Apheresis in Autoimmune Encephalitis and Autoimmune Dementia
Abstract
:1. Introduction
1.1. Antibody-Mediated AE
1.2. Paraneoplastic AE
1.3. Therapy for AE
2. Search Strategy
2.1. Inclusion Criteria
2.2. Search Strategy
3. Results
3.1. Therapeutic Apheresis in Autoimmune Encephalitides
3.2. Therapeutic Procedure for Apheresis
3.3. Initiation of Therapy with Apheresis and Prior Treatment
3.4. Effects of Treatment with Apheresis in Patients with AE
3.5. Future Treatment Options for Apheresis
3.6. Apheresis in Children with AE
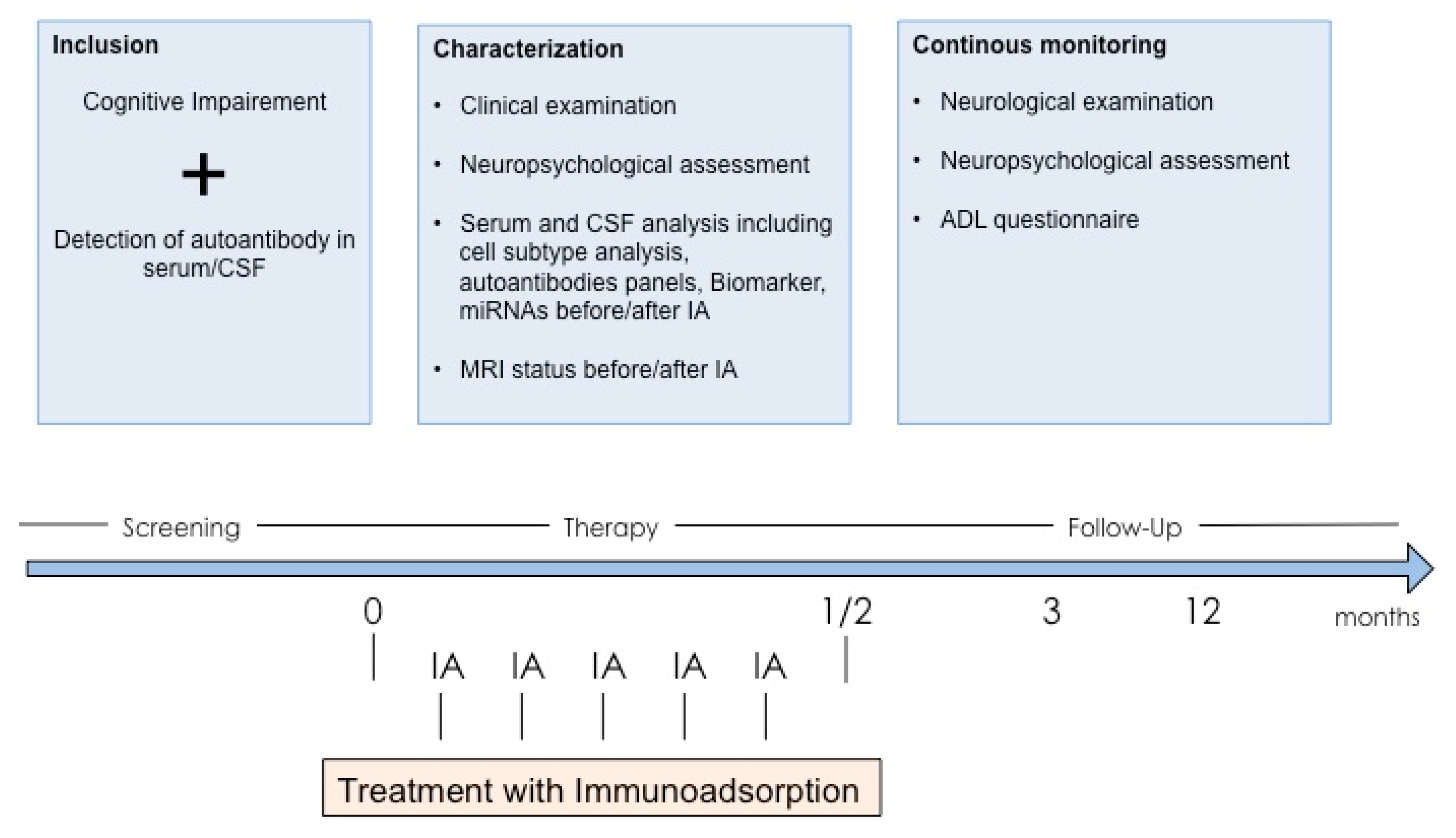
3.7. Autoimmune Dementia and Treatment with Apheresis

3.8. Closing Remarks and Outlook
Author Contributions
Funding
Conflicts of Interest
Ethics Approval
Consent for Publication
References
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Finke, C.; Prüss, H.; Heine, J.; Reuter, S.; Kopp, U.A.; Wegner, F.; Bergh, F.T.; Koch, S.; Jansen, O.; Münte, T.; et al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol. 2017, 74, 50. [Google Scholar] [CrossRef] [PubMed]
- Reintjes, W.; Romijn, M.D.; Hollander, D.; Ter Bruggen, J.P.; Van Marum, R.J. Reversible dementia: Two nursing home patients with voltage-gated potassium channel antibody-associated limbic encephalitis. J. Am. Med. Dir. Assoc. 2015, 16, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Marquetand, J.; Lessen, M.; Bender, B.; Reimold, M.; Elsen, G.; Stoecker, W.; Synofzik, M. Slowly progressive LGI1 encephalitis with isolated late-onset cognitive dysfunction: A treatable mimic of Alzheimer’s disease. Eur. J. Neurol. 2016, 23, 28–29. [Google Scholar] [CrossRef]
- Saiz, A.; Blanco, Y.; Sabater, L.; González, F.; Bataller, L.; Casamitjana, R.; Ramió-Torrentà, L.; Graus, F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: Diagnostic clues for this association. Brain 2008, 131, 2553–2563. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Graus, F. Antibody-mediated encephalitis. N. Engl. J. Med. 2018, 378, 840–851. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Armangué, T.; Planagumà, J.; Radosevic, M.; Mannara, F.; Leypoldt, F.; Geis, C.; Lancaster, E.; Titulaer, M.J.; Rosenfeld, M.R.; et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: Mechanisms and models. Lancet Neurol. 2019, 18, 1045–1057. [Google Scholar] [CrossRef]
- Gaig, C.; Graus, F.; Compta, Y.; Högl, B.; Bataller, L.; Brüggemann, N.; Giordana, C.; Heidbreder, A.; Kotschet, K.; Lewerenz, J.; et al. Clinical manifestations of the anti-IgLON5 disease. Neurology 2017, 88, 1736–1743. [Google Scholar] [CrossRef] [Green Version]
