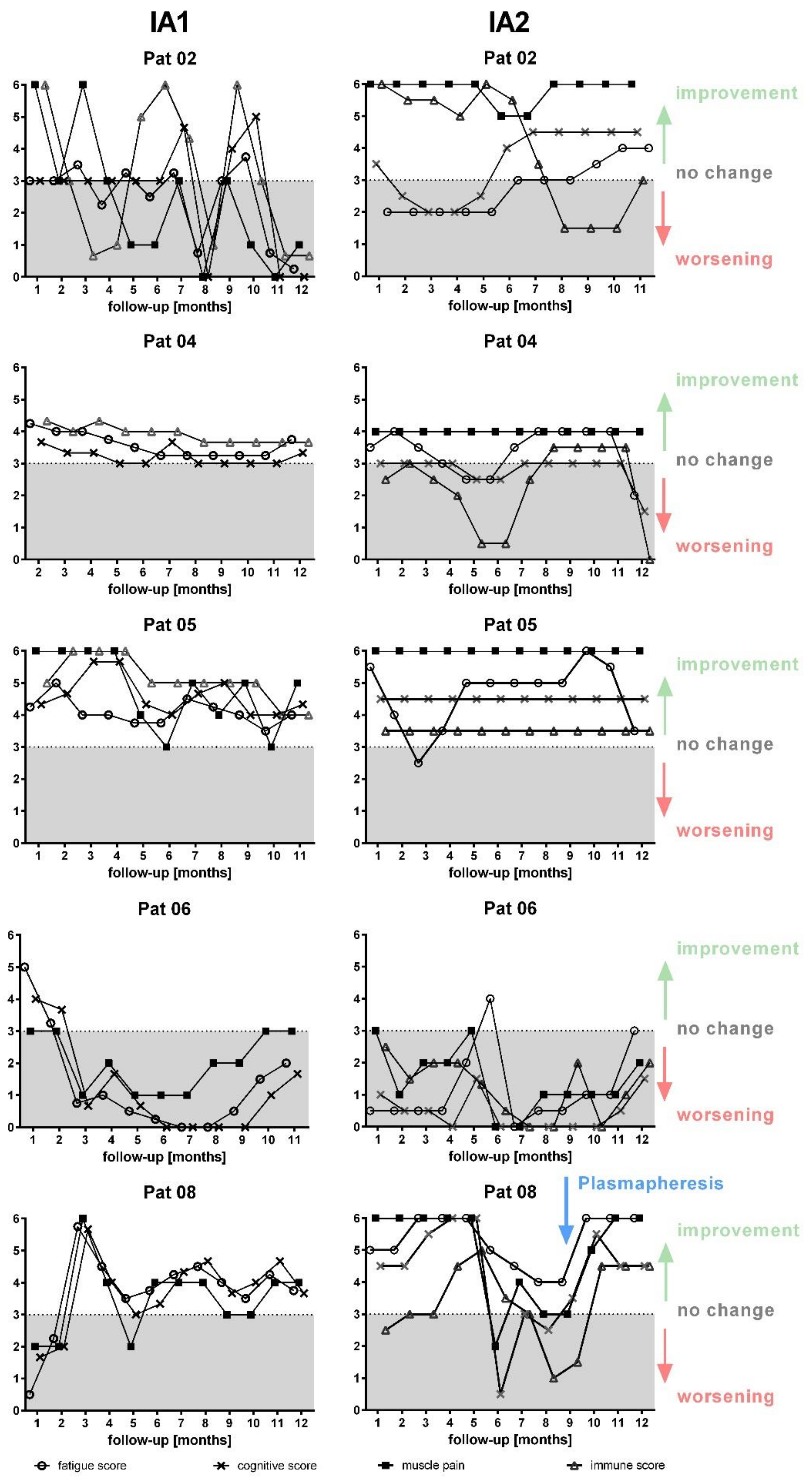
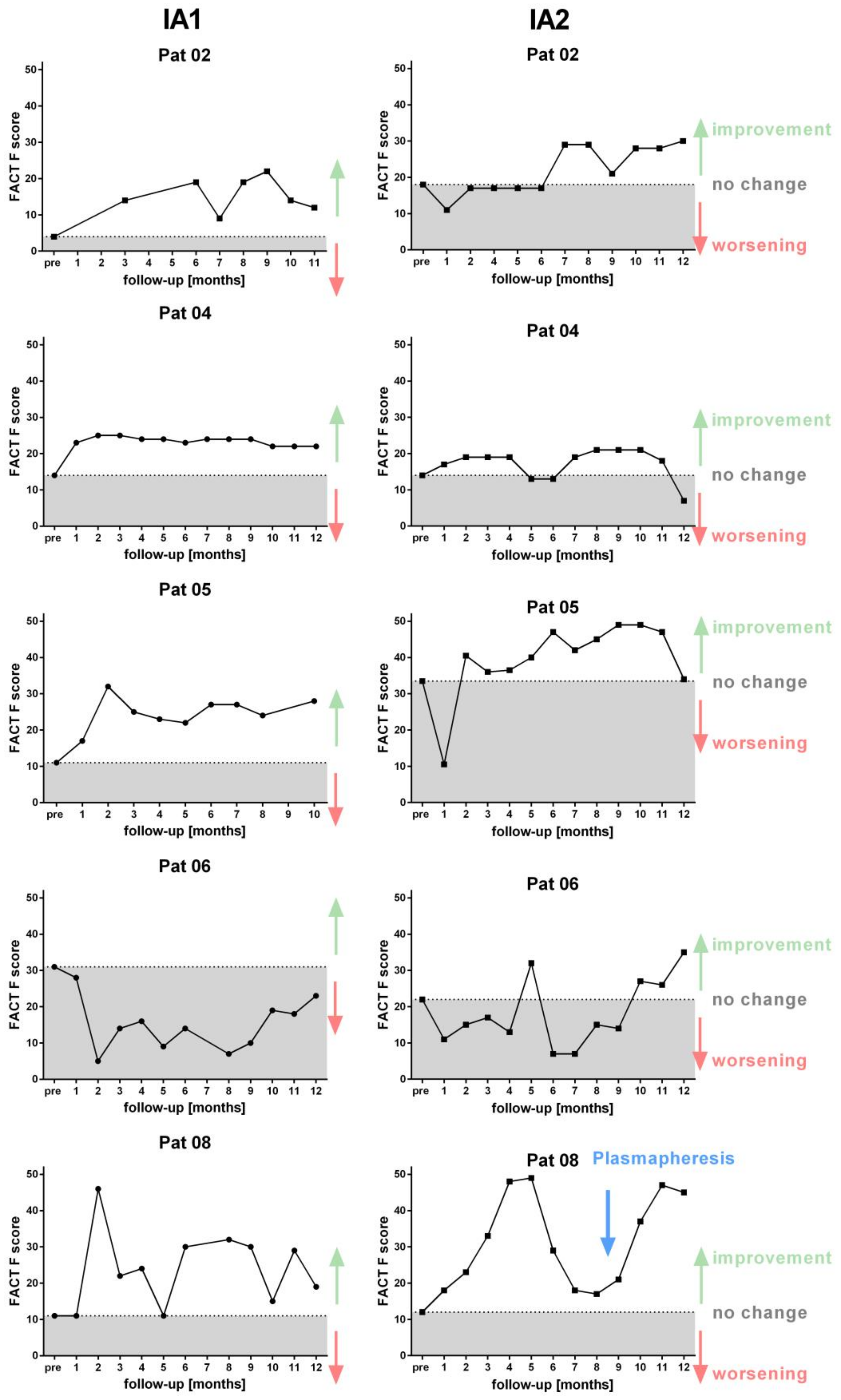
3. Results
3.1. Patient Characteristics and IA Treatment
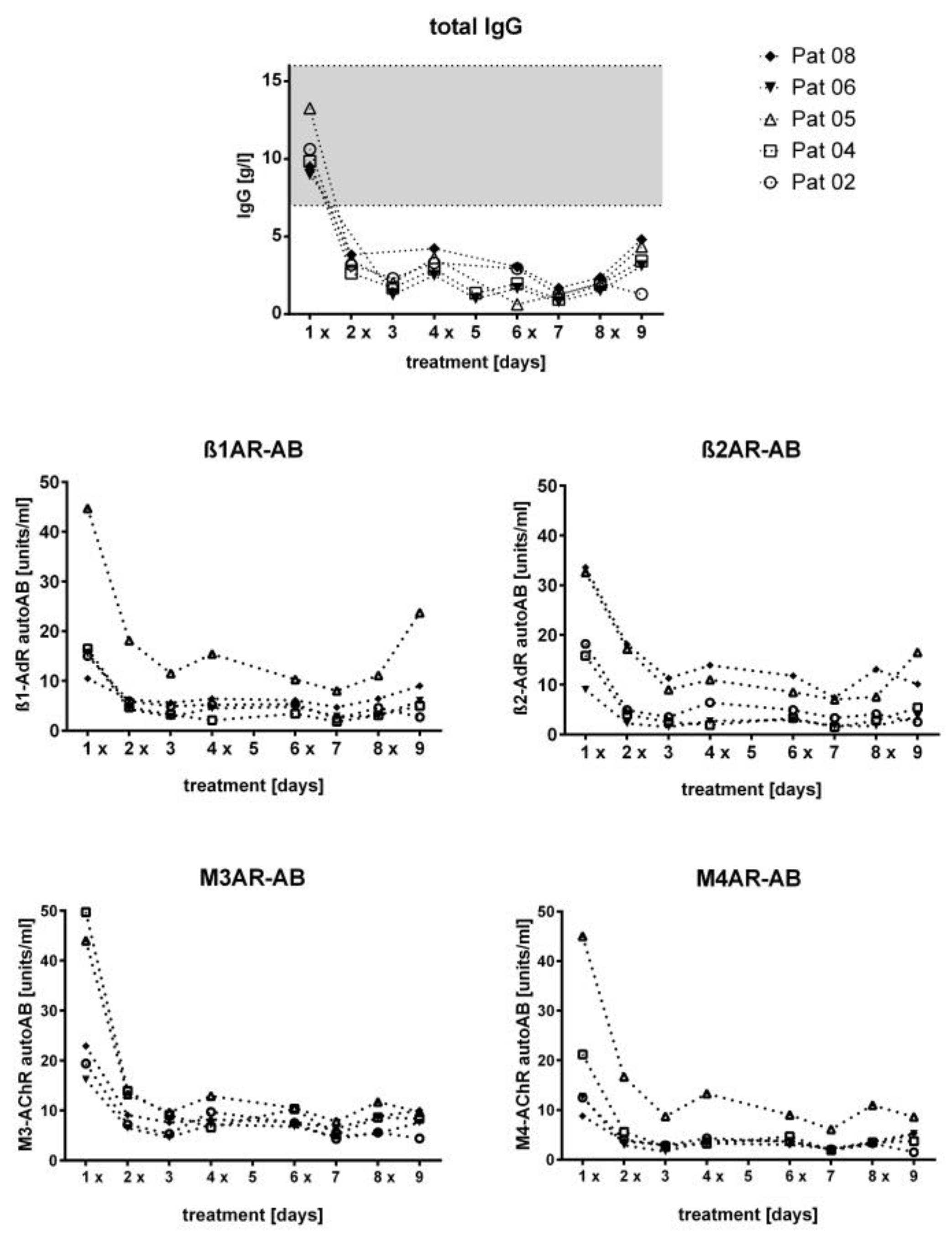
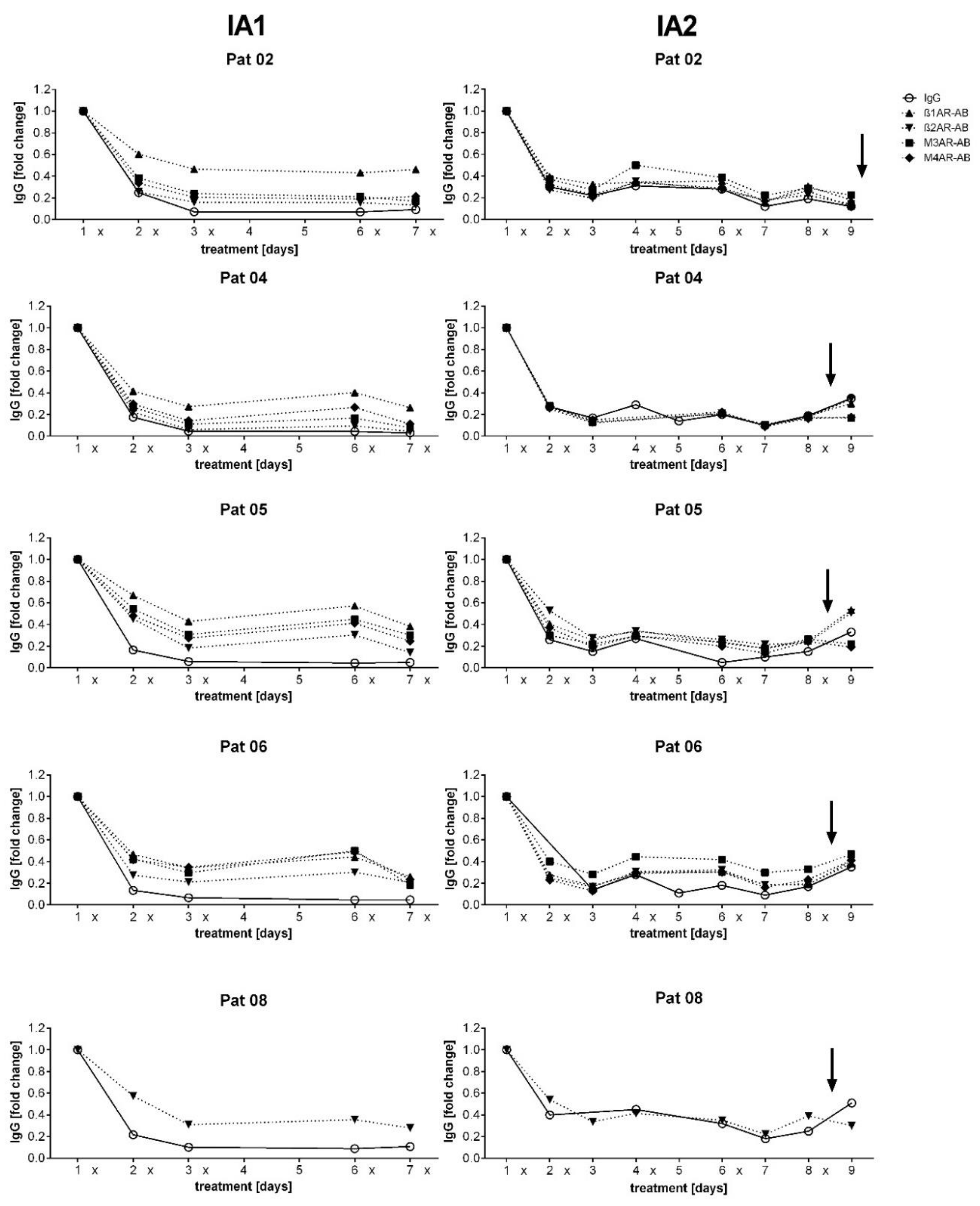
3.2. Course of IgG and Autoantibodies
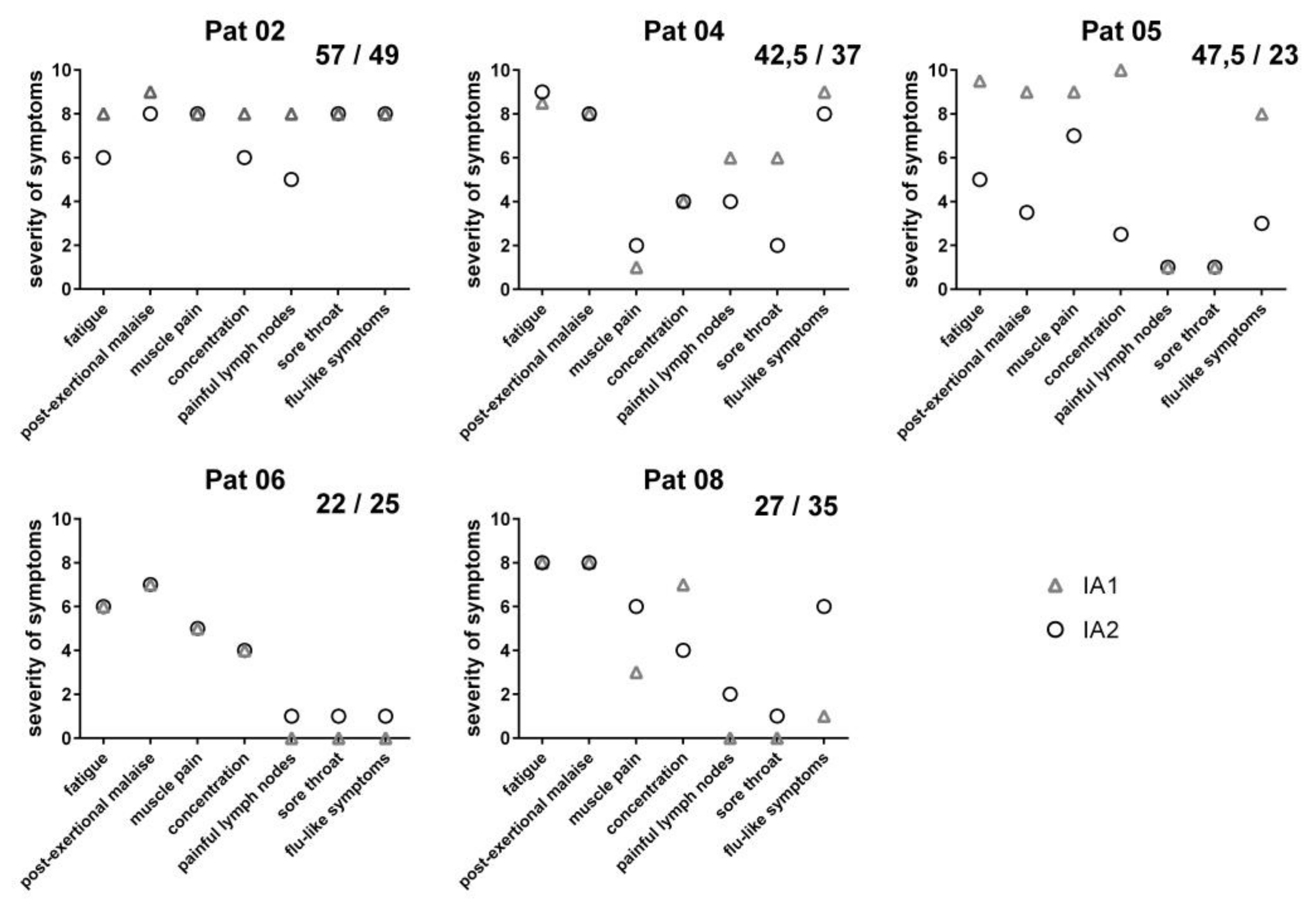
3.3. Clinical Course
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic Encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Chu, L.; Valencia, I.J.; Garvert, D.W.; Montoya, J.G. Onset Patterns and Course of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Pediatr. 2019, 7, 12. [Google Scholar] [CrossRef]
- Clayton, E.W.; Biaggionni, I.; Cockshell, S.; Vermeculen, R.; Snell, C.; Rove, K. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Rowe, P.C.; Underhill, R.; Friedman, K.J.; Gurwitt, A.; Medow, M.S.; Schwartz, M.S.; Speight, N.; Stewart, J.M.; Vallings, R.; Rowe, K.S. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Diagnosis and Management in Young People: A Primer. Front. Pediatr. 2017, 5. [Google Scholar] [CrossRef]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A.J.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2019, 6, 412. [Google Scholar] [CrossRef]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome—Evidence for an autoimmune disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Steiner, S.; Becker, S.C.; Hartwig, J.; Sotzny, F.; Lorenz, S.; Bauer, S.; Löbel, M.; Stittrich, A.B.; Grabowski, P.; Scheibenbogen, C. Autoimmunity-Related Risk Variants in PTPN22 and CTLA4 Are Associated With ME/CFS With Infectious Onset. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Klein, R.; A Berg, P. High incidence of antibodies to 5-hydroxytryptamine, gangliosides and phospholipids in patients with chronic fatigue and fibromyalgia syndrome and their relatives: Evidence for a clinical entity of both disorders. Eur. J. Med. Res. 1995, 1, 21–26. [Google Scholar]
- Maes, M.; Mihaylova, I.; Kubera, M.; Leunis, J.C.; Twisk, F.N.; Geffard, M. Igm-Mediated Autoimmune Responses Directed against Anchorage Epitopes Are Greater in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (Me/Cfs) Than in Major Depression. Metab. Brain Dis. 2012, 27, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Hernandez, O.-D.; Cuccia, M.; Bozzini, S.; Bassi, N.; Moscavitch, S.; Diaz-Gallo, L.M.; Blank, M.; Agmon-Levin, N.; Shoenfeld, Y. Autoantibodies, Polymorphisms in the Serotonin Pathway, and Human Leukocyte Antigen Class II Alleles in Chronic Fatigue Syndrome. Ann. N. Y. Acad. Sci. 2009, 1173, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Löbel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.