Multimodality Imaging in the Diagnostic Work-Up of Endocarditis and Cardiac Implantable Electronic Device (CIED) Infection
Abstract
1. Introduction
2. Clinical Diagnosis: From the Duke Criteria to the European Society of Cardiology (ESC) 2015 Criteria and the Novel 2019 International CIED Infection Criteria
3. Multidisciplinary Approach of Endocarditis Team
4. Left Heart Native Valve IE
4.1. Main Clinical Characteristics
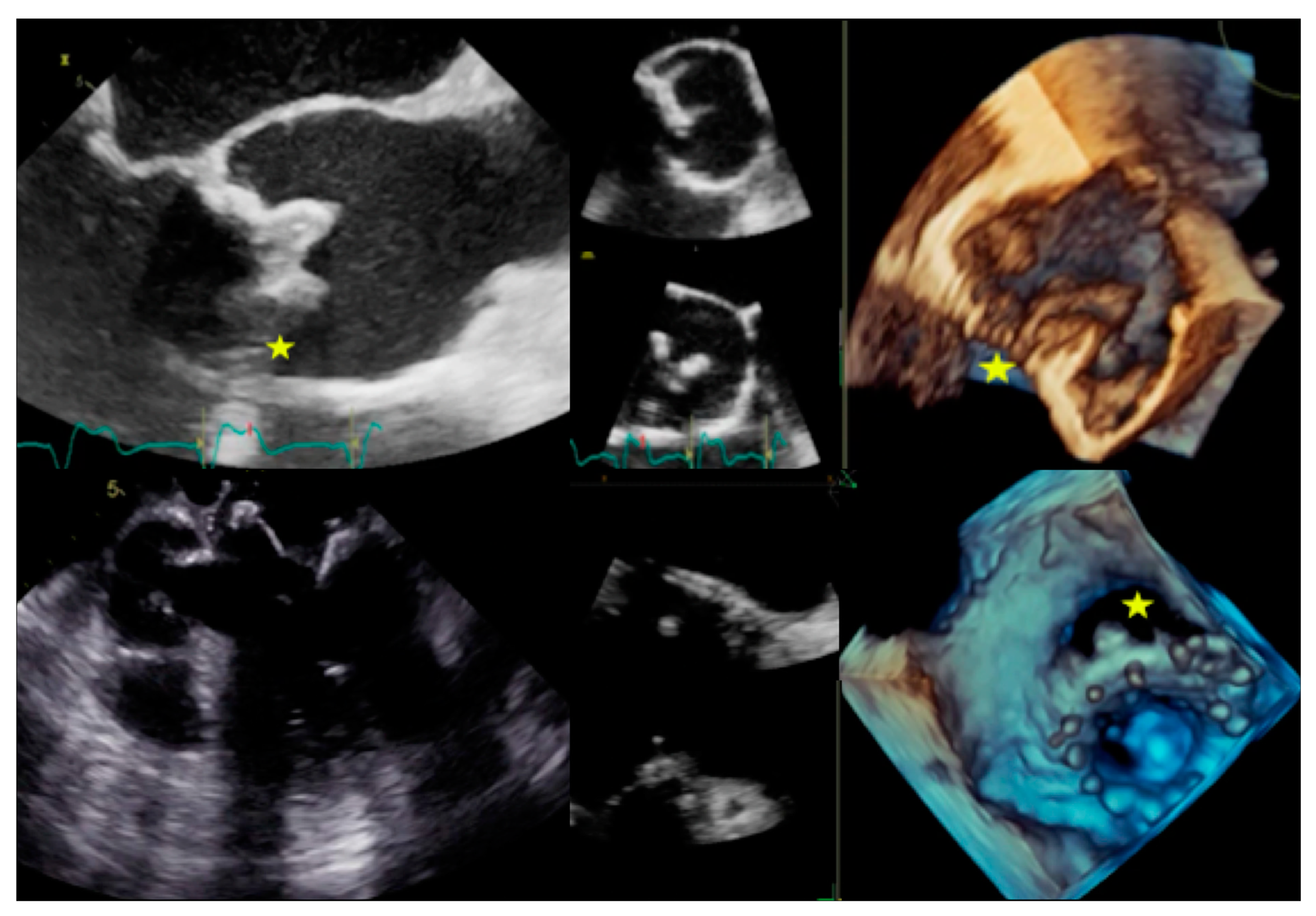
4.2. When to Ask for Transthoracic Echocardiography (TTE) and When to Ask for Transoesophageal Echocardiography (TOE)
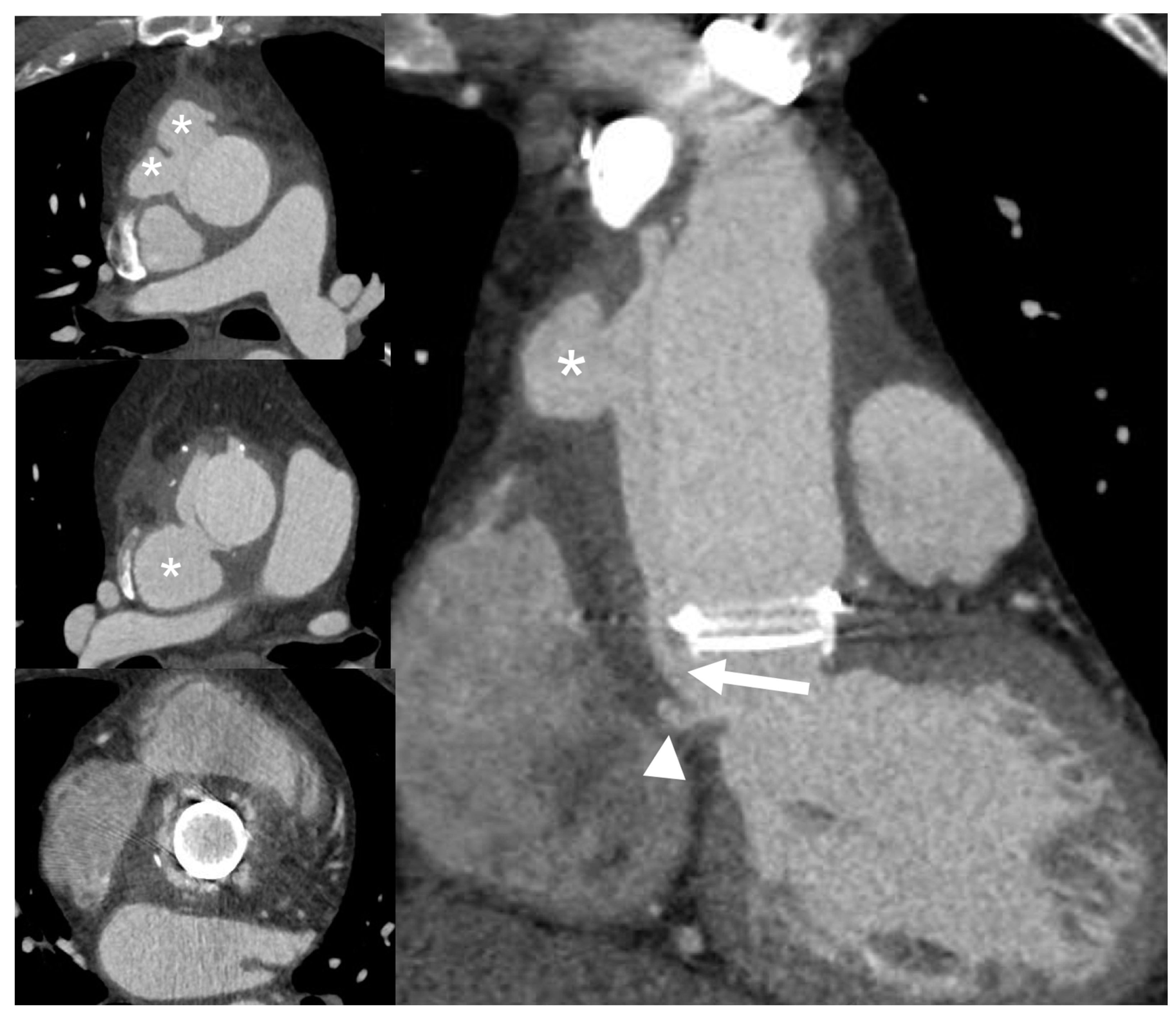
4.3. Role of CCTA in Diagnosing IE and Local Complications
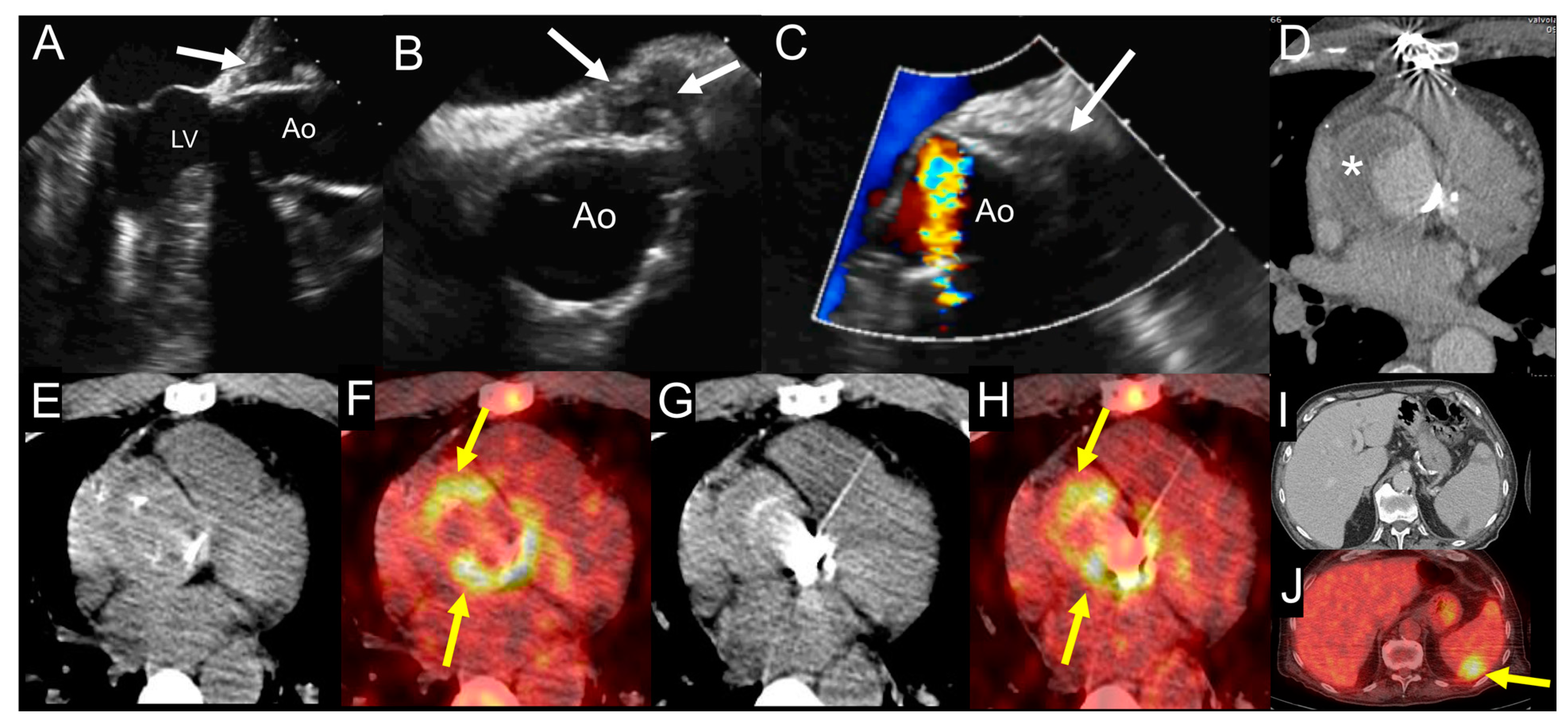
4.4. When to Ask for Nuclear Imaging
4.5. How to Search for Embolisms
4.6. Role of Cardiovascular Magnetic Resonance (CMR) in Diagnosing IE and Local Complications
4.7. Diagnostic Workflow Summary
- •
- The initial assessment of suspected left-sided native IE is based on Duke criteria.
- •
- The patient must receive TTE, TOE, and blood cultures.
- •
- If IE is rejected and the suspicious is low, no more investigations are needed.
- •
- If the diagnosis is definite, the patient should be investigated for silent embolism using CT or PET/CT scan, as well as MRI for cerebral involvement (embolism or hemorrhage), according with clinical status.
- •
- In the case of possible IE or rejected IE but high suspicion there is indication for repeating new TTE/TEE and blood cultures, further investigations, such as whole-body, contrast-enhanced CT or PET/CT scan, should be considered to detect silent embolism or metastatic infections and, therefore, to reclassify the patient, according with ESC 2015 modified diagnostic criteria [2].