- Irani, S.R.; Alexander, S.; Waters, P.; Kleopa, K.A.; Pettingill, P.; Zuliani, L.; Peles, E.; Buckley, C.; Lang, B.; Vincent, A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010, 133, 2734–2748. [Google Scholar] [CrossRef]
- Graus, F.; Delattre, J.Y.; Antoine, J.C.; Dalmau, J.; Giometto, B.; Grisold, W.; Honnorat, J.; Smitt, P.S.; Vedeler, C.; Verschuuren, J.; et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Gultekin, S.H.; Rosenfeld, M.R.; Voltz, R.; Eichen, J.; Posner, J.B.; Dalmau, J. Paraneoplastic limbic encephalitis: Neurological symptoms, immunological findings and tumour association in 50 patients. Brain 2000, 123, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, U.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorgun, M.H.; Erdogan, S.; Bay, M.; Ayyıldız, E.; Yücemen, N.; Iihan, O.; Yücesan, C.; Ayyildiz, E. Therapeutic plasma exchange in treatment of neuroimmunologic disorders: Review of 92 cases. Transfus. Apher. Sci. 2013, 49, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; O’Brien, P.C.; Petterson, T.M.; Noseworthy, J.H.; Lucchinetti, C.F.; Dodick, D.W.; Pineda, A.A.; Stevens, L.N.; Rodriguez, M. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann. Neurol. 1999, 46, 878–886. [Google Scholar] [CrossRef]
- Pinching, A.J.; Peters, D.K. Remission of myasthenia gravis following plasma-exchange. Lancet 1976, 308, 1373–1376. [Google Scholar] [CrossRef]
- Kreye, J.; Wenke, N.K.; Chayka, M.; Leubner, J.; Murugan, R.; Maier, N.; Jurek, B.; Ly, L.-T.; Brandl, D.; Rost, B.R.; et al. Human cerebrospinal fluid monoclonal N-methyl-D-aspartate receptor autoantibodies are sufficient for encephalitis pathogenesis. Brain 2016, 139, 2641–2652. [Google Scholar] [CrossRef] [Green Version]
- DeSena, A.D.; Noland, D.K.; Matevosyan, K.; King, K.; Phillips, L.; Qureshi, S.S.; Greenberg, B.M.; Graves, D. Intravenous methylprednisolone versus therapeutic plasma exchange for treatment of anti-n-methyl-d-aspartate receptor antibody encephalitis: A retrospective review. J. Clin. Apher. 2015, 30, 212–216. [Google Scholar] [CrossRef]
- Ehrlich, S.; Fassbender, C.; Blaes, C.; Finke, C.; Günther, A.; Harms, L.; Hoffmann, F.; Jahner, K.; Klingel, R.; Kraft, A.; et al. Therapeutische Apherese bei autoimmuner Enzephalitis. Der Nervenarzt 2013, 84, 498–507. [Google Scholar] [CrossRef]
- Heine, J.; Ly, L.-T.; Lieker, I.; Slowinski, T.; Finke, C.; Prüss, H.; Harms, L. Immunoadsorption or plasma exchange in the treatment of autoimmune encephalitis: A pilot study. J. Neurol. 2016, 263, 2395–2402. [Google Scholar] [CrossRef]
- Hempel, P.; Heinig, B.; Jerosch, C.; Decius, I.; Karczewski, P.; Kassner, U.; Kunze, R.; Steinhagen-Thiessen, E.; Bimmler, M. Immunoadsorption of agonistic autoantibodies against α1-adrenergic receptors in patients with mild to moderate dementia. Ther. Apher. Dial. 2016, 20, 523–529. [Google Scholar] [CrossRef]