-D.; Fluge, Ø.; et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kuratsune, H.; Hidaka, Y.; Hakariya, Y.; Tatsumi, K.-I.; Takano, T.; Kanakura, Y.; Amino, N. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. Int. J. Mol. Med. 2003, 12, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Ouchi, Y.; Nakatsuka, D.; Tahara, T.; Mizuno, K.; Tajima, S.; Onoe, H.; Yoshikawa, E.; Tsukada, H.; Iwase, M.; et al. Reduction of [11C](+)3-MPB Binding in Brain of Chronic Fatigue Syndrome with Serum Autoantibody against Muscarinic Cholinergic Receptor. PLoS ONE 2012, 7, e51515. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.; Sotzny, F.; Bauer, S.; Heidecke, H.; Riemekasten, G.; Dragun, D.; Meisel, C.; Dames, C.; Grabowski, P.; Scheibenbogen, C. IgG stimulated β2 adrenergic receptor activation is attenuated in patients with ME/CFS. Brain Behav. Immun. Health 2020, 3. [Google Scholar] [CrossRef]
- Wirth, K.; Scheibenbogen, C. A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun. Rev. 2020, 19, 102527. [Google Scholar] [CrossRef]
- Fujii, H.; Sato, W.; Kimura, Y.; Matsuda, H.; Ota, M.; Maikusa, N.; Suzuki, F.; Amano, K.; Shin, I.; Yamamura, T.; et al. Altered Structural Brain Networks Related to Adrenergic/Muscarinic Receptor Autoantibodies in Chronic Fatigue Syndrome. J. Neuroimaging 2020. [Google Scholar] [CrossRef]
