Abstract
The inflammatory neuropathies are disabling conditions with diverse immunological mechanisms. In some, a pathogenic role for immunoglobulin G (IgG)-class autoantibodies is increasingly appreciated, and immunoadsorption (IA) may therefore be a useful therapeutic option. We reviewed the use of and response to IA or plasma exchange (PLEx) in a cohort of 41 patients with nodal/paranodal antibodies identified from a total of 573 individuals with suspected inflammatory neuropathies during the course of routine diagnostic testing (PNAb cohort). 20 patients had been treated with PLEx and 4 with IA. Following a global but subjective evaluation by their treating clinicians, none of these patients were judged to have had a good response to either of these treatment modalities. Sequential serology of one PNAb+ case suggests prolonged suppression of antibody levels with frequent apheresis cycles or adjuvant therapies, may be required for effective treatment. We further retrospectively evaluated the serological status of 40 patients with either Guillain-Barré syndrome (GBS) or chronic inflammatory demyelinating polyneuropathy (CIDP), and a control group of 20 patients with clinically-isolated syndrome/multiple sclerosis (CIS/MS), who had all been treated with IgG-depleting IA (IA cohort). 32 of these patients (8/20 with CIDP, 13/20 with GBS, 11/20 with MS) were judged responsive to apheresis despite none of the serum samples from this cohort testing positive for IgG antibodies against glycolipids or nodal/paranodal cell-adhesion molecules. Although negative on antigen specific assays, three patients’ pre-treatment sera and eluates were reactive against different components of myelinating co-cultures. In summary, preliminary evidence suggests that GBS/CIDP patients without detectable IgG antibodies on routine diagnostic tests may nevertheless benefit from IA, and that an unbiased screening approach using myelinating co-cultures may assist in the detection of further autoantibodies which remain to be identified in such patients.
1. Introduction
The inflammatory neuropathies are a heterogeneous group of disorders in which peripheral nerve function and structure are disturbed by largely ill-defined immunological mechanisms [1]. They can broadly be divided into acute and chronic forms, typified by the umbrella terms Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP), respectively. Humoral and cellular immunity are likely to play a role in the pathogenesis of both syndromes. For some clinically defined subtypes, a role for the humoral immune system and pathogenic autoantibodies appears to be more prominent [2,3], but particularly at the level of the individual patient, a direct and consistent link between the clinical syndrome, serological profile, and underlying immunopathological mechanism remains difficult to establish.
Randomised controlled trials have demonstrated that therapeutic plasma exchange (PLEx) speeds up recovery from GBS [4], and provides at least a short-term improvement in disability in CIDP [5]. In both conditions there is evidence that intravenous immunoglobulin (IVIg) has similar efficacy [6,7]. Two small, randomised studies have compared immunoadsorption (IA) with PLEx or IVIg in CIDP. Response rates to IA (6/9 using tryptophan-based columns [8] and 4/5 using protein A [9]) were not significantly different to their respective comparators. The trial comparing IA (using protein A) with IVIg had a high drop-out rate and was excluded from the relevant Cochrane review due to a high risk of bias [9]. Two further reports described the crossover from PLEx to IA in CIDP, in a single patient each, reaching opposite conclusions about which was more efficacious [10,11]. A number of retrospective case series and case reports have favourably evaluated immunoadsorption in both GBS and CIDP [12,13,14,15,16,17,18,19,20,21]. A retrospective Japanese report of IA in GBS found that patients who received IA within 6 days of onset of their neuropathy had a more rapid improvement in disability compared to those who received supportive care alone, whereas patients who received IA later than this in their disease course did not [22]. However, high-quality evidence demonstrating the efficacy of IA in the inflammatory neuropathies is lacking [23]. There is also some evidence that apheresis can improve recovery from multiple sclerosis relapses, and these approaches are often used after inadequate responses to corticosteroids [24,25].
Certain subtypes of GBS are associated with immunoglobulin (Ig) G ganglioside antibodies [26], with a handful of small studies showing an effective reduction of antibody titres using IA [19,27]. More recently a subset of CIDP-like neuropathies have been linked to predominantly IgG4-subclass antibodies directed against nodal or paranodal cell-adhesion molecules [28,29,30,31,32]. It has been speculated that patients with such antibodies may respond particularly well to selective IgG immunoadsorption [33]. A recent case series of four patients with CIDP and neurofascin-155 (NF155) antibodies reported that PLEx was effective in 3, and partially effective in 1, whilst tryptophan-based IA was ineffective in one such patient [34].
There are of course substantial differences between PLEx and IA. The former removes a broad range of circulating molecules and requires the use of replacement fluid, typically fresh frozen plasma, or albumin. Replacement fluid is not required in IA, and the range of circulating factors removed is more limited. This is advantageous in reducing complications, such as those due to the unwanted removal of coagulation factors [35], but may also lead to a loss of therapeutic effect if this depends on the removal of pro-inflammatory cytokines, or other pathogenically-relevant molecules, rather than immunoglobulins. It is also important to appreciate that there are variations in the biological effects between the different types of IA, which may also influence their clinical efficacy. For example, Yuki and colleagues have previously demonstrated that tryptophan-based columns are more effective than phenylalanine for adsorbing anti-ganglioside antibodies [36]. IA using protein A or synthetic ligands has been proposed as a method to remove a larger fraction of circulating IgG more selectively and quickly, whilst more modestly affecting IgM and IgA levels, and leaving complement, albumin and fibrinogen largely unaffected [37].
Intuitively, it may be assumed that patients who respond to “Ig-selective” IA do so because pathogenic Ig is being removed from the circulation. However, previous assessments of IA efficacy rarely report serological status. It is therefore currently unclear as to whether the presence of known serum autoantibodies in GBS and CIDP prospectively identifies a subpopulation of patients who are likely to respond more favourably to IA. It is also unclear as to whether any particular IA system or treatment programme is more likely to produce a positive outcome.
In this study we provide a retrospective evaluation of apheresis in two serologically-defined patient cohorts. We first reviewed the subjective clinician-reported overall impression of response to IA or PLEx in a cohort of neuropathy patients identified during routine diagnostic testing (PNAb cohort), and compared patients in which nodal/paranodal antibodies were or were not detected. We present the detailed case history and parallel serological analysis of a patient with NF155 antibodies who was treated with IA. Finally, we perform a retrospective analysis of the serological status of a sample of 60 patients who had been treated with IgG-depleting IA (IA cohort) and compare this with clinician-reported outcomes.
2. Experimental Section
2.1. Paranodal Antibody (PNAb) Patient Cohort
Since 2015, 88 patients with confirmed or suspected inflammatory neuropathies presenting to the neuropathy clinic in Oxford have been recruited to an observational study. This study was approved by the National Health Service (NHS) National Research Ethics Service Committee (South Central–Oxford A, 14/SC/0280). Patients recruited prior to 2017 were tested retrospectively, and those recruited from 2017 prospectively, for nodal/paranodal antibodies by the methods described in Appendix A. Since August 2017, serum samples from a further 537 external patients with confirmed or suspected inflammatory neuropathies have been received for diagnostic nodal/paranodal antibody testing by the Oxford laboratory. Clinical information was requested for all patients, including details of treatments used, and a clinician-led, subjective, overall impression of their efficacy.
2.2. IA Patient Cohort
The IA cohort consisted of 60 subjects (20 with CIDP, 20 with GBS, and a control group of 20 with multiple sclerosis/clinically-isolated syndrome, MS/CIS) who were selected from patients treated with IA between June 2013 and January 2018 in the University of Ulm, Department of Neurology based on the inclusion criteria outlined below. The study was reviewed by the appropriate ethics committee of the University of Ulm (approval number 20/10) and was performed in accordance with the ethical standards of the Declaration of Helsinki from 1964. Written informed consent for the sample collection was obtained from all patients participating in this study.
2.2.1. CIDP
All patients with CIDP fulfilled the EFNS criteria for possible, probable, or definite CIDP, had a continuously progressive course of disease, and had previously received several cycles of steroids (n = 5), IVIg (n = 2) or both (n = 13), with insufficient response. Fifteen patients who had previously received IVIg showed further disease progression under IVIg therapy, therefore we opted for a new therapeutic approach with IA. In 5 patients who had never received IVIg we chose IA instead of IVIg based on our favourable clinical experience with IA in CIDP. Two patients had never been treated with prednisolone because of severe diabetes mellitus. Further treatments included azathioprine (n = 5), cyclophosphamide (n = 1), mycophenolate mofetil (n = 2), and methotrexate (n = 1). Assessment of the clinical outcome directly and 2 weeks after IA was based on the Inflammatory Neuropathy Cause and Treatment (INCAT) score [38] and the Ulmer CIDP score, which includes the INCAT, the Oxford muscle strength grading scale (Medical Research Council, MRC), and vibration sensitivity testing [33].
2.2.2. GBS
All patients with GBS showed the typical clinical picture including rapidly progressive bilateral limb weakness and sensory deficits, hypo-/areflexia, electrophysiological signs of demyelination, and increased protein levels in cerebrospinal fluid. Anti-ganglioside antibodies were not tested prospectively. In contrast to CIDP and MS, IA was a first-line therapy in 4 GBS patients, and used as an escalation therapy in 9 more. In order to establish equally sized subgroups, the GBS group included 7 patients who received PLEx rather than IA. Classification of the clinical outcome (no improvement, equivocal improvement, partial improvement, large improvement) directly after the last treatment was retrospectively based on the neurological examination as documented in the medical records (discharge letter) of each patient.
2.2.3. MS/CIS
All patients fulfilled the 2017 MacDonald diagnostic criteria for MS [39] or CIS. All patients treated with IA suffered from a steroid-refractory relapse, i.e., an acute relapse without complete remission after one or more cycles of high dose intravenous methylprednisolone (IVMP) therapy (at least 3 × 1000 mg). Assessment of the clinical outcome directly after the last treatment was based on the Expanded Disability Status Scale (EDSS).