- Kohler, W.; Ehrlich, S.; Dohmen, C.; Haubitz, M.; Hoffmann, F.; Schmidt, S.; Klingel, R.; Kraft, A.; Neumann-Haefelin, T.; Topka, H.; et al. Tryptophan immunoadsorption for the treatment of autoimmune encephalitis. Eur. J. Neurol. 2014, 22, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Onugoren, M.D.; Golombeck, K.S.; Bien, C.; Abu-Tair, M.; Brand, M.; Bulla-Hellwig, M.; Lohmann, H.; Münstermann, D.; Pavenstädt, H.; Thölking, G.; et al. Immunoadsorption therapy in autoimmune encephalitides. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; Balogun, R.A.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the use of therapeutic apheresis in clinical practice—evidence-based approach from the writing committee of the american society for apheresis: The eighth special issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef] [PubMed]
- Gresa-Arribas, N.; Titulaer, M.J.; Torrents, A.; Aguilar, E.; McCracken, L.; Leypoldt, F.; Gleichman, A.J.; Balice-Gordon, R.; Rosenfeld, M.R.; Lynch, D.; et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: A retrospective study. Lancet Neurol. 2013, 13, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Fassbender, C.; Klingel, R.; Köhler, W. Immunoadsorption for autoimmune encephalitis. Atheroscler. Suppl. 2017, 30, 257–263. [Google Scholar] [CrossRef]
- Sprenger, K.B.G.; Huber, K.; Kratz, W.; Henze, E. Nomograms for the prediction of patient’s plasma volume in plasma exchange therapy from height, weight and hematocrit. J. Clin. Apher. 1987, 3, 185–190. [Google Scholar] [CrossRef]
- Irani, S.R.; Vincent, A. NMDA receptor antibody encephalitis. Curr. Neurol. Neurosci. Rep. 2011, 11, 298–304. [Google Scholar] [CrossRef]
- Flanagan, E.P.; McKeon, A.; Lennon, V.A.; Boeve, B.F.; Trenerry, M.R.; Tan, K.M.; Drubach, D.A.; Josephs, K.A.; Britton, J.W.; Mandrekar, J.N.; et al. Autoimmune dementia: Clinical course and predictors of immunotherapy response. Mayo Clin. Proc. 2010, 85, 881–897. [Google Scholar] [CrossRef]
- Finke, C.; Kopp, U.A.; Pajkert, A.; Behrens, J.R.; Leypoldt, F.; Wuerfel, J.T.; Ploner, C.J.; Prüss, H.; Paul, F. Information, P.E.K.F.C. structural hippocampal damage following anti-n-methyl-d-aspartate receptor encephalitis. Biol. Psychiatry 2016, 79, 727–734. [Google Scholar] [CrossRef]