5. Right Heart Native Valve IE
5.1. Main Clinical Characteristics
5.2. When to Ask for TTE and When to Ask for TOE
5.3. Role of CCTA in Diagnosing IE and Local Complications
5.4. When to Ask for Nuclear Imaging
5.5. How to Search for Embolisms
5.6. Role of CMR in Diagnosing IE and Local Complications
5.7. Diagnostic Workflow Summary
- •
- Similarly to left-sided native IE, the workflow of suspected right-sided IE is based on TTE, TEE, and blood cultures to stratify patients according with Duke criteria. TTE has a central role in exploring tricuspid valve, while TOE is generally required in the case of pulmonary valve involvement.
- •
- Second-line imaging (CT, PET/CT, WBC SPECT/CT) is indicated to search for perivalvular and pulmonary complications, to detect distal embolisms, and to identify POE.
6. Early and Late Prosthetic Valve Infective Endocarditis (PVE)
6.1. Main Clinical Characteristics
6.2. When to Ask for TTE and When to Ask for TOE
6.3. Role of CCTA in Diagnosing PVE and Local Complication
6.4. When to Ask for Nuclear Imaging
6.5. How to Search for Embolisms
6.6. Role of CMR in Diagnosing PVE and Local Complications
6.7. Diagnostic Workflow Summary
- •
- As for native valve IE, in case of suspected PVE the initial assessment is based on Duke criteria, with the specific indication to perform both TTE and TOE for the higher accuracy of transesophageal approach.
- •
- If IE is rejected and the suspicious is low, no more investigations are needed.
- •
- If the diagnosis is definite, the patient should be investigated for silent embolism or metastatic infections using CT or PET/CT scan and with CCTA to detect periprosthetic extension. MRI to detect cerebral involvement (embolism or hemorrhage) is also indicated according with the clinical status.