2.3. IA Treatment
One cycle of IA consisted of five treatments on 5 consecutive days. The total plasma volume of each patient was calculated using body weight, height, and haematocrit. Two plasma volumes were processed during the first treatment, and 2.5 plasma volumes were processed during all the subsequent treatments. The Adsorber system (ADAsorb, medicap clinic GmbH, Ulrichstein, Germany) contained two regenerating protein A columns (Immunosorba, Fresenius Medical Care, Bad Homburg, Germany).
2.4. Sample Collection and Storage
Eluate samples were obtained during each IA treatment and buffered with bicarbonate (pH 7.0). Serum samples were obtained before and after each IA treatment. A standardized protocol for serum and eluate collection was applied as previously recommended [40]. All biosamples were stored according to the predefined standard operating procedure (SOPs) at the local biobank in Ulm at minus 80 °C within two hours. Later they were transferred for measurement on dry ice to Oxford for further analysis.
2.5. Serological Analysis
Sera and eluates from the 3 patient cohorts and from control subjects were analysed using a nodal/paranodal antibody cell-based assay, paranodal, ganglioside and sulfatide ELISA, and against myelinating co-cultures. Methodological details for these experiments are given in Appendix A.
3. Results
3.1. Nodal/Paranodal Antibody (PNAb) Diagnostic Cohort
3.1.1. Demographics, Clinical and Serological Characteristics
Since August 2018, serum samples from 537 different patients with confirmed or suspected inflammatory neuropathies have been received for diagnostic nodal/paranodal antibody testing by the Oxford laboratory, and we have tested a further 88 patients from our own research cohort. Overall, 42/625 patients (6.7%) were positive for nodal/paranodal antibodies (PNAb+), comprising 16 (2.6%) with NF155 specific antibodies, 1 (0.2%) with NF186 specific antibodies, 6 (1%) with pan-neurofascin antibodies, 12 (1.9%) with contactin-1 (CNTN1) antibodies and 7 (1.1%) with contactin-associated protein (Caspr1) or CNTN1/Caspr1-complex antibodies. The median age of the PNAb+ patients was 58 (range 15 to 79) and 30/42 (71.4%) were male. The initial clinical diagnosis was CIDP in 28 (66.6%), GBS in 13 (31.0%) and atypical multifocal motor neuropathy in 1 (2.4%). In one patient, the diagnosis of CIDP was subsequently revised to motor neuron disease; the diagnosis of an inflammatory neuropathy was retained at follow up in all other antibody positive cases. The remaining 583 patients were paranodal antibody negative (PNAb-negative), with clinical data available for 185 patients. The median age of the PNAb-negative patients was 62 (range 4 to 90) and 120/185 (64.9%) were male. The initial clinical diagnosis was CIDP in 100 (53.8%), combined central and peripheral demyelination in 3 (1.6%), GBS in 38 (20.4%), and multifocal motor neuropathy in 16 (8.6%). In 9/131 (6.9%) patients for whom follow up data was available, the diagnosis was subsequently revised away from that of an inflammatory neuropathy. Summary demographic and clinical details of the subgroups of apheresis treated PNAb-positive and PNAb-negative patients are given in Table 1. There was no significant difference in the median age, sex distribution, clinical diagnosis, or other serological results between the 2 subgroups. There was a non-significant trend towards more severe disease and more frequent IgG and less frequent IgM paraprotein detection in PNAb-positive patients. The frequencies of prior IVIg, steroid, PLEx and immunosuppressant use was also similar between the groups, while rituximab and IA were significantly more likely to have been used in the PNAb-positive group. PLEX aside, there was, however, no statistically significant difference in the clinician reported responses to these therapies between the 2 groups, although there was a trend to rituximab being more often judged effective in the PNAb-positive compared to PNAb-negative group.

Table 1.
Summary characteristics of apheresis treated patients from the PNAb cohort.
3.1.2. Physician-Reported Subjective Evaluation of Responses to Plasma Exchange or Immunoadsorption
Of the PNAb+ patients, 17 were treated with PLEx alone, 1 with IA alone, and 3 with both modalities. Protein A columns were used for three of the IA treated patients, the other (described in detail below) was treated with a GAM-peptide-ligand-based column (Globaffin, Fresenius Medical Care (UK) Ltd, Sutton-in-Ashfield, UK). Serial disability measures are available for only one other PNAb+ patient: a 68-year-old lady with a clinical diagnosis of GBS. Neither her overall neuropathy limitations score (ONLS, 12/12) nor inflammatory neuropathy Rasch-built overall disability score (iRODS, 0/48) improved following 2 cycles 5 treatments of PLEx starting on days 40 and 69 of her illness, prior to her death on day 110 from infectious complications. For all other PNab+ patients, only clinician-reported, retrospective, and subjective evaluations of response were available. None of the treating clinicians judged that either PLEx or IA had produced a subjectively “good” response in any of the PNAb+ patients. With PLEx, 5 patients (25.0%) were reported as having had a partial response, 2 (10.0%) an equivocal response, 12 (60.0%) no response, and one to have deteriorated (5.0%). With IA, 1 (25%) partial response, and 1 (25%) equivocal response were reported, with 2 patients (50%) reported as showing no response (Figure 1A,B). The proportion of PNAb+ patients subjectively judged as showing a partial or better response to PLEx (25.0%) versus IA (25.0%) was identical.
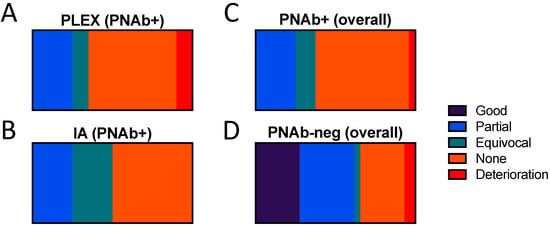
Figure 1.
Physician-reported subjective evaluation of response to plasma exchange or immuno-adsorption in paranodal antibody positive and negative patients. Paranodal antibody positive patients treated with (A) plasma exchange (n = 17), (B) immunoadsorption (n = 4), or (C) either modality (n = 21), compared to (D) paranodal antibody negative patients (n = 33) (all treated with plasma exchange).
Of the PNAb-negative patients, 33 were treated with PLEx: 8 patients (24.2%) were subjectively reported as having a good response, 10 (30.3%) a partial response, 1 (3.0%) an equivocal response, 8 (24.2%) no response, and 2 (6.1%) as deteriorating. For 4 patients, the response to PLEx was not reported. Amongst the 3 ganglioside antibody positive patients, 2 were reported as having a partial response, and 1 no response, to PLEx. Apheresis, with or without IA, was significantly more likely to have been reported by treating clinicians to have produced partial or better response in the PNAb-negative patients (62.1%) compared to the PNAb+ patients (25.0%) (p = 0.01, Fisher’s exact test, OR 4.9 (95% CI 1.52 to 14.88) (Figure 1C,D). It should be emphasised that this is a comparison of the physicians’ subjective overall impression of response, rather than an evaluation of the true efficacy, or otherwise, of these treatments.
3.2. Detailed Profile of an NF155 Antibody Positive Patient Treated with Immunoadsorption
This 46-year-old male first presented to neurology in July 2019 with a 6-week history of ascending numbness and paraesthesia in his feet, then hands. He had lost the ability to run and found walking to be unsteady. On examination, power was full, but there was global areflexia with distal sensory loss to temperature, pin-prick, vibration and proprioception. His gait was broad-based and unsteady and Rhomberg’s test was positive after 20 s of eye closure. There was a postural tremor of both hands without cerebellar or extrapyramidal signs. The presentation was felt to be consistent with sensory ataxic CIDP. Neurofascin-155 antibody mediated disease was high in the differential. A positive result on the NF155 CBA and ELISA was duly returned 2 days later, at an initial titre of 1:6400. IgG4 was the dominant subclass, with IgG1 and IgG2 also represented (Figure 2A,B). CSF was acellular with an elevated protein (1.8 g/L). Nerve conduction studies showed absent median but preserved sural sensory nerve action potentials. Distal motor latencies and F-wave latencies were significantly prolonged, with slowing of intermediate motor conduction velocities. There was conduction block without temporal dispersion in the sampled peroneal nerve between the ankle and fibular head. Pulsed dexamethasone was commenced 4 days later (40mg per day for 4 days every 4 weeks for 3 cycles). There was no change in the examination findings. A progressive deterioration in symptoms and disability measures prompted a trial of IVIg (2 g/kg over 5 days) which resulted in a pompholyx-type skin rash, and no neurological benefit over the next 6 weeks. Approval was then sought for rituximab, and IA was arranged as a potential temporising measure.
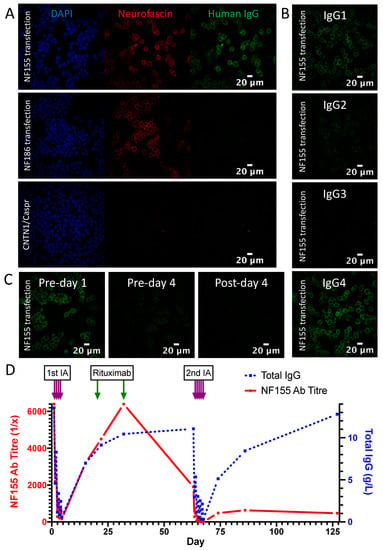
Figure 2.
Serological results of NF155 antibody positive patient at baseline and during IA treatment. (A) Serum contains IgG (green) which binds to the cell membrane of NF155-transfected HEK293T cells, and co-localises with a commercial pan-neurofascin antibody (red). No signal is seen with NF186 or CNTN1/Caspr1-transfected cells. (B) The predominant IgG subclass of the NF155 antibodies is IgG4, with IgG1>IgG2 also represented. (C) The antibody signal intensity at 1:100 before, during and immediately after the first cycle of IA shows a progressive decline. (D) NF155 antibody titre (red) and total IgG levels (blue) over 2 cycles of IA, before and after rituximab.