- Bektaş, Ö.; Yılmaz, A.; Kendirli, T.; Şıklar, Z.; Deda, G.; Yilmaz, A.; Kendirli, T. Hashimoto encephalopathy causing drug-resistant status epilepticus treated with plasmapheresis. Pediatr. Neurol. 2012, 46, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Pari, E.; Rinaldi, F.; Premi, E.; Codella, M.; Rao, R.; Paghera, B.; Panarotto, M.B.; De Maria, G.; Padovani, A. A follow-up 18F-FDG brain PET study in a case of Hashimoto’s encephalopathy causing drug-resistant status epilepticus treated with plasmapheresis. J. Neurol. 2014, 261, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.K.; Rabinstein, A.A.; Hocker, S.E.; Pittock, S.J.; Wijdicks, E.F.M.; McKeon, A. Autoimmune encephalitis in the ICU: Analysis of phenotypes, serologic findings, and outcomes. Neurocritical Care 2015, 24, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Dubovsky, A.N.; Arvikar, S.; Stern, T.A.; Axelrod, L. The neuropsychiatric complications of glucocorticoid use: Steroid psychosis revisited. Psychosomatics 2012, 53, 103–115. [Google Scholar] [CrossRef]
- Berger, B.; Hottenrott, T.; Leubner, J.; Dersch, R.; Rauer, S.; Stich, O.; Prüss, H. Transient spurious intrathecal immunoglobulin synthesis in neurological patients after therapeutic apheresis. BMC Neurol. 2015, 15, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jurek, B.; Chayka, M.; Kreye, J.; Lang, K.; Kraus, L.; Fidzinski, P.; Kornau, H.; Dao, L.; Wenke, N.K.; Long, M.; et al. Human gestational N -methyl- d -aspartate receptor autoantibodies impair neonatal murine brain function. Ann. Neurol. 2019, 86, 656–670. [Google Scholar] [CrossRef] [Green Version]
- Dahm, L.; Ott, C.; Steiner, J.; Stepniak, B.; Teegen, B.; Saschenbrecker, S.; Hammer, C.; Borowski, K.; Begemann, M.; Lemke, S.; et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann. Neurol. 2014, 76, 82–94. [Google Scholar] [CrossRef]
- Coutinho, E.; Jacobson, L.; Pedersen, M.G.; Benros, M.E.; Nørgaard-Pedersen, B.; Mortensen, P.B.; Harrison, P.J.; Vincent, A. CASPR2 autoantibodies are raised during pregnancy in mothers of children with mental retardation and disorders of psychological development but not autism. J. Neurol. Neurosurg. Psychiatry 2017, 88, 718–721. [Google Scholar] [CrossRef] [Green Version]
- Colpo, A.; Marson, P.; Pavanello, F.; Tison, T.; Gervasi, M.T.; Zambon, A.; Ruffatti, A.; De Silvestro, G.; Hoxha, A. Therapeutic apheresis during pregnancy: A single center experience. Transfus. Apher. Sci. 2019, 58, 652–658. [Google Scholar] [CrossRef]
- Özkale, M.; Erol, I.; Özkale, Y.; Kozanoğlu, I. Overview of therapeutic plasma exchange in pediatric neurology: A single-center experience. Acta Neurol. Belg. 2018, 118, 451–458. [Google Scholar] [CrossRef]
- Armangue, T.; Olivé-Cirera, G.; Martínez-Hernandez, E.; Sepulveda, M.; Ruiz-Garcia, R.; Muñoz-Batista, M.; Ariño, H.; González-Álvarez, V.; Felipe-Rucián, A.; Martínez-González, M.J.; et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: A multicentre observational study. Lancet Neurol. 2020, 19, 234–246. [Google Scholar] [CrossRef]