- Fluge, Ø.; Bruland, O.; Risa, K.; Storstein, A.; Kristoffersen, E.K.; Sapkota, D.; Næss, H.; Dahl, O.; Nyland, H.; Mella, O. Benefit from B-Lymphocyte Depletion Using the Anti-CD20 Antibody Rituximab in Chronic Fatigue Syndrome. A Double-Blind and Placebo-Controlled Study. PLoS ONE 2011, 6, e26358. [Google Scholar] [CrossRef]
- Fluge, Ø.; Risa, K.; Lunde, S.; Alme, K.; Rekeland, I.G.; Sapkota, D.; Kristoffersen, E.K.; Sørland, K.; Bruland, O.; Dahl, O.; et al. B-Lymphocyte Depletion in Myalgic Encephalopathy/Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment. PLoS ONE 2015, 10, e0129898. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; Balogun, R.A.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef]
- Stummvoll, G.; Schmaldienst, S.; Smolen, J.S.; Derfler, K.; Biesenbach, P. Lupus nephritis: Prolonged immunoadsorption (IAS) reduces proteinuria and stabilizes global disease activity. Nephrol. Dial. Transplant. 2011, 27, 618–626. [Google Scholar] [CrossRef][Green Version]
- Dandel, M.; Wallukat, G.; Englert, A.; Hetzer, R. Immunoadsorption therapy for dilated cardiomyopathy and pulmonary arterial hypertension. Atheroscler. Suppl. 2013, 14, 203–211. [Google Scholar] [CrossRef]