Four treatment sessions of 2–2.5 plasma volumes were given on 4 consecutive days using a multiple pass, GAM-peptide-ligand-based column (Globaffin, Fresenius Medical Care Ltd, Sutton-in-Ashfield, UK). IA was effective in rapidly and substantially reducing the NF155 antibody titre (Figure 2C), but this had returned to baseline by 1 month (Figure 2D) and there was no observed clinical benefit. Rituximab was then given (1g on 2 occasions 2 weeks apart) followed by a second cycle of 5 treatments sessions of IA 1 month later. This was again associated with a rapid and substantial reduction in NF155 antibody titre, which on this occasion recovered more slowly and incompletely (Figure 2D). This more persistent suppression of antibody titres was associated with a progressive improvement in symptoms and disability, which is currently ongoing (Figure 3).
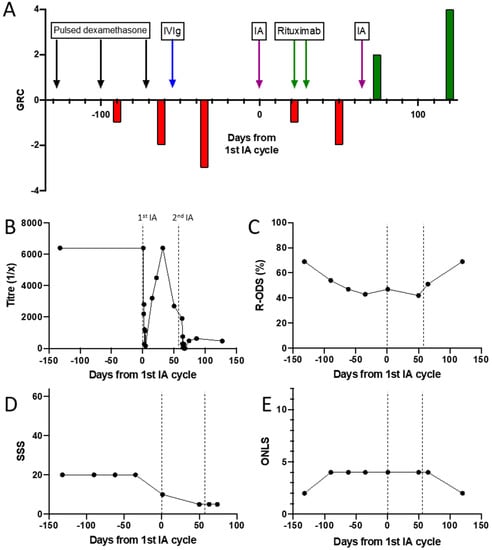
Figure 3.
Antibody titres and outcome/disability measures during treatment of a patient with an NF155-antibody-mediated neuropathy. (A) Patient global rating of change after treatment with dexamethasone, IVIg, IA and rituximab. (B) NF155 antibody titre. (C) Inflammatory neuropathy Rasch-built Overall Disability Score. (D) Sensory sum score. (E) Overall neuropathy limitations score.
3.3. Demographics and Clinical Characteristics of the IA Treated Cohort
3.3.1. CIDP
Details of this cohort are given in Appendix B (Table A1). Sixteen of these 20 CIDP patients have been described in a previous publication [33]. 16/20 (80%) were male. At the start of IA treatment, the cohort had a median age of 66 (range 27 to 80), and a median disease duration of 95.5 months (range 63 to 139). All had progressive disease and met the European Federation of Neurological Societies (EFNS) criteria for definite, probable, or possible CIDP [41]. 18/20 had been previously treated with corticosteroids and 14/20 with IVIg, with sub-optimal responses. Six patients were treated with at least one of azathioprine, cyclophosphamide, mycophenolate mofetil or methotrexate. Nine patients received multiple (range 2–9) cycles of IA. Five patients showed improvements in their Inflammatory Neuropathy Cause and Treatment (INCAT) disability score when assessed 2 weeks after initial IA treatment, and 8 patients showed substantial improvements (at least 10 points) in the CIDP score.
3.3.2. GBS
Details of this cohort are given in Appendix B (Table A2). 10/20 patients (50%) were male. At the start of IA or PLEx treatment, the cohort had a median age of 66 (range 31 to 89). IA was applied to 13/20 patients. IA was used as a first-line therapy in 3, as a second-line therapy (after unsuccessful treatment with IVIg) in 9, and as a third-line therapy (after both IVIg and PLEx) in 1 patient. This subgroup was supplemented with 7 patients who received PLEx, instead of IA. In these patients, PLEx was used as a first-line therapy in 6, and as a second-line therapy (after IVIg) in 1 patient. 18/20 patients received 1 cycle of IA or PLEx, and only 2 patients received 2 cycles. 4/20 (3/13 IA, 1/7 PLEx) patients showed no clinical improvement after the last treatment, 3 patients (2/13 IA, 1/7 PLEx) showed equivocal improvement, 8 patients (4/13 IA, 4/7 PLEx) showed partial improvement, and 5 patients (4/13 IA, 1/ PLEx) showed large improvement.
3.3.3. MS/CIS
Details of this cohort are given in Appendix B (Table A3). 15/20 patients (75%) were female. At the start of IA treatment, the cohort had a median age of 29 (range 15 to 57). Patients were diagnosed with MS (16/20) or CIS (4/20), and had all been treated unsuccessfully with at least one cycle of high-dose intravenous methyl prednisolone (MP). 8 patients had received 2 or more cycles of high-dose IVMP. 11/20 patients showed an improvement of EDSS after the last IA treatment, while 9/20 patients did not improve.
3.4. Glycolipid and Nodal/Paranodal Antibodies in the IA Cohort
Pre-treatment serum samples from the IA cohort were tested for sulfatide and GM1- and GQ1b-ganglioside IgG antibodies by ELISA. None of these sera were positive on these assays. Serum samples taken pre and post-treatment, as well as first treatment session eluates from the IA cohort (20 CIDP, 20 GBS and 20 MS/CIS patients), were tested by both cell-based assay (CBA) and ELISA for antibodies to nodal (neurofascin-186) and paranodal (neurofascin-155, contactin-1 and Caspr) cell adhesion molecules. None of the sera were positive on either assay. One eluate from the MS/CIS cohort (patient 09) was positive on the neurofascin-155 CBA (blind scored as ‘2+’ at 1:100, end-point titre 1:200, Figure 4A) (For scoring method see Appendix A.1). The sole detected subclass was IgG1. The corresponding pre-treatment serum was negative for NF155 antibodies at 1:100, the standard screening titre for this assay, but scored 3+ when repeated at 1:20. Two further eluates, one from the MS/CIS cohort and one from the CIP cohort, also produced faint membrane binding (1+) on the neurofascin-155 CBA that was not sufficient to be called positive at 1:100. Repeat testing of these eluates at 1:20 increased the signal to 2+ and 3+ respectively. However, this titre is below the usual positivity cut-off for this assay, and no signal was produced with any of the IgG subclass-specific secondary antibodies. All of these eluates were negative on the neurofascin-155 ELISA and negative for all other antigens by both CBA (including neurofascin-186, Figure 4B) and ELISA (results not shown).
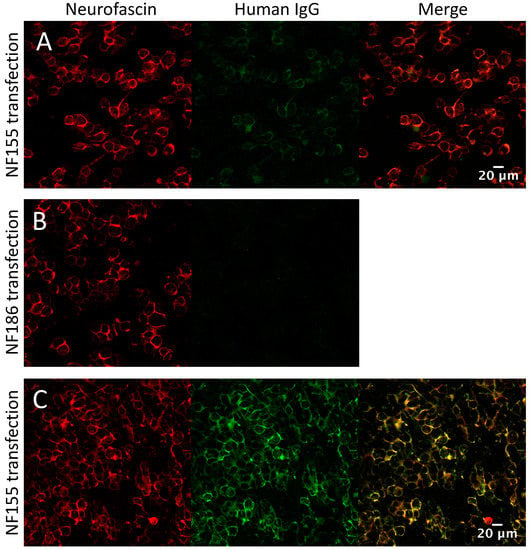
Figure 4.
Nodal/paranodal cell-based assays. (A) MS/CIS eluate weakly positive on the neurofascin-155 CBA at 1:100 (Score 2+, end-point titre 1:200) and (B) negative on the neurofascin-186 CBA. (C) Strong positive at 1:100 (Score 4+, end-point titre 1:3200) from the antibody positive CIDP cohort shown for comparison.
3.5. Screening the IA Eluates for Novel Antibodies Using Myelinating Co-Cultures
In this experiment, eluates from the first treatment session of each IA cohort were compared with purified IgG from the serum of 22 healthy control volunteers (gratefully received from A/Prof Sarosh Irani, University of Oxford) isolated by Protein G purification. Serum was not available in sufficient quantities from PNAb cohort to purify IgG and these samples were therefore not tested in this experiment. IgG from IA eluates (1:50 dilution) and protein G purification (1:12.5 dilution) were applied to myelinated human sensory neuron cultures in a 96 well, flat-bottom imaging plate format enabling high-throughput staining and imaging. The mean IgG concentration after dilution was not significantly different between the groups (One-Way ANOVA: F(3,78) = 1.500, p = 0.2211) (Figure 5A). Out of 82 samples tested, 1 CIDP (patient 11), 1 GBS (patient 07, who was also concurrently identified as HIV positive, see Appendix B.2 for further detail) and 1 MS/CIS (patient 13) sample were scored as ‘positive’ for either axonal, glial or nodal IgG deposition by an observer blinded to the patient group; a further 1 MS/CIS patient sample with weak IgG labelling was marked ‘equivocal’. All 4 of these sera and IA eluates were negative on the glycolipid and paranodal antibody assays, as above. Pre-treatment serum from MS patient 13 was also negative on our in-house live CBAs for aquapaorin-4 and MOG antibodies. Neither of the MS/CIS eluates which produced a weak signal on the neurofascin-155 CBA were positive on the co-culture assay.
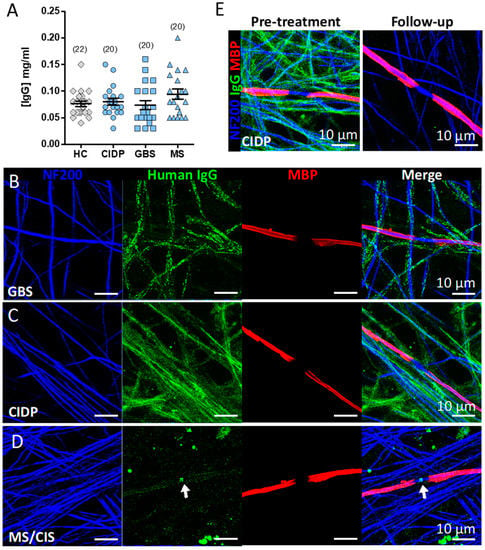
Figure 5.
IgG deposition in myelinated co-cultures. (A) IgG concentration of dilution-adjusted eluates used for screening on myelinated cultures. (B–D) Immunofluorescence images of IgG binding patterns in myelinating co-cultures of IA eluates (1:50) from three patients with neurological disease identified in the screening assay: B) GBS (patient 07), C) CIDP (patient 11), and D) MS/CIS (patient 13) (arrow indicates IgG deposition at the node of Ranvier). (E) IgG labelling in myelinated co-cultures of serum (1:50) sampled from the CIDP (patient 11) before (Pre-treatment) and after IA (Follow-up). Note all IgG immunoreactivity is lost at follow-up. NF200, neurofilament 200; MBP, myelin basic protein.