- Doss, S.; Wandinger, K.-P.; Hyman, B.T.; Panzer, J.A.; Synofzik, M.; Dickerson, B.; Mollenhauer, B.; Scherzer, C.R.; Ivinson, A.J.; Finke, C.; et al. High prevalence of NMDA receptor IgA/IgM antibodies in different dementia types. Ann. Clin. Transl. Neurol. 2014, 1, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.P.; Drubach, D.A.; Boeve, B.F. Autoimmune dementia and encephalopathy. Handb. Clin. Neurol. 2016, 133, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Miske, R.; Gross, C.C.; Scharf, M.; Golombeck, K.S.; Hartwig, M.; Bhatia, U.; Schulte-Mecklenbeck, A.; Bönte, K.; Strippel, C.; Schöls, L.; et al. Neurochondrin is a neuronal target antigen in autoimmune cerebellar degeneration. Neurol. Neuroimmunol. Neuroinflamm. 2016, 4, e307. [Google Scholar] [CrossRef] [Green Version]
- Weisenhorn, D.M.V.; Floss, T.; Wurst, W.; Istvánffy, R. Expression of neurochondrin in the developing and adult mouse brain. Dev. Genes Evol. 2004, 214, 206–209. [Google Scholar] [CrossRef]

| Antigen | Clinical Presentation | Age/Gender | Tumor Type |
|---|---|---|---|
| Antibodies against neurotransmitter receptors [6] | |||
| NMDAR [7] | Schizophreniform psychosis, perioral dyskinesia, epileptic seizures, coma, dystonia, hypoventilation | All ages, peak in childhood and youth, 75% women | Ovarian teratoma |
| GABAaR | Epileptic seizures, schizophreniform syndrome, refractory status epilepticus and epilepsia partialis continua | Younger adults; m > f (1.5:1) | Hodgkin lymphoma |
| GABAbR | LE with frequent epileptic seizures | Older adults f = m | 50% lung cancer (SCLC) |
| AMPAR | LE, Epileptic seizures, memory deficits, psychosis | Older Adults f > m (2.3:1) | In 70% lung/breast cancer |
| mGluR5 | LE, Ophelia syndrome (depression, agitation, hallucination, memory deficits, personality changes) | Young adults, m > f (1.5:1) | Hodgkin lymphoma |
| GlycinR | PERM (progressive encephalomyelitis with rigidity and myoclonus), SPS, cognitive deficits | Older adults f = m | Thymoma (<10%) |
| DPPX | LE with tremor, myoclonus, hallucinations, therapy refractory diarrhea | Older adults f < m (1:2.3) | Not known |
| Antibodies against ion channel subunits or cell adhesion molecules [8,9] | |||
| LGI1 | Facio-brachial dystonic seizures (FBDS), amnesia, psychosis, LE, hyponatremia | Adults > 40 years, m > f (2:1) | Rare |
| Caspr2 | LE, neuro-myotonia, Morvan syndrome, can slowly progress over up to 1 year;similar to LGI1, but no hyponatremia | Elderly m > f (9:1) | Thymoma possible |