- •
- In case of possible IE or rejected IE but high suspicion, there is indication for repeating new TTE/TEE and blood cultures. CCTA or PET/CT are recommended to detect periprosthetic extension. A whole-body, contrast-enhanced CT or PET/CT or WBC SPECT/CT is indicated to detect silent embolism or metastatic infections. All these methods contribute to reclassify the patient according with ESC 2015-modified diagnostic criteria [2].
7. CIED-Related Infective Endocarditis
7.1. Main Clinical Characteristics
7.2. When to Ask for TTE and When to Ask for TOE
7.3. Role of CCTA in Diagnosing CIED-IE and Local Complications
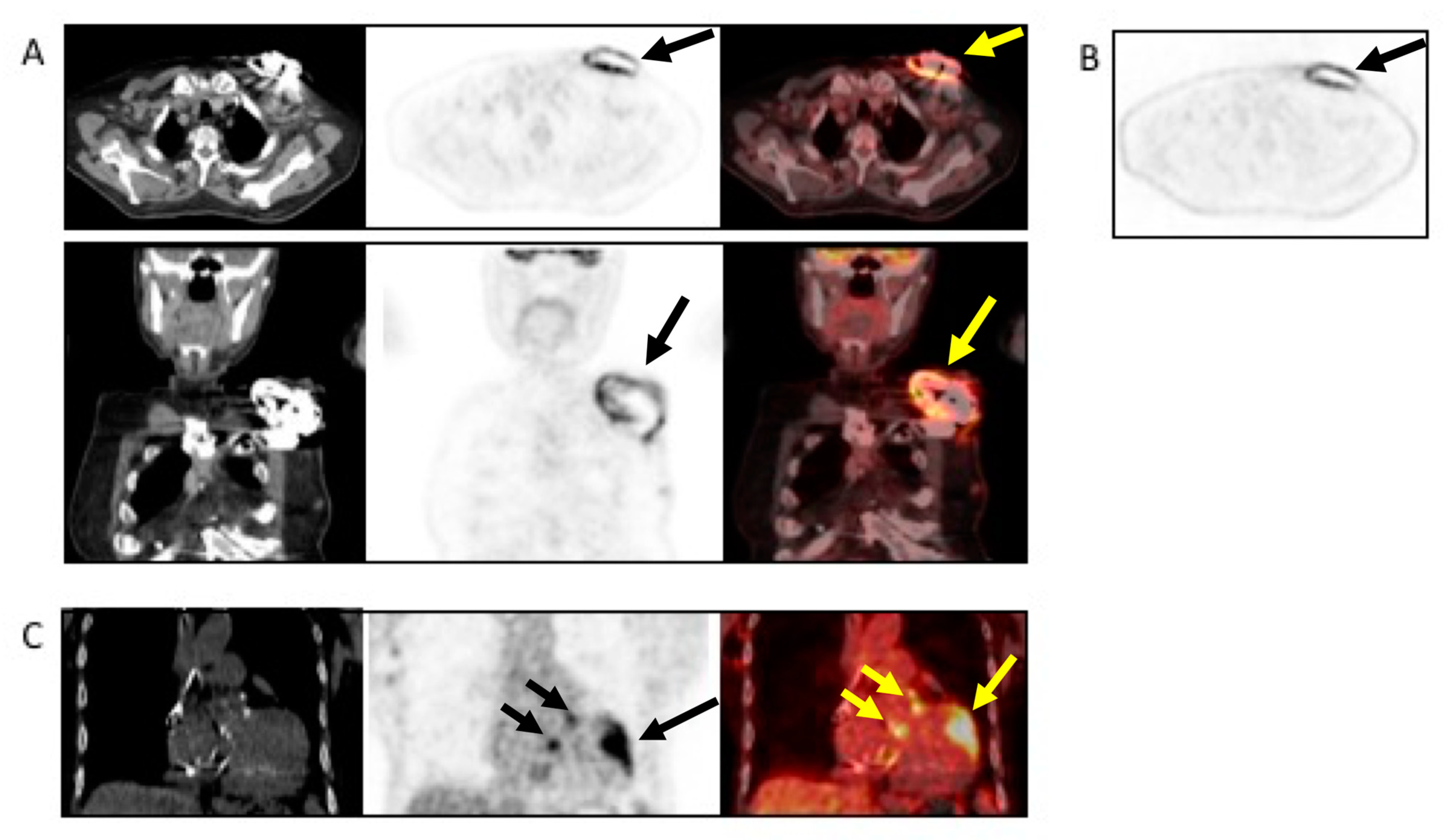
7.4. When to Ask for Nuclear Imaging
7.5. Diagnostic Workflow Summary
- •
- In case of suspected CIED-IE, the physician should address the Duke criteria, being aware of the significantly limited diagnostic accuracy. However, blood cultures, TTE, and eventually TOE should be considered also in case of suspected infection limited to the pocket.
- •
- In case of possible CIED-IE or rejected CIED-IE but persistent high clinical suspicion there is indication to repeat TTE/TOE and blood cultures.
- •
- Chest CT has a specific role in searching for pulmonary embolism, infarct or abscess. The (18F)FDG PET/CT and WBC SPECT/CT have a central role in detecting pocket infection, lead infection, and pulmonary embolism.