Serum samples taken pre- and post-IA from the four candidate patients (1:50 dilution) were further validated on myelinated cultures plated on 13 mm coverslips with careful attention paid to media changes and washing steps. Strong IgG deposition aligned with neurofilament positive axons was observed in the serum and IA eluate of the GBS (patient 07) (Figure 5B) and CIDP (patient 11) (Figure 5C) patients. We confirmed nodal reactive IgG in the serum and IA eluate of one MS patient (Figure 5D and Video S1), which was absent from post-IA serum. The post-treatment follow-up serum from the CIDP (patient 11) patient was negative for any IgG reactivity (Figure 5E). No IgG reactivity was observed in the serum or eluate of the MS/CIS patient 13 previously marked as equivocal, confirming this as a false positive. Clinical vignettes describing the patients with IgG deposition on co-cultures are given in Appendix B.
4. Discussion
In the PNAb cohort, we found that PLEx or IA were more often subjectively judged to have been effective in seronegative cases, and that in contrast, detection of at least one of the known nodal/paranodal antibodies in patients with inflammatory neuropathies was not associated with clinicians perceiving a positive response to either treatment. The proportion of PNAb-negative patients judged to have had a partial or better response (62.1%) was similar to the proportion of patients judged to have had a partial or better response in the IA cohort (52.5% overall), all of whom were also negative for known nodal/paranodal antibodies. We emphasise that the evaluation of the PNAb cohort is limited by the retrospective and subjective nature of the patient assessment. In addition, the small number of cases precludes us from reaching any conclusions regarding the objective benefits of one treatment modality compared to the other in this setting. In addition, improvement in neurological symptoms following IA/PLEX may occur after a delay, which may not be reflected in the immediate judgement of the treating physician. Blinding, randomisation, standardised follow up, as well as a control group to judge the natural history of these heterogeneous diseases, are required for a definitive evaluation of apheresis treatment efficacy in these patient groups. However, it is notable that treating physicians were less likely to think that apheresis had been effective in PNAb-positive patients.
Why seropositive patients were rarely assessed to have responded positively to either IA or PLEx is unclear. Our close monitoring of a prospectively-identified neurofascin-155 positive individual showed that while IA given as a mono-therapy was able to effectively reduce antibody titres, levels quickly rebounded and reached pre-treatment levels inside 4 weeks. This transient serological effect was not sufficient to reduce disability. More prolonged suppression of antibody titres, with frequent apheresis cycles or adjuvant therapies, may therefore be required for effective treatment in such cases.
Rituximab has previously been suggested as an effective treatment for paranodal antibody positive patients [42,43], but may take several weeks (or even months) to produce benefit. In this case, a second cycle of IA, 4 weeks after a course of rituximab, produced a more persistent suppression of antibody titres, which was associated with clinical improvement. The extent to which IA contributed to this effect is unclear. Theoretically, the more rapid action of IA might be complementary to the delayed but more sustained effects of rituximab. Whether this combination of treatment offers significant benefit over rituximab alone requires further investigation.
Retrospective analysis of serum samples from 60 IA-treated patients failed to identify any individuals who would have been classified as positive on routine diagnostic testing for previously described nodal/paranodal and glycolipid antibodies. A small number of first-treatment IA eluates did produce a low-level signal on the neurofascin-155 CBA. Whilst the diagnostic importance of low-titre, non-IgG4 results has been doubted [44], a pathogenic role for these antibodies cannot be ruled out.
The apparently better response of seronegative patients to apheresis, particularly IA, has several possible explanations. One is that these differences simply reflect variation in the disease characteristics and natural progression of seropositive versus seronegative inflammatory neuropathies: Overall, seropositive patients tend to have more severe, aggressive disease that is refractory to treatment [30,31,32]. Conversely, less severely affected, seronegative, patients may be more likely to have a monophasic disease course and stabilise or improve, independent of any particular therapy. Indeed, the median peak disability, measured by nadir modified Rankin score (mRs), of apheresis-treated PNAb+ patients in our series was higher, albeit non-significantly, than that of the apheresis-treated seronegative group (median nadir mRs 5 v 4, p = 0.1, Mann-Witney test, Table 1), although there was no significant difference in the use of, or clinician evaluated response to, other treatment modalities. There was also no significant difference in the proportion of patients initially diagnosed as GBS (28.6% and 30.3%, p > 0.99) compared to CIDP (66.7% and 54.5%, p = 0.41) in the PNAb+ and PNAb-negative groups, respectively (Fisher’s exact test, Table 1). However, this does not exclude the possibility that patients in the seronegative group may often have a shorter disease course, with less irreversible axonal degeneration.
Another explanation for perceived apheresis efficacy in seronegative patients is the presence of antibodies below the threshold for positive detection on diagnostic testing, leading to a correspondingly slower rebound in titres following PLEx/IA and a more sustained suppression of antibody levels. A further possibility is that the response to IA in diagnostically seronegative patients is due to the therapeutic removal of as-yet uncharacterised, pathologically relevant antibodies in these patient groups. We therefore tested for further nerve-related antigens by screening eluates from the IA cohort against myelinating co-cultures. Three positive IA eluate samples were identified in the original 96-well co-culture screen and were further validated in a larger 24-well format, confirming similar binding patterns. IgG from one GBS patient co-localised with NF200 suggesting an axonal antigen. One CIDP patient serum and IA eluate showed IgG binding that aligned with NF200-positive axons but may also reflect deposition on non-myelinating Schwann cells.
One patient’s serum and IA eluate from the MS/CIS group revealed nodal specific IgG binding. The presence of antibodies against nodal antigens such as neurofascin, has precedence in MS, and although uncommon, is more predominant in chronic progressive forms of the disease [45]. However, this sample was negative for antibodies against both the glial/paranodal and nodal/axonal isoforms of neurofascin (NF155 and NF186, respectively). The original focus on peripheral neuropathies led us to use a sensory neuron system for the myelinating cultures. Nevertheless, multiple peripheral nerve antigens are also found in the CNS (and vice versa), including NF155, CNTN1 and the ganglioside GM1. Therefore, it is quite feasible for the unknown antigen targeted by IgG in this CIS/MS patient to be mutually expressed in the peripheral and central nervous systems (CNS). Other autoantibodies against nerve and glial structures in the CNS including myelin basic protein, myelin-associated lipids, contactin-2, and KIR4.1 are among those proposed in MS patients [46]; however, their presence may not be specific to the disease [47]. For this reason, the inclusion of MS/CIS patients as a control group is potentially problematic. However, as patients with non-autoimmune neurological disease essentially never receive apheresis treatment, the inclusion of this group was a pragmatic way to obtain non-neuropathy IA eluates for use in our unbiased screening assays. With some similarity to the discovery of nodal/paranodal antibodies in chronic neuropathies, MS has recently been separated from other distinct, serologically-defined disorders, characterised by the presence of aquaporin-4 or myelin oligodendrocyte glycoprotein (MOG) directed autoantibodies. Whether the nodal antigen targeted by antibodies in this MS patient has a pathogenic role and might similarly define a non-MS disease entity is currently unknown. Further investigation using brain tissue may help elucidate the antigen target, pathological potential, and clinical relevance. Unfortunately, purified Ig/eluate was not available from the PNAb-negative apheresis cohort, and it is possible that novel autoantibodies are also present in some of these patients.
The two patients for whom follow-up samples were available (CIDP and MS/CIS) had no detectable IgG labelling in their serum after IA compared to pre-treatment. Thus, IA is effective at removing both established and potentially novel pathogenic autoreactive IgG from the circulation. Follow-up serum samples at later time points will help correlate any changes in disease progress with antibody titres.
Development of myelinated hiPSC-derived neuronal cultures in a 96-well format allowed for efficient simultaneous screening of >80 IgG eluates from patients and controls. The benefits of using live cultures for screening are the presence of complex structures including nodes of Ranvier, paranodal and juxtaparanodal regions, and compact myelin internodes, that provide an unbiased substrate for antibody screening against nerve-related antigens in their native conformation. IgG binding patterns ranged from broad axonal coverage to focal nodal localisation, reflecting morphologically distinct antigens. Images were acquired by an experienced observer who was blind to the sample identity. Although time-consuming, acquisition in such a supervised manner aids the detection of localised signals, such as the node-specific labelling identified in one MS/CIS patient.
A single sample that was marked as ‘equivocal’ on the 96-well assay was subsequently confirmed as negative. The minimal occurrence of non-specific IgG labelling in the 96 well format may reflect a lower washing efficiency in the smaller volume of the 96-well plate. Nevertheless, no healthy control samples were identified as positive in the screen, suggesting the cultures are useful as a selective substrate for nerve-targeted autoantibodies.
IA is rarely performed on healthy subjects; therefore control IgG were prepared from the sera of healthy volunteers by protein G purification. IgG concentrations in the healthy samples were normalised to the patient IA eluates such that the mean IgG concentrations were not significantly different, however the variation within each group was maintained in order to reflect the original sample. The detection of specific signals in both the serum and IA eluate of each of the three positive patients suggests that a uniform dilution of 1:50 is sufficient for antibody screening within IA eluates. We cannot, however, exclude the possibility of further antibodies below the level of detection. In summary, our findings of nerve antigen reactive antibodies in three ‘seronegative’ neurological patients suggest the utility of an unbiased screening system such as we have described here for the myelinating co-cultures. The development of equivalent cultures containing CNS antigens and cell-types may be of further benefit to relevant MS cases.
5. Conclusions
Currently available serological tests do not unambiguously identify patients who are likely to respond to IA or PLEx. In patients with nodal/paranodal antibody associated neuropathies, frequent plasmapheresis and/or additional therapies may be required to produce an acceptable level and duration of clinical improvement. Prospective longitudinal studies involving standardized and validated outcome measures, with serial monitoring of auto-antibodies, are needed to optimise apheresis treatment regimens and accurately assess efficacy.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/7/2025/s1, Video S1: 3D reconstruction of IgG deposition (green) at the node of Ranvier after incubation with MS serum.