| IgLON5 | REM- and non-REM sleep disorders, sleep apnea, stridor, dysarthria, dysphagia, dysautonomia, movement disorders, dementia | Older adults, f = m | Not known |
| Antibodies against intracellular (onconeural) antigens [10,11] | |||
| Hu (ANNA-1) | Encephalomyelitis, brainstem encephalitis, LE, Denny-Brown syndrome | Large variability, depending on tumor type | >90%, SCLC |
| Ri (ANNA-2) | OMS, CS, encephalomyelitis | >90%, Ovary, breast cancer | |
| Yo (PCA-1) | CS | >90%, Ovary cancer | |
| Ma2 | LE, CS, diencephalic/hypothalamic involvement | >90%, Testicular, lung cancer | |
| CV2 (CRMP5) | Encephalomyelitis, LE, CS | >90%, SCLC, thymoma | |
| Amphiphysin | SPS | >90%, Breast, SCLC | |
| GAD | SPS, LE, ataxia | Middle aged, f > m (4:1) | Tumor association rare |
| Author | Year | Journal | Study Type | AE Type | Sample Size | Procedure | Outcome Measurement | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| DeSena AD | 2015 | J Clin Aph | Retrospective | NMDAR | 10 | PE | Modified Rankin scale (mRS) | Steroids alone not as effective as steroids followed by PE | [17] |
| Ehrlich S | 2012 | Nervenarzt | Retrospective | Antibody-mediated, paraneoplastic | 30 | PE, IA | mRS | Improvement of mRS after PE or IA | [18] |
| Heine J | 2016 | J Neurol | Prospective | NMDAR, LGI1, Caspr2, GAD, mGluR5, Hu | 21 | PE, IA | mRS | Improvement of mRS in 60% of patients | [19] |
| Hempel P | 2016 | Ther Apher Dial | Prospective | agAAB | 8 | IA | Neuropsychological test | Stabilized cognitive performance after 4-day treatment | [20] |
| Köhler W | 2014 | Eur J Neurol | Retrospective | NMDAR, GABA, LGI1, GAD | 13 | IA | mRS | Improvement of mRS in 11/13 patients | [21] |
| Onugoren MD | 2016 | Neurol Neuroimmunol Neuroinflamm | Retrospective | LGI1, Caspr2, NMDAR, GAD | 19 | IA | mRS | Improvement of mRS in patients with LGI1, Caspr2, NMDAR, no improvement in patients with GAD | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rössling, R.; Prüss, H. Apheresis in Autoimmune Encephalitis and Autoimmune Dementia. J. Clin. Med. 2020, 9, 2683. https://doi.org/10.3390/jcm9092683
Rössling R, Prüss H. Apheresis in Autoimmune Encephalitis and Autoimmune Dementia. Journal of Clinical Medicine. 2020; 9(9):2683. https://doi.org/10.3390/jcm9092683
Chicago/Turabian StyleRössling, Rosa, and Harald Prüss. 2020. "Apheresis in Autoimmune Encephalitis and Autoimmune Dementia" Journal of Clinical Medicine 9, no. 9: 2683. https://doi.org/10.3390/jcm9092683
APA StyleRössling, R., & Prüss, H. (2020). Apheresis in Autoimmune Encephalitis and Autoimmune Dementia. Journal of Clinical Medicine, 9(9), 2683. https://doi.org/10.3390/jcm9092683