8. Current Challenges and Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Durack, D.T.; Lukes, A.S.; Bright, D.K. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Duke endocarditis service. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Habib, G.; Derumeaux, G.; Avierinos, J.F.; Casalta, J.P.; Jamal, F.; Volot, F.; Garcia, M.; Lefevre, J.; Biou, F.; Maximovitch-Rodaminoff, A.; et al. Value and limitations of the duke criteria for the diagnosis of infective endocarditis. J. Am. Coll. Cardiol. 1999, 33, 2023–2029. [Google Scholar] [CrossRef]
- Swart, L.E.; Scholtens, A.M.; Tanis, W.; Nieman, K.; Bogers, A.; Verzijlbergen, F.J.; Krestin, G.P.; Roos-Hesselink, J.W.; Budde, R.P.J. 18F-fluorodeoxyglucose positron emission/computed tomography and computed tomography angiography in prosthetic heart valve endocarditis: From guidelines to clinical practice. Eur. Heart J. 2018, 39, 3739–3749. [Google Scholar] [CrossRef]
- Pizzi, M.N.; Roque, A.; Fernandez-Hidalgo, N.; Cuellar-Calabria, H.; Ferreira-Gonzalez, I.; Gonzalez-Alujas, M.T.; Oristrell, G.; Gracia-Sanchez, L.; Gonzalez, J.J.; Rodriguez-Palomares, J.; et al. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F-fluordeoxyglucose positron emission tomography/computed tomography angiography: Initial results at an infective endocarditis referral center. Circulation 2015, 132, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Pizzi, M.N.; Cuellar-Calabria, H.; Aguade-Bruix, S. 18F-FDG-PET/CT angiography for the diagnosis of infective endocarditis. Curr. Cardiol. Rep. 2017, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Blomstrom-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.C.; et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). EP Eur. 2020, 22, 515–549. [Google Scholar]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, e57–e185. [Google Scholar] [CrossRef]
- Sollini, M.; Berchiolli, R.; Delgado Bolton, R.C.; Rossi, A.; Kirienko, M.; Boni, R.; Lazzeri, E.; Slart, R.; Erba, P.A. The “3M” approach to cardiovascular infections: Multimodality, multitracers, and multidisciplinary. Semin. Nucl. Med. 2018, 48, 199–224. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Correa de Sa, D.D.; Tleyjeh, I.M.; Anavekar, N.S.; Schultz, J.C.; Thomas, J.M.; Lahr, B.D.; Bachuwar, A.; Pazdernik, M.; Steckelberg, J.M.; Wilson, W.R.; et al. Epidemiological trends of infective endocarditis: A population-based study in olmsted county, minnesota. Mayo Clin. Proc. 2010, 85, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.H.; Cabell, C.H.; Benjamin, D.K., Jr.; Kuniholm, E.F.; Fowler, V.G., Jr.; Engemann, J.; Sexton, D.J.; Corey, G.R.; Wang, A. Early predictors of in-hospital death in infective endocarditis. Circulation 2004, 109, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the esc-eorp euro-endo (european infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Tornos, P.; Iung, B.; Permanyer-Miralda, G.; Baron, G.; Delahaye, F.; Gohlke-Barwolf, C.; Butchart, E.G.; Ravaud, P.; Vahanian, A. Infective endocarditis in europe: Lessons from the euro heart survey. Heart 2005, 91, 571–575. [Google Scholar] [CrossRef]
- Nadji, G.; Rusinaru, D.; Remadi, J.P.; Jeu, A.; Sorel, C.; Tribouilloy, C. Heart failure in left-sided native valve infective endocarditis: Characteristics, prognosis, and results of surgical treatment. Eur. J. Heart Fail. 2009, 11, 668–675. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, Y.J.; Kim, S.H.; Sun, B.J.; Kim, D.H.; Yun, S.C.; Song, J.M.; Choo, S.J.; Chung, C.H.; Song, J.K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef]
- Vilacosta, I.; Graupner, C.; San Roman, J.A.; Sarria, C.; Ronderos, R.; Fernandez, C.; Mancini, L.; Sanz, O.; Sanmartin, J.V.; Stoermann, W. Risk of embolization after institution of antibiotic therapy for infective endocarditis. J. Am. Coll. Cardiol. 2002, 39, 1489–1495. [Google Scholar] [CrossRef]
- Eudailey, K.; Lewey, J.; Hahn, R.T.; George, I. Aggressive infective endocarditis and the importance of early repeat echocardiographic imaging. J. Thorac. Cardiovasc. Surg. 2014, 147, e26–e28. [Google Scholar] [CrossRef]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Erba, P.A.; Sadeghpour, A.; Meshaal, M.; Sambola, A.; Furnaz, S.; Citro, R.; Ternacle, J.; Donal, E.; et al. The esc-eorp euro-endo (european infective endocarditis) registry. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 202–207. [Google Scholar] [CrossRef] [PubMed]