Author Contributions
Conceptualization, S.R. and J.D.; methodology, A.J.D., J.F., H.T., J.D., S.R., M.S.; validation, A.J.D., J.F., H.T., M.S., J.D., S.R.; formal analysis, A.J.D., J.D., S.R.; investigation, A.J.D., J.F., H.T., M.S., J.D., S.R.; resources, J.D., S.R.; data curation, A.J.D., J.F., J.D., S.R.; writing—original draft preparation, A.J.D., S.R.; writing—review and editing, A.J.D., J.F., H.T., J.D., S.R., M.S.; visualization, A.J.D., S.R.; supervision, S.R.; project administration, S.R.; funding acquisition, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical Research Council, grant number MR/P008399/1 (awarded to S.R.) and the GBS/CIDP Foundation International Benson Fellowship (awarded to J.F.). A.D. is also supported by a Human Immune Discovery Initiative grant (BRCRCF19-04) from the National Institute for Health Research. Support for assay development was received from the John Fell Fund, University of Oxford.
Acknowledgments
The authors would like to thank Sarosh Irani, University of Oxford, for providing serum samples from control subjects used to prepare the control IgG, and all clinicians who sent samples and completed request forms for nodal/paranodal antibody testing.
Conflicts of Interest
S.R. runs a not-for-profit diagnostic testing service for nodal/paranodal antibodies. He has received a speaker’s honorarium and travel expenses from Fresenius Medical Care. A.D. is named inventor on a patent for immune cell therapy in nerve injury and has received travel grants from IASP and Biolegend. M.S. has received consulting and/or speaker honoraria from Bayer, Biogen, Merck, Roche, and Sanofi Genzyme. She has received research funding from the Hertha-Nathorff-Program. J.D. reports research funds and speaker’s honoraria from Fresenius Medical Care GmbH and Fresenius Medical Care Deutschland GmbH. The Globaffin IA column used to treat the NF155 PNAb+ patient was provided free of charge by Fresenius on a trial basis. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Detailed Experimental Methods
Appendix A.1. Nodal/Parnodal Cell-Based Assays
All sera and IA eluates were screened for IgG antibodies to neurofascin-155, neurofascin-186, contatctin-1 and Caspr1 using a live, cell-based assay (CBA), following previously described methods with slight modification [32]. In brief, HEK293T cells on poly-L-lysine coated 13mm coverslips at 80–90% confluence were transiently transfected with human neurofascin-155 (RC228652, Origene) or human neurofascin-186 (courtesy of Jerome Devaux, University of Marseille) mammalian-expression vectors, or co-transfected with both human contactin-1 (CNTN1, EXA1153-MO29 Genecopoeia, Maryland, US) and human Caspr1 (EXMO417-MO2 Genecopoeia, Maryland, US) at equimolar concentrations, using Jet-PEI transfection reagent (101-10; Polyplus). After 16 h, the cells were washed and replaced with Dulbecco’s Modified Eagle Medium (DMEM) (Gibco) containing 10% fetal bovine serum (FBS). 24 hours later, sera and eluates diluted 1:100 in DMEM + BSA (1%) were incubated with the cells for 1 h at room temperature. Co-incubation with commercial chicken anti-neurofascin primary antibody, (1:1000) (Cat no. AF3235; R&D Systems, Bio-Techne) was used to confirm successful transfection and to assess for co-localisation with any bound human IgG. Following serum/eluate incubation, cells were washed 3 times with DMEM + HEPES (20 mM), and fixed for 5 minutes in 4% PFA. Secondary antibody incubation was with goat anti-human IgG-Fc specific-Alexa Fluor 488 (1:750) (Cat no. H10120; Life Tech) and goat anti-Chicken Alexa Fluor 546 1:1000 (Cat no. A11040; Life Tech). To determine antibody subclass unconjugated mouse anti-human IgG subclass 1-4 antibodies were used at 1:100 (Cat nos. I2513, I25635, I7260 I7385; Sigma-Aldrich, Merck) followed by a fluorescently tagged tertiary antibody goat anti-mouse Alexa Fluor 488 (1:750) (Cat no. A11029; Life Tech). Positivity was assessed by an observer blinded to the clinical data using fluorescence microscopy. Taking into account the intensity of the membrane signal and co-localisation of the human IgG signal with the commercial antibody, the assay was scored on a 5 point scale as follows: 4+ very strong positive, 3+ strong positive, 2+ positive, 1+ negative (non-specific background or faint/poorly co-localised human IgG signal only), 0 no human IgG binding seen.
Appendix A.2. Nodal/Paranodal ELISA
Individual wells of Nunc Maxisorp ELISA plates (Fisher Scientific) were coated overnight at 4 C with either human recombinant neurofascin-155 (NF155) (8208-NF; R&D systems), NF186 (TP329070; OriGene Technologies) or CNTN1 (10383-H08H; Sino Biological Inc) diluted to 1 g/ml in PBS. The coating solution was then removed and the plate blocked with 5% milk in PBS for 1 h at room temperature. Serum or eluates diluted 1:100 in 5% milk were then applied for 1h at room temperature then washed by 5 cycles of immersion in PBS. Anti-human IgG (Fc specific) peroxidase-conjugated anti-human IgG (A0170; Sigma) was used as the secondary antibody at 1:3000. The detection reaction was performed using 50 l o-Phenylenediamine dihydrochloride solution (OPD fast, Sigma), stopped after 20 minutes by the application of 25 l 4M sulphuric acid, and optical densities measured at 492 nm using a FLUOstar Omega plate reader (BMG Labtech). Wells with ODs greater than 0.1 above uncoated (PBS only) control wells were considered positive.
Appendix A.3. Ganglioside and Sulfatide ELISA
Ganglioside and sulfatide ELISAs were performed using Immunolon 2HB 96 well plates [48]. Wells were coated with 100 ul of GM1 or GQ1b bovine gangliosides diluted to 2 g/ml, or sulfatide to 5 g/ml, in methanol. Negative control wells contained methanol only. Plates were then air-dried overnight in the fume hood, placed at 4 C, and blocked with 2% BSA/PBS. Sera/eluates were diluted 1:100 in 1% BSA/PBS and incubated for 2 h at 4 C. Secondary antibodies and detection were the same as the nodal/paranodal ELISA, except that secondary antibody incubation was performed at 4 C.
Appendix A.4. Protein G IgG Purification
Healthy control sera (100 µl) were diluted 1:1 in sterile PBS, added to protein G columns (Cat. 28-4083-47, Ab SpinTrap, GE Healthcare) prepared according to the manufacturer’s instructions. Briefly, samples were incubated 15 min at 4 °C on rollers to bind IgG. Serum was then removed by centrifugation (100 g, 30 s) and columns washed twice with binding buffer (20 mM Na2PO4, pH 7.0). IgG were eluted with 320 µl 0.1M glycine (pH 2.6) and neutralised with 80 µl Tris-HCl (pH 8.0). Elution was repeated once more and samples taken forward for IgG quantification.
Appendix A.5. IgG ELISA
IgG in serum and eluates was quantified by enzyme-linked immunosorbent assay (ELISA) using a human IgG ELISA quantification kit (Cat. E80-104, Bethyl Laboratories Inc. TX, US) according to the manufacturer’s instructions. Briefly, 96 well plates (Maxisorp, Nunc) were coated with goat anti-human IgG-Fc capture antibody (10 µg/ml) in coating buffer (0.05M carbonate-bicarbonate, pH 9.6) (100 µl/well) for 1h at room temperature (RT). Plates were washed 5 times by immersion in wash buffer (50mM Tris, 0.14M NaCl, 0.05% Tween 20, pH 8.0), blocked for 1h at RT in blocking buffer (50mM Tris, 0.14M NaCl, 1% BSA, pH 8.0), followed again by immersion 5 times in washing buffer. Serum samples and IgG eluates were prepared at 1:10,000 dilution in sample diluent, as well as a dilution series of human reference serum standards (50mM Tris, 0.14M NaCl, 1% BSA, 0.05% Tween 20, pH 8.0). All samples and standards were prepared in duplicate (100 µL/well) and incubated 1h at RT. After 5x immersion washes 100 μL of HRP-conjugated goat anti-human IgG-Fc Detection Antibody (1:200,000) was incubated 1h at RT followed by 5 immersion washes. Plates were developed by the addition of 100 μL of TMB substrate solution (20 min, RT) and reaction stopped by adding equal volume of 0.18M H2SO4. Absorbance values were read immediately on a plate reader (FLUO Star Omega, BMG Labtech) at 450nm (signal) and 630nm (background). A standard curve was constructed from the background subtracted absorbance (OD) values obtained from the human serum standards using a 4-parameter function (https://mycurvefit.com/). IgG concentrations of each sample were calculated from averages of the duplicate, background-subtracted OD values, multiplied by the original dilution. All values for diluted samples fell within the standard curve (1–1000 ng/mL).
Appendix A.6. Myelinating Co-Cultures
Myelinating co-cultures were prepared using human induced pluripotent stem cells (hiPSC)-derived sensory neurons and primary rat Schwann cells with some modifications to previously described methods [49]. hiPSCs from control subjects were obtained via the StemBANCC consortium at the University of Oxford (https://www.ndcn.ox.ac.uk/research/stembancc). In brief, hiPSCs were differentiated to sensory neurons using a combination of small-molecule mediated dual-SMAD inhibition and wnt activation. On day 11 of differentiation, sensory neuron precursors were seeded onto 13 mm diameter glass coverslips (approximately 20,000 cells per coverslip) or 96-well flat, glass-bottom imaging plates (Sensoplate Microplate, Greiner-Bio) (approximately 5000 neurons per well) previously coated with poly-D-lysine (PDL) (10 µg/mL) overnight and reduced growth-factor matrigel (Corning). Neurons were maintained in neurobasal media supplemented with N2, B27, Glutamax and anti-anti (all Gibco, Life Technologies) (‘complete’ neurobasal) plus recombinant human β-NGF (rhNGF) (Cat. 450-01, Peprotech), NT3 (Cat. 450-03, Peprotech), GDNF (Cat. 450-10, Peprotech), and BDNF (Cat. PHC7074, Life Technologies) (all growth factors 25 ng/ml), supplemented with Rho-associated, coiled-coil containing protein kinase (ROCK) inhibitor (10 µM) (Tocris, Bio-Techne) on days 11–12, CHIR99021 (3 µM) (Sigma) on days 11–14 and cytosine arabinoside (Ara-C) (1 µM) (Sigma) on days 12–14. Neurons were incubated at 37 °C in 5% CO2 for 4 weeks with twice-weekly medium changes prior to addition of Schwann cells for myelination.