- Wallukat, G.; Müller, J.; Hetzer, R. Specific Removal of β1-Adrenergic Autoantibodies from Patients with Idiopathic Dilated Cardiomyopathy. N. Engl. J. Med. 2002, 347, 1806. [Google Scholar] [CrossRef] [PubMed]
- Scheibenbogen, C.; Loebel, M.; Freitag, H.; Krueger, A.; Bauer, S.; Antelmann, M.; Doehner, W.; Scherbakov, N.; Heidecke, H.; Reinke, P.; et al. Immunoadsorption to remove ß2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLoS ONE 2018, 13, e0193672. [Google Scholar] [CrossRef] [PubMed]
- Cella, D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: A new tool for the assessment of outcomes in cancer anemia and fatigue. Semin. Hematol. 1997, 34, 13–19. [Google Scholar] [PubMed]
- Bell, D.S. The Doctor’s Guide to Chronic Fatigue Syndrome: Understanding, Treating, and Living with Cfids; Reprint, Reprint edition (18 January 1995); Da Capo Press: Boston, MA, USA, 1995. [Google Scholar]
- Scherbakov, N.; Szklarski, M.; Hartwig, J.; Sotzny, F.; Lorenz, S.; Meyer, A.; Grabowski, P.; Doehner, W.; Scheibenbogen, C. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020, 7, 1064–1071. [Google Scholar] [CrossRef]
- Heine, J.; Ly, L.-T.; Lieker, I.; Slowinski, T.; Finke, C.; Prüss, H.; Harms, L. Immunoadsorption or plasma exchange in the treatment of autoimmune encephalitis: A pilot study. J. Neurol. 2016, 263, 2395–2402. [Google Scholar] [CrossRef]