- El Kadi, S.; van den Buijs, D.M.F.; Meijers, T.; Gilbers, M.D.; Bekkers, S.; van Melle, J.P.; Riezebos, R.K.; Blok, W.L.; Tanis, W.; Wahadat, A.R.; et al. Infective endocarditis in the netherlands: Current epidemiological profile and mortality: An analysis based on partial esc eorp collected data. Neth. Heart J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Grob, A.; Thuny, F.; Villacampa, C.; Flavian, A.; Gaubert, J.Y.; Raoult, D.; Casalta, J.P.; Habib, G.; Moulin, G.; Jacquier, A. Cardiac multidetector computed tomography in infective endocarditis: A pictorial essay. Insights Imaging 2014, 5, 559–570. [Google Scholar] [CrossRef]
- Kim, I.C.; Chang, S.; Hong, G.R.; Lee, S.H.; Lee, S.; Ha, J.W.; Chang, B.C.; Kim, Y.J.; Shim, C.Y. Comparison of cardiac computed tomography with transesophageal echocardiography for identifying vegetation and intracardiac complications in patients with infective endocarditis in the era of 3-dimensional images. Circ. Cardiovasc. Imaging 2018, 11, e006986. [Google Scholar] [CrossRef]
- Bruun, N.E.; Habib, G.; Thuny, F.; Sogaard, P. Cardiac imaging in infectious endocarditis. Eur. Heart J. 2014, 35, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Feuchtner, G.M.; Stolzmann, P.; Dichtl, W.; Schertler, T.; Bonatti, J.; Scheffel, H.; Mueller, S.; Plass, A.; Mueller, L.; Bartel, T.; et al. Multislice Computed Tomography in Infective Endocarditis. J. Am. Coll. Cardiol. 2009, 53, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Entrikin, D.W.; Gupta, P.; Kon, N.D.; Carr, J.J. Imaging of infective endocarditis with cardiac CT angiography. J. Cardiovasc. Comput. Tomogr. 2012, 6, 399–405. [Google Scholar] [CrossRef]
- Kestler, M.; Garcia-Pavia, P.; Rodríguez-Créixems, M.; Rotger, A.; Jimenez-Requena, F.; Mari, A.; Orcajo, J.; Hernández, L.; Alonso, J.C.; Bouza, E.; et al. Role of 18F-FDG PET in patients with infectious endocarditis. J. Nucl. Med. 2014, 55, 1093–1098. [Google Scholar] [CrossRef]
- Kouijzer, I.J.; Vos, F.J.; Janssen, M.J.; van Dijk, A.P.; Oyen, W.J.; Bleeker-Rovers, C.P. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1102–1107. [Google Scholar] [CrossRef]
- Granados, U.; Fuster, D.; Pericas, J.M.; Llopis, J.L.; Ninot, S.; Quintana, E.; Almela, M.; Pare, C.; Tolosana, J.M.; Falces, C.; et al. Diagnostic accuracy of 18F-FDG PET/CT in infective endocarditis and implantable cardiac electronic device infection: A cross-sectional study. J. Nucl. Med. 2016, 57, 1726–1732. [Google Scholar] [CrossRef]
- Erba, P.A.; Pizzi, M.N.; Roque, A.; Salaun, E.; Lancellotti, P.; Tornos, P.; Habib, G. Multimodality imaging in infective endocarditis: An imaging team within the endocarditis team. Circulation 2019, 140, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Gradon, J.D. Right-side endocarditis in injection drug users: Review of proposed mechanisms of pathogenesis. Clin. Infect. Dis. 2000, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Carozza, A.; De Santo, L.; Romano, G.; Della Corte, A.; Ursomando, F.; Scardone, M.; Caianiello, G.; Cotrufo, M. Infective endocarditis in intravenous drug abusers: Patterns of presentation and long-term outcomes of surgical treatment. J. Heart Valve Dis. 2006, 15, 125–131. [Google Scholar] [PubMed]
- Nandakumar, R.; Raju, G. Isolated tricuspid valve endocarditis in nonaddicted patients: A diagnostic challenge. Am. J. Med Sci. 1997, 314, 207–212. [Google Scholar] [PubMed]
- Yuan, S.M. Right-sided infective endocarditis: Recent epidemiologic changes. Int. J. Clin. Exp. Med. 2014, 7, 199–218. [Google Scholar]
- De Rosa, F.G.; Cicalini, S.; Canta, F.; Audagnotto, S.; Cecchi, E.; Di Perri, G. Infective endocarditis in intravenous drug users from Italy: The increasing importance in HIV-infected patients. Infection 2007, 35, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Chrissoheris, M.P.; Libertin, C.; Ali, R.; Ghantous, A.; Bekui, A.; Donohue, T. Endocarditis complicating central venous catheter bloodstream infections: A unique form of health care associated endocarditis. Clin. Cardiol. 2009, 32, E48–E54. [Google Scholar] [CrossRef]