Primary Schwann cells were isolated from the sciatic nerves of rat pups (P2-3). Mother and pups were killed by rising concentration of CO2 in accordance with Schedule 1 of the UK Home Office Animals (Scientific Procedures) Act 1986. Sciatic nerves were rapidly dissected and digested in a mixture of collagenase (3mg/ml) (Worthington, Lorne Labs) and dispase II (3.5mg/mL) (Roche) for 1 h at 37 °C with frequent gentle agitation. Nerves were washed in DMEM + FBS (10%) and gently triturated using a fire-polished glass Pasteur pipette. Dissociated cells were seeded into tissue culture flasks overnight and expanded in Schwann cell expansion medium containing charcoal-stripped FBS (10%) (Sigma), Forskolin (4 µM), recombinant human NRG1-β1 EGF domain (80 ng/mL) (Cat. 396-HB, R&D Systems) and recombinant murine NGF (10 ng/ml) (Cat. 450-34, Peprotech) in DMEM/F12 (Gibco). Cells were serially treated with 5–10 µM Ara-C to eliminate fibroblasts. Expanded Schwann cells were added to the neuronal cultures (25,000 cells per coverslip or 5000 cell per 96-well) and allowed to proliferate and align with the axons for 1 week in basal media containing: (CS-FBS) (10%), insulin (5 mg/ml) (Sigma), holo-transferrin (100 mg/mL) (Sigma), rhNGF (25 ng/mL) (Peprotech) (Sigma), Selenium (25 ng/mL) (Sigma), 25 ng/ml thyroxine (Sigma), progesterone (30 ng/ml) (Sigma), triiodothyronine (25 ng/mL) (Sigma) and putrescine 8 mg/mL (Sigma) in DMEM/F12 media (Gibco, Life Technologies). From this point on, cultures were maintained in ‘myelination medium’ containing: 5% CS-FBS, ascorbic acid (25 µg/mL), phenol-free matrigel (1:300) (Corning) and hrNGF (25 ng/mL) in ‘complete’ neurobasal medium. Myelinating cultures were matured for at least 4 weeks before use in subsequent experiments.
Appendix A.7. Myelinated Co-Culture Immunreactivity Screening
Sera or IgG eluates were diluted in neurobasal ‘complete’ media (including 1% BSA and human NGF, 50 ng/mL), added to myelinated co-cultures either in a 96 well plate (100 µL/well) or coverslips in a 24 well plate (300 µL/well) format and incubated for 1h at 37 °C. Serum containing antibodies to known antigens, as well as normal human serum, were run as positive and negative controls, respectively. For 96-well plate screening, serum samples were blinded by an independent investigator. Cultures were then washed 4x with pre-warmed PBS and fixed with 2% PFA in PBS for 30 min at RT. Wells were washed with PBS followed by DMEM plus HEPES (20 mM). Cultures were then labelled with Alex488-conjugated goat anti-human IgG (H+L) (A11013, Life Technologies) secondary antibody (1:750) in DMEM/HEPES plus 1% BSA, 1h at RT followed by washing 2x with DMEM/HEPES and 3x PBS. Cultures were then permeabilised with ice cold methanol (45 min on ice), blocked with 5% normal goat serum and incubated with chicken anti-neurofilament (NF)200 (1:10,000) (ab4680, Abcam) and rat anti-myelin basic protein (MBP) (1:500) (ab7349, Abcam) primary antibodies overnight at 4 °C. After washing in PBS antibodies were labelled with goat anti-chicken biotin (1:500) (BA-9010, Vector Laboratories) and goat anti-rat Alexa 546 (1:1000) (A11081, Life Technologies) secondary antibodies for 1h at RT, followed by streptavidin pacific blue (1:500) (S11222, Life Technologies) 45–60 min at RT. After washing in PBS coverslips were mounted onto glass slides (SuperFrost, ThermoScientific) with Vectorshield (H1000, Vector Laboratories) and stored at −20 °C prior to imaging. 96-well plates were flooded with PBS containing 0.02% NaN3 and sealed with plate-sealing film. Plates were stored at 4 °C until imaging. Confocal images were acquired with a x63 oil-immersion lens (1024 × 1024 resolution) and exported as maximum intensity projection of 4–5 × 1 µm interval z-section images. Plates were allowed to reach room temperature before imaging.
Appendix B. Baseline Clinical Features of IA Cohorts and Clinical Vignettes of Patients with IgG Deposition in Co-Cultures

Table A1.
Baseline characteristics and response to IA treatment of the CIDP cohort.
Table A1.
Baseline characteristics and response to IA treatment of the CIDP cohort.
| ID | Age | Sex | Disease Duration (mo) | Steroids Yes/No | IVIg Yes/No | Other Immunosuppression Used 1 | IA Cycles | CIDP-Score Baseline | CIDP Score at 2 Weeks | Progression before IA | Progression after IA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 * | 59 | M | 83 | Y | Y | 1 | 296 | 298 | |||
| 02 * | 61 | M | 114 | Y | Y | AZA | 3 | 376 | 416 | 2.9 | 0 |
| 03 * | 58 | M | 158 | Y | N | AZA, CPM, MPM | 9 | 102 | 118 | 4.1 | 0.1 |
| 04 * | 65 | M | 114 | Y | N | 1 | 434 | 438 | |||
| 05 * | 67 | F | 112 | Y | Y | 1 | 435 | 435 | |||
| 06 * | 60 | M | 104 | Y | Y | 1 | 268 | 313 | |||
| 07 * | 80 | M | 66 | Y | N | AZA | 3 | 306 | 342 | 19.3 | 0.8 |
| 08 * | 62 | M | 73 | N | Y | 5 | 231 | 361 | 13.1 | 0.7 | |
| 09 * | 68 | M | 67 | Y | Y | 4 | 373 | 364 | 6.7 | 0 | |
| 10 * | 75 | M | 134 | Y | Y | 1 | 326 | 326 | |||
| 11 * | 66 | M | 166 | Y | Y | AZA, MTX | 1 | 308 | 316 | ||
| 12 * | 72 | M | 65 | N | Y | 3 | 314 | 330 | 8.7 | 2.0 | |
| 13 * | 66 | F | 64 | Y | N | 1 | 314 | 282 | |||
| 14 * | 60 | M | 102 | Y | N | 2 | 297 | 382 | 3.0 | 0 | |
| 15 * | 66 | F | 86 | Y | Y | 1 | 393 | 405 | |||
| 16 * | 68 | M | 62 | Y | Y | MPM | 1 | 264 | 264 | ||
| 17 | 67 | M | 65 | Y | N | 3 | N/A | N/A | N/A | N/A | |
| 18 | 53 | M | 97 | Y | Y | 1 | N/A | N/A | N/A | N/A | |
| 19 | 67 | F | 94 | Y | Y | AZA | 2 | N/A | N/A | N/A | N/A |
| 20 | 67 | M | 201 | Y | Y | 1 | N/A | N/A | N/A | N/A |
* These patients were included in a previous publication [33]. 1 AZA=azathioprine, CPM=cyclophosphamide, MPM=mycophenolate-mofetil, MTX=methotrexate.

Table A2.
Baseline characteristics and response to IA treatment of the GBS cohort.
Table A2.
Baseline characteristics and response to IA treatment of the GBS cohort.
| ID | Age | Sex | 1st/2nd/3rd-Line | PLEx Yes/No | IVIg Yes/No | PLEx / IA Cycles | Clinical Outcome |
|---|---|---|---|---|---|---|---|
| 01 | 76 | F | 2 | N | Y | 1 | 0 |
| 02 | 73 | M | 3 | Y | Y | 2 | (+) |
| 03 | 36 | F | 2 | N | Y | 1 | (+) |
| 04 | 76 | M | 2 | N | Y | 2 | ++ |
| 05 | 64 | M | 2 | N | Y | 1 | 0 |
| 06 | 31 | F | 1 | N | N | 1 | ++ |
| 07 | 52 | M | 1 | Y | N | 1 | + |
| 08 | 33 | F | 1 | N | N | 1 | 0 |
| 09 | 53 | F | 2 | N | Y | 1 | + |
| 10 | 38 | F | 2 | N | Y | 1 | ++ |
| 11 | 89 | M | 2 | N | Y | 1 | + |
| 12 | 75 | M | PLEx (1) | Y | N | 1 | + |
| 13 | 66 | F | PLEx (1) | Y | Y | 1 | 0 |
| 14 | 66 | F | PLEx (1) | Y | N | 1 | ++ |
| 15 | 42 | M | PLEx (1) | Y | N | 1 | + |
| 16 | 77 | F | PLEx (1) | Y | N | 1 | + |
| 17 | 67 | M | 2 | N | Y | 1 | ++ |
| 18 | 77 | M | PLEx (2) | Y | Y | 1 | (+) |
| 19 | 62 | M | PLEx (1) | Y | N | 1 | + |
| 20 | 66 | F | 2 | N | Y | 1 | + |
Outcome: 0 no response; (+) equivocal response; + partial response; ++ good response.

Table A3.
Baseline characteristics and response to IA treatment of the MS cohort.
Table A3.