- Dandel, M.; Wallukat, G.; Englert, A.; Lehmkuhl, H.; Knosalla, C.; Hetzer, R. Long-Term Benefits of Immunoadsorption in Β1-Adrenoceptor Autoantibody-Positive Transplant Candidates with Dilated Cardiomyopathy. Eur. J. Heart. Fail. 2012, 14, 1374–1388. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Marques, A.; Giil, L.M.; De Vito, R.; Rademacher, J.; Günther, J.; Lange, T.; Humrich, J.Y.; Klapa, S.; Schinke, S.; et al. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat. Commun. 2018, 9, 5224. [Google Scholar] [CrossRef]
- Perez, E.E.; Orange, J.S.; Bonilla, F.; Chinen, J.; Chinn, I.K.; Dorsey, M.; El-Gamal, Y.; Harville, T.O.; Hossny, E.; Mazer, B.; et al. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 2017, 139, S1–S46. [Google Scholar] [CrossRef]
- Whiting, P.F.; Bagnall, A.-M.; Sowden, A.J.; Cornell, J.E.; Mulrow, C.D.; Ramirez, G. Interventions for the Treatment and Management of Chronic Fatigue Syndrome. JAMA 2001, 286, 1360–1368. [Google Scholar] [CrossRef]
| Patient No. from 2016 Study | Gender | Age | ME/CFS Onset | Disease Severity Bell Score before IA1 | Disease Severity Bell Score before IA2 |
|---|---|---|---|---|---|
| 2 | f | 60 | 2011 | 30 | 45 |
| 4 | m | 50 | 2000 | 20 | 35 |
| 5 | f | 37 | 2012 | 40 | 75 |
| 6 | f | 32 | 2002 | 50 | 60 |