- Moller, J.H.; Anderson, R.C. 1000 consecutive children with a cardiac malformation with 26- to 37-year follow-up. Am. J. Cardiol. 1992, 70, 661–667. [Google Scholar] [CrossRef]
- Niwa, K.; Nakazawa, M.; Tateno, S.; Yoshinaga, M.; Terai, M. Infective endocarditis in congenital heart disease: Japanese national collaboration study. Heart 2005, 91, 795–800. [Google Scholar] [CrossRef]
- Kuijpers, J.M.; Koolbergen, D.R.; Groenink, M.; Peels, K.C.; Reichert, C.L.; Post, M.C.; Bosker, H.A.; Wajon, E.M.; Zwinderman, A.H.; Mulder, B.; et al. Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: Focus on the use of prosthetic material. Eur. Heart J. 2017, 38, 2048–2056. [Google Scholar] [CrossRef]
- Saremi, F.; Gera, A.; Ho, S.Y.; Hijazi, Z.M.; Sánchez-Quintana, D. CT and MR imaging of the pulmonary valve. Radiogrphics 2014, 34, 51–71. [Google Scholar] [CrossRef]
- Vongpatanasin, W.; Hillis, L.D.; Lange, R.A. Prosthetic heart valves. N. Engl. J. Med. 1996, 335, 407–416. [Google Scholar] [CrossRef]
- Tuñón, V.P.; Gutiérrez, C.; Curiel, L.; Baruque, B.; Tárrago, C.P.; Corchado, E.; Rodríguez, M.A.M.; Ibáñez, A.F.; Lasso, M.C.; Abad, S.M.; et al. Genetic algorithms to simplify infective endocarditis outcome. Eur. J. Intern. Med. 2011, 22, S76–S77. [Google Scholar] [CrossRef]
- Piper, C.; Körfer, R.; Horstkotte, D. Prosthetic valve endocarditis. Heart 2001, 85, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Thuny, F.; Avierinos, J.-F. Prosthetic Valve Endocarditis: Current Approach and Therapeutic Options. Prog. Cardiovasc. Dis. 2008, 50, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, T.; Park, L.; Tribouilloy, C.; Cortés, C.; Casillo, R.; Chu, V.; Delahaye, F.; Durante-Mangoni, E.; Edathodu, J.; Falces, C.; et al. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA 2011, 306, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Vázquez, A.; Fariñas, M.C.; García-Palomo, J.D.; Bernal, J.M.; Revuelta, J.M.; González-Macías, J. Evaluation of the Duke criteria in 93 episodes of prosthetic valve endocarditis: Could sensitivity be improved? Arch. Intern. Med. 2000, 160, 1185–1191. [Google Scholar] [CrossRef]
- Rajiah, P.; Moore, A.; Saboo, S.; Goerne, H.; Ranganath, P.; MacNamara, J.; Joshi, P.; Abbara, S. Multimodality imaging of complications of cardiac valve surgeries. Radiographics 2019, 39, 932–956. [Google Scholar] [CrossRef]
- Tanis, W.; Scholtens, A.; Habets, J.; Brink, R.B.V.D.; Van Herwerden, L.A.; Chamuleau, S.A.; Budde, R.P. CT Angiography and 18F-FDG-PET Fusion Imaging for Prosthetic Heart Valve Endocarditis. JACC Cardiovasc. Imaging 2013, 6, 1008–1013. [Google Scholar] [CrossRef]
- Cantoni, V.; Sollini, M.; Green, R.; Berchiolli, R.N.; Lazzeri, E.; Mannarino, T.; Acampa, W.; Erba, P.A. Comprehensive meta-analysis on [18F] FDG PET/CT and radiolabelled leukocyte SPECT–SPECT/CT imaging in infectious endocarditis and cardiovascular implantable electronic device infections. Clin. Transl. Imaging 2018, 6, 3–18. [Google Scholar] [CrossRef]
- Swart, L.; Gomes, A.; Scholtens, A.; Sinha, B.; Tanis, W.; Lam, M.G.; Van Der Vlugt, M.J.; Streukens, S.A.F.; Aarntzen, E.H.; Bucerius, J.; et al. Improving the diagnostic performance of 18 F-Fluorodeoxyglucose Positron-Emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018, 138, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Mikaïl, N.; Benali, K.; Iung, B.; Duval, X.; Nataf, P.; Jondeau, G.; Hyafil, F.; Le Guludec, D.; Rouzet, F. Characterization of 18F-Fluorodeoxyglucose Uptake Pattern in Noninfected Prosthetic Heart Valves. Circ. Cardiovasc. Imaging 2017, 10, e005585. [Google Scholar] [PubMed]