Baseline characteristics and response to IA treatment of the MS cohort.
| ID | Age | Sex | Diagnosis | DMT | Symptoms | MP | IA Cycles | EDSS before IA | EDSS after IA |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 44 | F | CIS | none | ON | 5×1g iv 5×2g iv | 1 | 2.0 | 0.0 |
| 02 | 21 | M | MS | none | Sensory deficits UE+LE | 5×1g iv | 1 | 4.0 | 3.0 |
| 03 | 48 | M | MS | none | Sensory deficits UE+LE | 5×1g iv | 1 | 4.0 | 3.0 |
| 04 | 18 | F | CIS | none | ON | 7×1g iv | 1 | 1.0 | 1.0 |
| 05 | 30 | F | MS | dimethyl fumarate | Sensory deficits UE | 2×5x1g iv 2×5x1g it | 1 | 1.0 | 1.0 |
| 06 | 46 | F | MS | none | Sensory deficits UE+LE, gait ataxia | 5×1g iv | 1 | 6.5 | 6.5 |
| 07 | 28 | F | MS | dimethyl fumarate | Sensomotoric deficits UE+LE | 5×1g iv 5×1g iv | 1 | 6.5 | 6.5 |
| 08 | 26 | F | MS | dimethyl fumarate | Sensory deficits UE | 4×1g iv | 1 | 3.0 | 3.0 |
| 09 | 19 | M | MS | none | Organic psycho syndrome | 5×1g iv | 2 | 5.5 | 3.0 |
| 10 | 20 | F | MS | none | ON | 5×1g iv | 1 | 2.0 | 1.0 |
| 11 | 47 | F | MS | fingolimod | Motor deficits UE+LE | 5×1g iv | 1 | 7.0 | 6.0 |
| 12 | 19 | F | MS | none | ON (bilateral), hemihypesthesia | 5×1g iv 5×2g iv | 1 | 2.5 | 2.5 |
| 13 | 49 | F | MS | fingolimod | ON, paraparesis | 5×1g iv | 1 | 4.5 | 4.5 |
| 14 | 23 | F | CIS | none | ON | 5×1g iv 5×1g iv | 1 | 2.0 | 1.0 |
| 15 | 50 | F | MS | none | ON (bilateral), gait ataxia | 5×1g iv 5×2g iv | 1 | 5.0 | 4.0 |
| 16 | 15 | M | MS | none | Dysarthria, dysphagia | 12×1g iv | 1 | 4.0 | 3.0 |
| 17 | 17 | F | CIS | none | ON | 5×1g iv 5×1g iv | 1 | 1.0 | 1.0 |
| 18 | 46 | F | MS | interferon beta 1a | Sensory deficits UE+LE, gait ataxia | 5×1g iv | 1 | 3.5 | 3.0 |
| 19 | 57 | F | MS | none | Paraparesis, hemihypesthesia | 5×1g iv | 1 | 4.0 | 4.0 |
| 20 | 37 | M | MS | none | Paresis LE | 5×1g iv 5×2g iv | 1 | 6.0 | 5.5 |
MS—Multiple Sclerosis; CIS—Clinically Isolated Syndrome; ON—optic neuritis; UE—upper extremities; LE—lower extremities; MP—methyl prednisolone; DMT—actual disease-modifying treatment; EDSS—Expanded Disability Status Scale.
Appendix B.1. CIDP (Patient 11)
This 66-year-old male first developed sensory deficits, myalgia, and gait disturbance in 2007, followed in 2009 by asymmetric distal weakness in the legs then arms, and after 3 years, worsening neuropathic pain and trigeminal nerve dysfunction. Routine bloods, serum protein electrophoresis with immunofixation, and an extensive autoantibody screen revealed no abnormalities. Neve conduction studies showed a demyelinating, sensory-motor neuropathy (reduced nerve conduction velocities, prolonged motor distal latencies, prolonged F-wave latencies, and temporal dispersion in multiple nerves), meeting the EFNS criteria for definite CIDP. EMG showed no evidence of myopathy. First line treatment with high dose then tapering corticosteroids was initiated in 2007. This produced some improvement in myalgia but no other benefit and was stopped after a few weeks due to unacceptable side effects (multiple infections). Further progression in 2009 led to the use of IVIg and the introduction of azathioprine, which was again stopped after a few weeks due to adverse reactions. High-dose, pulsed, corticosteroids were again used in 2011, and methotrexate was also introduced. The clinical picture stabilised but these therapies could not be continued due to recurrent urosepsis. The patient then received 1 cycle (5 treatment sessions and 12 plasma volumes in total) of IA in 2015 without further improvement in his clinical picture after 2 weeks.
Appendix B.2. GBS (Patient 07)
This 52-year-old male presented in 2017 with neuropathic pain, limb-weakness, and facio-bulbar cranial nerve dysfunction. There was a rapid worsening over the next few days to complete tetraplegia, with autonomic involvement (bradycardia) and respiratory insufficiency, necessitating transfer to intensive care for ventilatory support. Nerve conduction studies showed a demyelinating, sensory-motor neuropathy. The CSF protein was elevated at 1.2 g/L, as was the CSF white cell count at 19 per mm3. The white cells were classified as activated lymphocytes and monocytes. No infectious organisms were identified in the CSF despite extensive testing. A subsequent serological HIV test was positive, initially showing 376000 HIV RNA copies per ml. This confirmed a new diagnosis of HIV infection, and raises the possibility that this gentleman’s GBS was associated with HIV seroconversion. However, in the absence of serial serological testing, we cannot confirm this unequivocally. The CD4/CD8 ratio was 0.34 (reduced). Standard IVIg treatment did not produce any immediate improvement. Antiretroviral therapy was commenced with an associated decline in viral load over the next few weeks, reducing HIV RNA copies to 100/ml. IA therapy had to be delayed multiple times due to recurrent infections and other complications. It was finally started about 6 weeks after onset of symptoms. Following 5 days of IA, there was a slow improvement in strength over the next 14 days, with a limited return of movement in the arms and legs. After a subsequent cycle of plasma exchange, this slow improvement continued. The patient was transferred to an early rehabilitation clinic about 3 months after onset of symptoms.
Appendix B.3. MS (Patient 13)
This 37-year-old male was diagnosed with highly active multiple sclerosis in 1998. This followed a relapsing-remitting course, with an accumulation of residual deficits producing a persistent spastic tetraparesis. Brain and spinal MRI were performed, showing multiple supra- and infratentorial, as well as spinal T2-hyperintense lesions with Gadolinum-enhancement in the cervical cord. Aquaporin-4- and MOG- antibodies were negative. The patient had previously received multiple disease modifying therapies, including beta-interferon, natalizumab, and currently fingolimod, but continued to experience relapses in the last year. A 2015 relapse with left sided optic neuritis was treated with high-dose prednisolone. This was associated with partial improvement and was followed with 5 days of IA. Further outcome data is not available.
References
- Rinaldi, S.; Bennett, D.L. Pathogenic mechanisms in inflammatory and paraproteinaemic peripheral neuropathies. Curr. Opin. Neurol. 2014, 27, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.J. The immunobiology of Guillain-Barre syndromes. J. Peripher. Nerv. Syst. 2005, 10, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Fehmi, J.; Scherer, S.S.; Willison, H.J.; Rinaldi, S. Nodes, paranodes and neuropathies. J. Neurol. Neurosurg. Psychiatry 2017, 89, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Chevret, S.; Hughes, R.A.; Annane, D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst. Rev. 2017, 2017, CD001798. [Google Scholar] [CrossRef]
- Mehndiratta, M.M.; Hughes, R.A.C.; Pritchard, J. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst. Rev. 2015, 2015, CD003906. [Google Scholar] [CrossRef]
- Eftimov, F.; Winer, J.B.; Vermeulen, M.; De Haan, R.; Van Schaik, I.N. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst. Rev. 2013, CD001797. [Google Scholar] [CrossRef]
- Hughes, R.A.C.; Raphaël, J.C.; Swan, A.V.; A Doorn, P. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst. Rev. 2004, CD002063. [Google Scholar] [CrossRef]
- Lieker, I.; Slowinski, T.; Harms, L.; Hahn, K.; Klehmet, J. A prospective study comparing tryptophan immunoadsorption with therapeutic plasma exchange for the treatment of chronic inflammatory demyelinating polyneuropathy. J. Clin. Apher. 2017, 32, 486–493. [Google Scholar] [CrossRef]
- Zinman, L.; Sutton, D.; Ng, E.; Nwe, P.; Ngo, M.; Bril, V. A pilot study to compare the use of the Excorim staphylococcal protein immunoadsorption system and IVIG in chronic inflammatory demyelinating polyneuropathy. Transfus. Apher. Sci. 2005, 33, 317–324. [Google Scholar] [CrossRef]
- Hadden, R.D.M.; Bensa, S.; Lunn, M.; Hughes, R. Immunoadsorption inferior to plasma exchange in a patient with chronic inflammatory demyelinating polyradiculoneuropathy. J. Neurol. Neurosurg. Psychiatry 2002, 72, 644–646. [Google Scholar] [CrossRef]
- Ullrich, H.; Mansouri-Taleghani, B.; Lackner, K.J.; Schalke, B.; Bogdahn, U.; Schmitz, G. Chronic inflammatory demyelinating polyradiculoneuropathy: Superiority of protein A immunoadsorption over plasma exchange treatment. Transfus. Sci. 1998, 19, 33–38. [Google Scholar] [CrossRef]