| 8 | f | 32 | 2005 | 10 | 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tölle, M.; Freitag, H.; Antelmann, M.; Hartwig, J.; Schuchardt, M.; van der Giet, M.; Eckardt, K.-U.; Grabowski, P.; Scheibenbogen, C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Efficacy of Repeat Immunoadsorption. J. Clin. Med. 2020, 9, 2443. https://doi.org/10.3390/jcm9082443
Tölle M, Freitag H, Antelmann M, Hartwig J, Schuchardt M, van der Giet M, Eckardt K-U, Grabowski P, Scheibenbogen C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Efficacy of Repeat Immunoadsorption. Journal of Clinical Medicine. 2020; 9(8):2443. https://doi.org/10.3390/jcm9082443
Chicago/Turabian StyleTölle, Markus, Helma Freitag, Michaela Antelmann, Jelka Hartwig, Mirjam Schuchardt, Markus van der Giet, Kai-Uwe Eckardt, Patricia Grabowski, and Carmen Scheibenbogen. 2020. "Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Efficacy of Repeat Immunoadsorption" Journal of Clinical Medicine 9, no. 8: 2443. https://doi.org/10.3390/jcm9082443
APA StyleTölle, M., Freitag, H., Antelmann, M., Hartwig, J., Schuchardt, M., van der Giet, M., Eckardt, K.-U., Grabowski, P., & Scheibenbogen, C. (2020). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Efficacy of Repeat Immunoadsorption. Journal of Clinical Medicine, 9(8), 2443. https://doi.org/10.3390/jcm9082443