- Roque, A.; Pizzi, M.N.; Fernández-Hidalgo, N.; Permanyer, E.; Cuellar-Calabria, H.; Romero-Farina, G.; Ríos, R.; Almirante, B.; Castell-Conesa, J.; Escobar, M.; et al. Morpho-metabolic post-surgical patterns of non-infected prosthetic heart valves by [18F]FDG PET/CTA: “normality” is a possible diagnosis. Eur. Heart J. Cardiovasc. Imaging 2019, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- San, S.; Ravis, E.; Tessonier, L.; Philip, M.; Cammilleri, S.; Lavagna, F.; Norscini, G.; Arregle, F.; Martel, H.; Oliver, L.; et al. Prognostic value of (18)F-Fluorodeoxyglucose positron emission tomography/computed tomography in infective endocarditis. J. Am. Coll. Cardiol. 2019, 74, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Vos, F.J.; Bleeker-Rovers, C.P.; Sturm, P.D.; Krabbe, P.F.M.; Van Dijk, A.; Cuijpers, M.L.H.; Adang, E.M.M.; Wanten, G.J.A.; Kullberg, B.J.; Oyen, W. 18F-FDG PET/CT for detection of metastatic infection in Gram-positive bacteremia. J. Nucl. Med. 2010, 51, 1234–1240. [Google Scholar] [CrossRef]
- Gomes, A.; Glaudemans, A.W.J.M.; Touw, D.J.; Van Melle, J.P.; Willems, T.P.; Maass, A.H.; Natour, E.; Prakken, N.H.J.; Borra, R.J.H.; Van Geel, P.P.; et al. Diagnostic value of imaging in infective endocarditis: A systematic review. Lancet Infect. Dis. 2017, 17, e1–e14. [Google Scholar] [CrossRef]
- Orvin, K.; Goldberg, E.; Bernstine, H.; Groshar, D.; Sagie, A.; Kornowski, R.; Bishara, J. The role of FDG-PET/CT imaging in early detection of extra-cardiac complications of infective endocarditis. Clin. Microbiol. Infect. 2015, 21, 69–76. [Google Scholar] [CrossRef]
- Uslan, D.Z.; Sohail, M.R.; Sauver, J.L.S.; Friedman, P.A.; Hayes, D.L.; Stoner, S.M.; Wilson, W.R.; Steckelberg, J.M.; Baddour, L.M. Permanent pacemaker and implantable cardioverter defibrillator infection. Arch. Intern. Med. 2007, 167, 669. [Google Scholar] [CrossRef]
- Erba, P.A.; Sollini, M.; Conti, U.; Bandera, F.; Tascini, C.; De Tommasi, S.M.; Zucchelli, G.; Doria, R.; Menichetti, F.; Bongiorni, M.G.; et al. Radiolabeled WBC scintigraphy in the diagnostic workup of patients with suspected device-related infections. JACC Cardiovasc. Imaging 2013, 6, 1075–1086. [Google Scholar] [CrossRef]
- Klug, D.; Balde, M.; Pavin, D.; Hidden-Lucet, F.; Clementy, J.; Sadoul, N.; Rey, J.L.; Lande, G.; Lazarus, A.; Victor, J.; et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators. Circulation 2007, 116, 1349–1355. [Google Scholar] [CrossRef]
- Klug, D.; Wallet, F.; Lacroix, D.; Marquié, C.; Kouakam, C.; Kacet, S.; Courcol, R. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart 2004, 90, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. EP Eur. 2018, 20, 1217. [Google Scholar] [CrossRef] [PubMed]
- Vilacosta, I.; Sarriá, C.; Roman, J.A.S.; Jiménez, J.; Castillo, J.A.; Iturralde, E.; Rollan, M.J.; Elbal, L.M. Usefulness of transesophageal echocardiography for diagnosis of infected transvenous permanent pacemakers. Circulation 1994, 89, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Lancellotti, P.; Vilacosta, I.; Gaemperli, O.; Rouzet, F.; Hacker, M.; Signore, A.; Slart, R.H.; Habib, G. Recommendations on nuclear and multimodality imaging in IE and CIED infections. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1795–1815. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Cha, Y.-M.; Wilson, W.R. Infections of Cardiovascular Implantable Electronic Devices. N. Engl. J. Med. 2012, 367, 842–849. [Google Scholar] [CrossRef]
- Bensimhon, L.; Lavergne, T.; Hugonnet, F.; Mainardi, J.L.; Latremouille, C.; Maunoury, C.; Lepillier, A.; Le Heuzey, J.Y.; Faraggi, M. Whole body [18F]fluorodeoxyglucose positron emission tomography imaging for the diagnosis of pacemaker or implantable cardioverter defibrillator infection: A preliminary prospective study. Clin. Microbiol. Infect. 2011, 17, 836–844. [Google Scholar] [CrossRef]
- Sarrazin, J.-F.; Philippon, F.; Tessier, M.; Guimond, J.; Molin, F.; Champagne, J.; Nault, I.; Blier, L.; Nadeau, M.; Charbonneau, L.; et al. Usefulness of Fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J. Am. Coll. Cardiol. 2012, 59, 1616–1625. [Google Scholar] [CrossRef]
- Cautela, J.; Alessandrini, S.; Cammilleri, S.; Giorgi, R.; Richet, H.; Casalta, J.-P.; Habib, G.; Raoult, D.; Mundler, O.; Deharo, J.-C. Diagnostic yield of FDG positron-emission tomography/computed tomography in patients with CEID infection: A pilot study. EP Eur. 2012, 15, 252–257. [Google Scholar] [CrossRef]
- Graziosi, M.; Nanni, C.; Lorenzini, M.; Diemberger, I.; Bonfiglioli, R.; Pasquale, F.; Ziacchi, M.; Biffi, M.; Martignani, C.; Bartoletti, M.; et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: A prospective study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1617–1623. [Google Scholar] [CrossRef]