- Galldiks, N.; Burghaus, L.; Dohmen, C.; Teschner, S.; Pollok, M.; Leebmann, J.; Frischmuth, N.; Höllinger, P.; Nazli, N.; Fassbender, C.; et al. Immunoadsorption in Patients with Chronic Inflammatory Demyelinating Polyradiculoneuropathy with Unsatisfactory Response to First-Line Treatment. Eur. Neurol. 2011, 66, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, T.; Suzuki, N. Can immunoadsorption plasmapheresis be used as the first choice therapy for neuroimmunological disorders? Ther. Apher. 1997, 1, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Pernat, A.M.; Svigelj, V.; Ponikvar, R.; Buturović-Ponikvar, J. Guillain-Barré Syndrome Treated by Membrane Plasma Exchange and/or Immunoadsorption. Ther. Apher. Dial. 2009, 13, 310–313. [Google Scholar] [CrossRef]
- Arakawa, H.; Yuhara, Y.; Todokoro, M.; Kato, M.; Mochizuki, H.; Tokuyama, K.; Kunimoto, F.; Morikawa, A. Immunoadsorption therapy for a child with Guillain-Barre syndrome subsequent to Mycoplasma infection: A case study. Brain Dev. 2005, 27, 431–433. [Google Scholar] [CrossRef]
- Okamiya, S.; Ogino, M.; Ogino, Y.; Irie, S.; Kanazawa, N.; Saito, T.; Sakai, F. Tryptophan-immobilized Column-based Immunoadsorption as the Choice Method for Plasmapheresis in Guillain-Barre Syndrome. Ther. Apher. Dial. 2004, 8, 248–253. [Google Scholar] [CrossRef]
- Haupt, W.; Rosenow, F.; Van Der Ven, C.; Birkmann, C. Immunoadsorption in Guillain-Barré syndrome and myasthenia gravis. Ther. Apher. 2000, 4, 195–197. [Google Scholar] [CrossRef]
- Uetakagaito, M.; Horikawa, H.; Yoshinaka, H.; Tagawa, Y.; Yuki, N. Two Patients with Acute Guillain-Barré Syndrome Treated with Different Apheresis Methods. Ther. Apher. 1997, 1, 340–342. [Google Scholar] [CrossRef]
- Hirai, K.; Kihara, M.; Nakajima, F.; Miyanomae, Y.; Yoshioka, H. Immunoadsorption Therapy in Guillain-Barré Syndrome. Pediatric Neurol. 1998, 19, 55–57. [Google Scholar] [CrossRef]
- Chida, K.; Takase, S.; Itoyama, Y. Development of facial palsy during immunoadsorption plasmapheresis in Miller Fisher syndrome: A clinical report of two cases. J. Neurol. Neurosurg. Psychiatry 1998, 64, 399–401. [Google Scholar] [CrossRef]
- Ruiz, J.C.; Berciano, J.; Polo, J.M.; De Francisco, A.L.M.; Arias, M. Treatment of Guillain-Barré syndrome with protein-A immunoadsorption: Report of two cases. Ann. Neurol. 1992, 31, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Komaba, Y.; Araki, T.; Iino, Y.; Katayama, Y. Plasma Immunoadsorption Therapy for Guillain-Barré Syndrome: Critical Day for Initiation. J. Nippon. Med. Sch. 2002, 69, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Mahdi-Rogers, M.; Brassington, R.; A Gunn, A.; A Van Doorn, P.; Hughes, R.A. Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst. Rev. 2017, 2017, CD003280. [Google Scholar] [CrossRef] [PubMed]
- Rolfes, L.; Pfeuffer, S.; Ruck, T.; Melzer, N.; Pawlitzki, M.; Heming, M.; Brand, M.; Wiendl, H.; Meuth, S. Ruck Therapeutic Apheresis in Acute Relapsing Multiple Sclerosis: Current Evidence and Unmet Needs—A Systematic Review. J. Clin. Med. 2019, 8, 1623. [Google Scholar] [CrossRef] [PubMed]
- Lipphardt, M.; Wallbach, M.; Koziolek, M.J. Plasma Exchange or Immunoadsorption in Demyelinating Diseases: A Meta-Analysis. J. Clin. Med. 2020, 9, 1597. [Google Scholar] [CrossRef]
- Willison, H.J.; Yuki, N. Peripheral neuropathies and anti-glycolipid antibodies. Brain 2002, 125, 2591–2625. [Google Scholar] [CrossRef]
- Tagawa, Y.; Yuki, N.; Hirata, K. Ability to remove immunoglobulins and anti-ganglioside antibodies by plasma exchange, double-filtration plasmapheresis and immunoadsorption. J. Neurol. Sci. 1998, 157, 90–95. [Google Scholar] [CrossRef]
- Ng, J.K.M.; Malotka, J.; Kawakami, N.; Derfuss, T.; Khademi, M.; Olsson, T.; Linington, C.; Odaka, M.; Tackenberg, B.; Prüss, H.; et al. Neurofascin as a target for autoantibodies in peripheral neuropathies. Neurology 2012, 79, 2241–2248. [Google Scholar] [CrossRef]
- Querol, L.; Nogales-Gadea, G.; Rojas-García, R.; Martinez-Hernandez, E.; Diaz-Manera, J.; Suárez-Calvet, X.; Navas, M.; Araque, J.; Gallardo, E.; Illa, I. Antibodies to contactin-1 in chronic inflammatory demyelinating polyneuropathy. Ann. Neurol. 2012, 73, 370–380. [Google Scholar] [CrossRef]
- Querol, L.; Nogales-Gadea, G.; Rojas-Garcia, R.; Diaz-Manera, J.; Pardo, J.; Ortega-Moreno, A.; Sedano, M.J.; Gallardo, E.; Berciano, J.; Blesa, R.; et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology 2014, 82, 879–886. [Google Scholar] [CrossRef]
- Doppler, K.; Appeltshauser, L.; Villmann, C.; Martin, C.; Peles, E.; Krämer, H.H.; Haarmann, A.; Buttmann, M.; Sommer, C. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain 2016, 139, 2617–2630. [Google Scholar] [CrossRef]
- Delmont, E.; Manso, C.; Querol, L.; Cortese, A.; Berardinelli, A.; Lozza, A.; Belghazi, M.; Malissart, P.; Labauge, P.; Taieb, G.; et al. Autoantibodies to nodal isoforms of neurofascin in chronic inflammatory demyelinating polyneuropathy. Brain 2017, 140, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Dorst, J.; Ludolph, A.C.; Senel, M.; Tumani, H. Short-term and long-term effects of immunoadsorption in refractory chronic inflammatory demyelinating polyneuropathy: A prospective study in 17 patients. J. Neurol. 2018, 265, 2906–2915. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Suzuki, H.; Oka, N.; Ogata, H.; Yanagimoto, S.; Sadakane, S.; Fukumoto, Y.; Yamana, M.; Yuhara, Y.; Yoshikawa, K.; et al. ELectron microscopic abnormality and therapeutic efficacy in chronic inflammatory demyelinating polyneuropathy with anti-neurofascin155 immunoglobulin G4 antibody. Muscle Nerve 2017, 57, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Süfke, S.; Lehnert, H.; Gebauer, F.; Uhlenbusch-Körwer, I. Safety Aspects of Immunoadsorption in IgG Removal Using a Single-Use, Multiple-pass Protein A Immunoadsorber (LIGASORB): Clinical Investigation in Healthy Volunteers. Ther. Apher. Dial. 2017, 21, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Watanabe, S.; Okita, N.; Takase, S.; Tagawa, Y.; Yuki, N. Immunoadsorption therapy for Fisher’s syndrome: Analysis of the recovery process of external ophthalmoplegia and the removal ability of anti-GQ1b antibodies. Rinsho Shinkeigaku 1996, 36, 551–556. [Google Scholar]
- Belak, M.; Borberg, H.; Jimenez, C.; Oette, K. Technical and clinical experience with Protein A Immunoadsorption columns. Transfus. Sci. 1994, 15, 419–422. [Google Scholar] [CrossRef]
- Merkies, I.S.; Schmitz, P.I.M.; A Van Der Meché, F.G.; Samijn, J.; A Van Doorn, P. Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J. Neurol. Neurosurg. Psychiatry 2002, 72, 596–601. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2017, 17, 162–173. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef]
- Pns, J.T.F.O.T.E.A.T.; Efns, J.T.F.O.T.; Pns, T. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society-First Revision. J. Peripher. Nerv. Syst. 2010, 15, 1–9. [Google Scholar] [CrossRef]
- Querol, L.; Rojas-García, R.; Diaz-Manera, J.; Barcena, J.; Pardo, J.; Ortega-Moreno, A.; Sedano, M.J.; Seró-Ballesteros, L.; Carvajal, A.; Ortiz-Castellon, N.; et al. Rituximab in treatment-resistant CIDP with antibodies against paranodal proteins. Neurol. Neuroimmunol. Neuroinflammation 2015, 2, e149. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, C.; Franciotta, D.; Cortese, A.; Callegari, I.; Serrati, C.; Mancardi, G.L.; Schenone, A.; Leonardi, A.; Benedetti, L. Remarkable Rituximab Response on Tremor Related to Acute-Onset Chronic Inflammatory Demyelinating Polyradiculoneuropathy in an Antineurofascin155 Immunoglobulin G4-Seropositive Patient. Mov. Disord. Clin. Pr. 2018, 5, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Cortese, A.; Lombardi, R.; Briani, C.; Callegari, I.; Benedetti, L.; Manganelli, F.; Luigetti, M.; Ferrari, S.; Clerici, A.M.; Marfia, G.A.; et al. Antibodies to neurofascin, contactin-1, and contactin-associated protein 1 in CIDP: Clinical relevance of IgG isotype. Neurol. Neuroimmunol. Neuroinflammation 2019, 7, e639. [Google Scholar] [CrossRef]
- Stich, O.; Perera, S.; Berger, B.; Jarius, S.; Wildemann, B.; Baumgartner, A.; Rauer, S. Prevalence of neurofascin-155 antibodies in patients with multiple sclerosis. J. Neurol. Sci. 2016, 364, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Häusser-Kinzel, S.; Weber, M.S. The Role of B Cells and Antibodies in Multiple Sclerosis, Neuromyelitis Optica, and Related Disorders. Front. Immunol. 2019, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Prineas, J.W.; Parratt, J.D. Multiple sclerosis: Serum anti-CNS autoantibodies. Mult. Scler. J. 2017, 24, 610–622. [Google Scholar] [CrossRef]
- Willison, H.J.; Veitch, J.; Swan, A.V.; Baumann, N.; Comi, G.; Gregson, N.A.; Iiia, I.; Jacobs, B.C.; Zielasek, J.; Hughes, R.A.C. Inter-laboratory validation of an ELISA for the determination of serum anti-ganglioside antibodies. Eur. J. Neurol. 1999, 6, 71–77. [Google Scholar] [CrossRef]
- Clark, A.; Kaller, M.; Galino, J.; Willison, H.J.; Rinaldi, S.; Bennett, D.L. Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination. Brain 2017, 140, 898–913. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).