- Tlili, G.; Amraoui, S.; Mesguich, C.; Riviere, A.; Bordachar, P.; Hindié, E.; Bordenave, L. High performances of 18F-fluorodeoxyglucose PET-CT in cardiac implantable device infections: A study of 40 patients. J. Nucl. Cardiol. 2015, 22, 787–798. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; James, J.; Cunnington, C.; Motwani, M.; Fullwood, C.; Hooper, J.; Burns, P.; Qamruddin, A.; Al-Bahrani, G.; Armstrong, I.; et al. Early diagnosis of cardiac implantable electronic device generator pocket infection using 18F-FDG-PET/CT. Eur. Hear. J. Cardiovasc. Imaging 2015, 16, 521–530. [Google Scholar] [CrossRef]
- Juneau, D.; Golfam, M.; Hazra, S.; Erthal, F.; Zuckier, L.S.; Bernick, J.; Wells, G.A.; Beanlands, R.S.; Chow, B.J. Molecular Imaging for the diagnosis of infective endocarditis: A systematic literature review and meta-analysis. Int. J. Cardiol. 2018, 253, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ma, G.; Li, J.; Zheng, K.; Dang, Y.; Shi, X.; Sun, Y.; Li, F.; Zhu, Z. In vivo cell tracking via 18F-fluorodeoxyglucose labeling: A review of the preclinical and clinical applications in cell-based diagnosis and therapy. Clin. Imaging 2013, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, M.; Prenant, C.; Ellis, B.; Boutin, H.; McMahon, A.; Brown, G.; Locatelli, P.; Jones, A.K. A new technique for the radiolabelling of mixed leukocytes with zirconium-89 for inflammation imaging with positron emission tomography. J. Label. Compd. Radiopharm. 2016, 59, 270–276. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Wu, Z.; Wang, H.; Niu, G.; Chen, X. 64Cu-Labeled PEGylated Polyethylenimine for cell trafficking and tumor imaging. Mol. Imaging Boil. 2009, 11, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, A.A.; Weinstein, E.A.; Bambarger, L.E.; Saini, V.; Chang, Y.S.; Demarco, V.P.; Klunk, M.H.; Urbanowski, M.E.; Moulton, K.L.; Murawski, A.M.; et al. A Systematic Approach for Developing Bacteria-Specific Imaging Tracers. J. Nucl. Med. 2016, 58, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, E.A.; Ordonez, A.A.; Demarco, V.P.; Murawski, A.M.; Pokkali, S.; Macdonald, E.M.; Klunk, M.; Mease, R.C.; Pomper, M.G.; Jain, S.K. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6, 259ra146. [Google Scholar] [CrossRef]
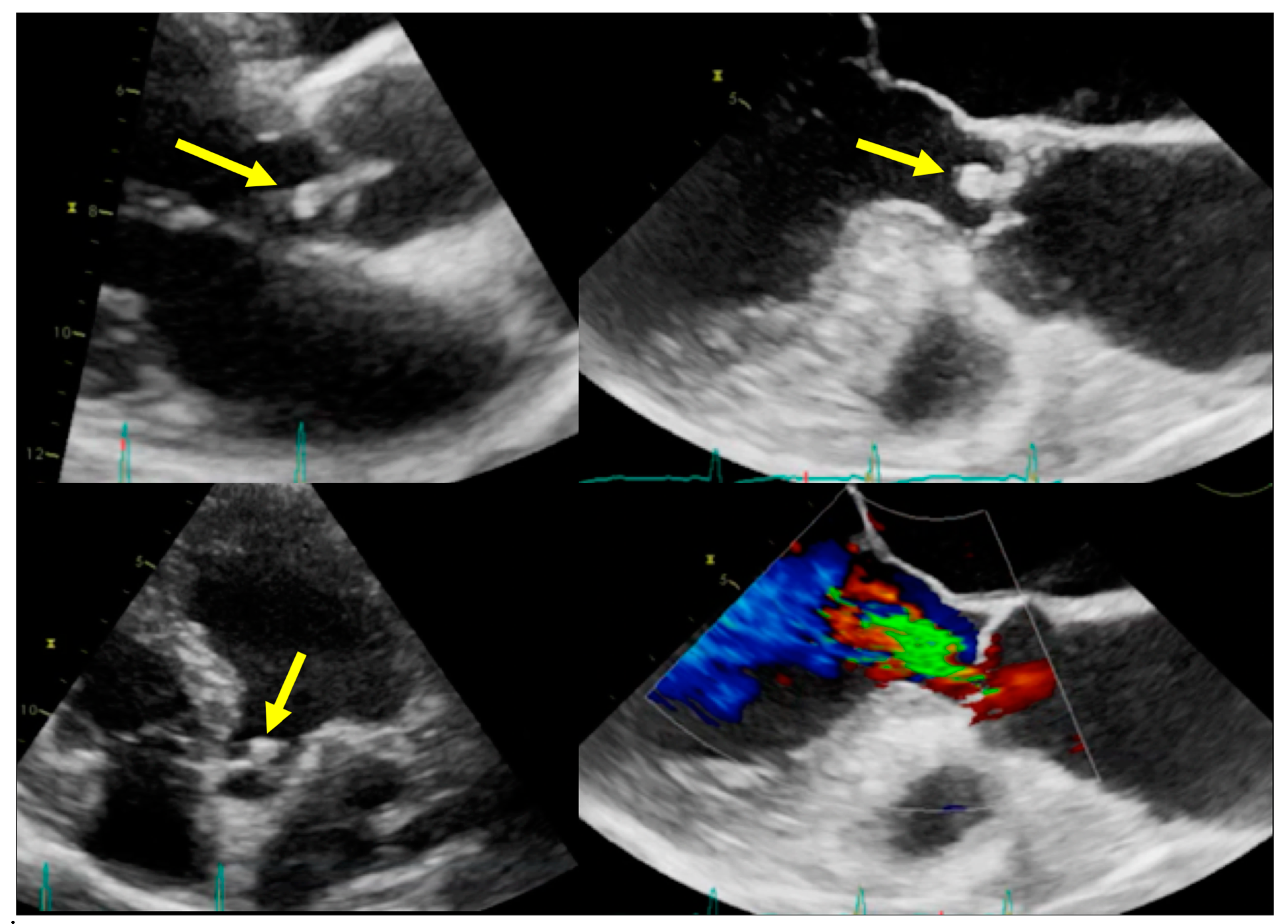
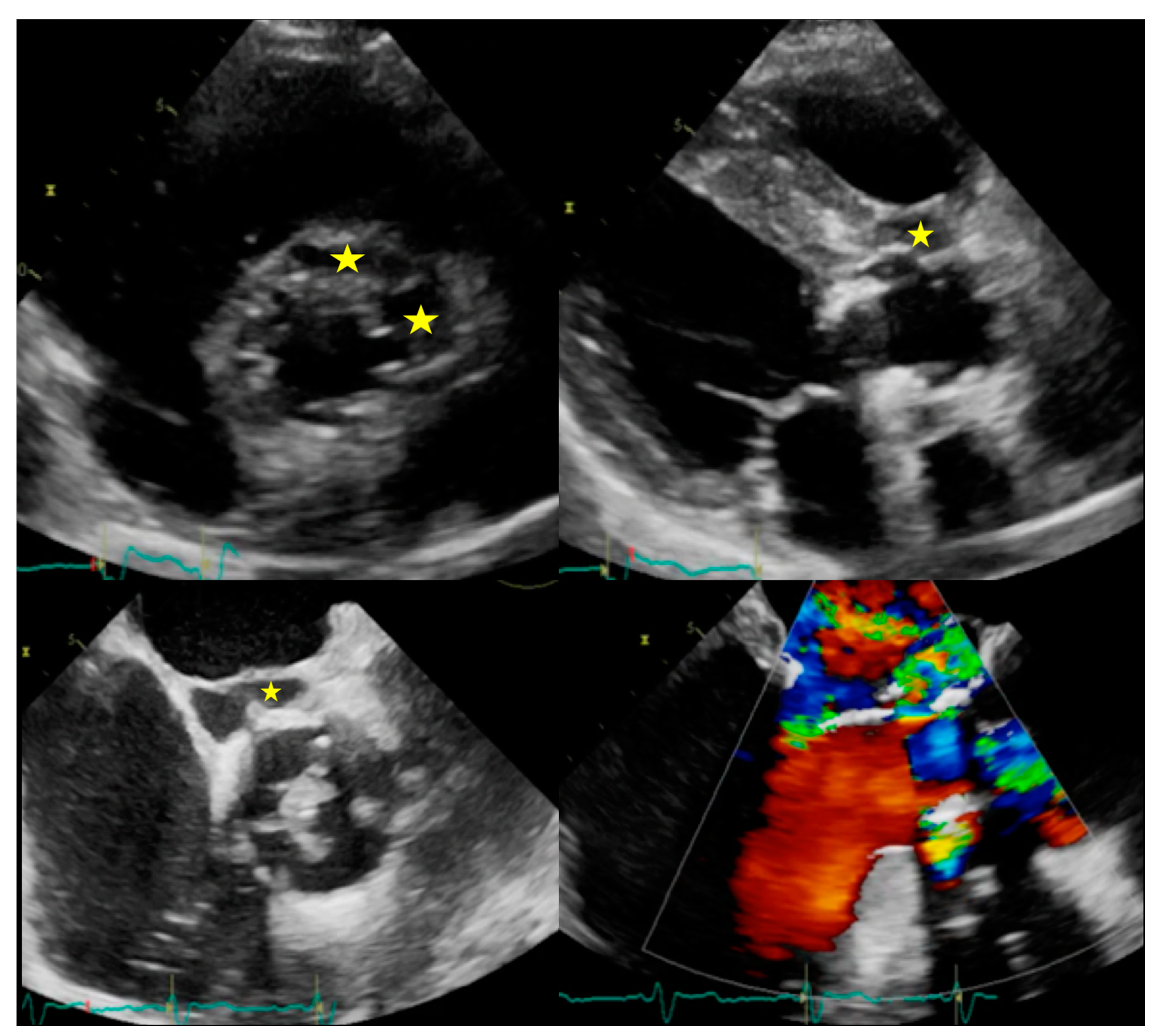
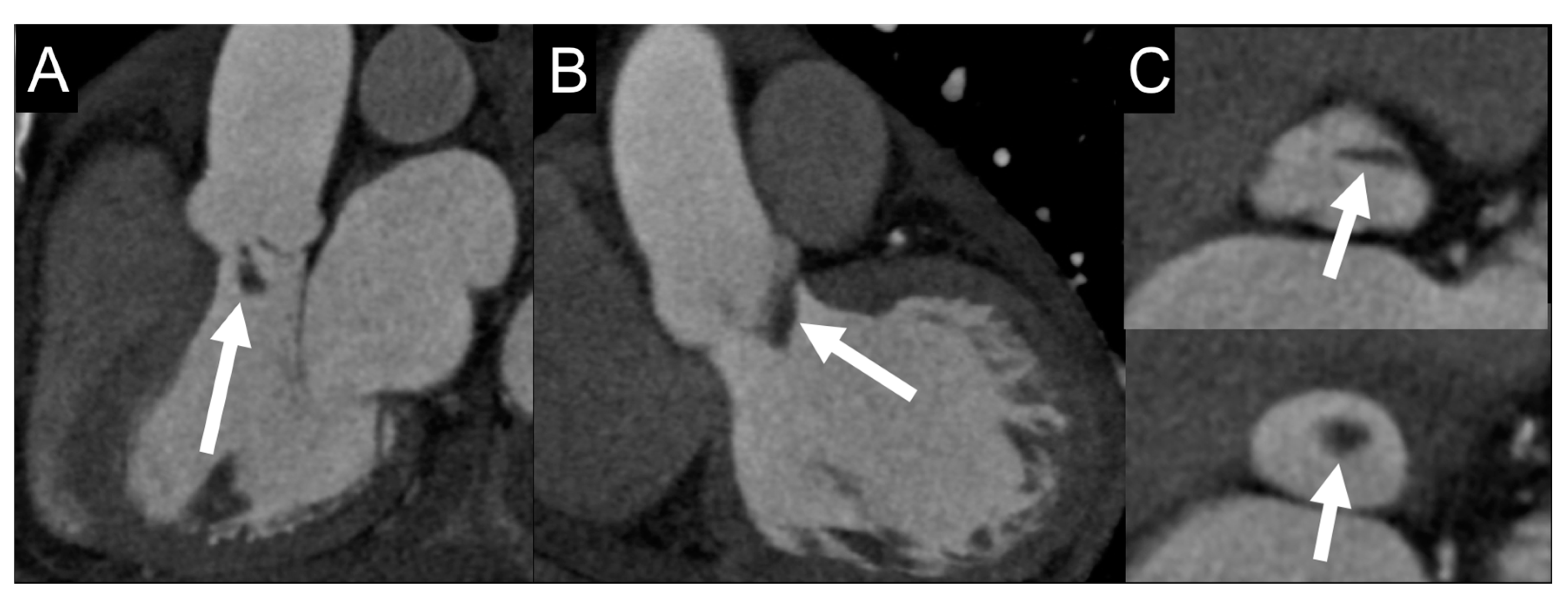
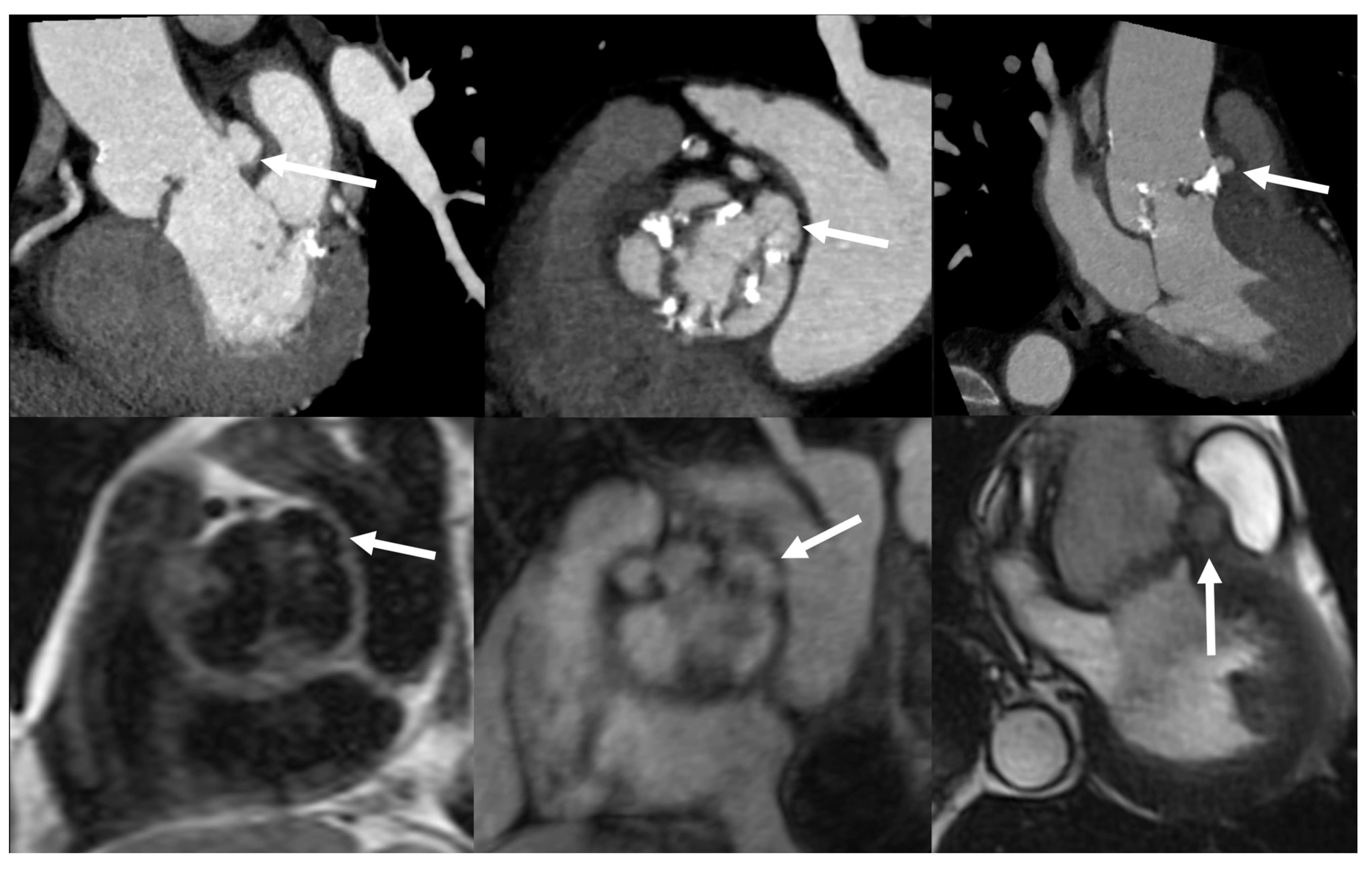
| Echocardiography | CCTA | PET/CT | WBC SPECT/CT | CMR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons | Pro | Cons | |
| General Comments | The first-line diagnostic tool. Diagnostic significance: providing information major Duke/ESC criteria. Prognostic significance: complication and prediction of the risk of embolism Able to assess treatment response. Widely available and unexpensive. TTE can be easily repeated. | Diagnostic accuracy of TTE/TOE is operator-related. TOE requires patient sedation, not always feasible. Limiting factors: poor acoustic window (COPD, thorax conformation), artifacts due to calcium/metals. | Diagnostic significance: major ESC criteria. Possibility to study coronary arteries at the same time. Prognostic assessment: embolisms detection with whole body contrast enhanced CT scan. Wide availability. | Radiation exposure. Risk of contrast-induced nephropathy. | Combination of metabolic evaluation and anatomic assessment. Diagnostic significance: major ESC criteria. Prognostic assessment: Simultaneous detection of embolism, metastatic lesions, portal of entry. Good availability. Easy to perform. Possibility to combine with CCTA evaluation of coronary tree at the same time. | Radiation Exposure. Patient preparation for myocardial suppression. If iodinate contrast is not administrated limited value for brain assessment. Prolonged antimicrobial treatment reduce intensity of [18F]FDG uptake. Pattern of uptake is important. | Combination of metabolic and anatomic assessment. High specificity for infection. Diagnostic significance: major ESC criteria. Prognostic assessment: simultaneous detection of embolism, metastatic lesions, portal of entry. | Radiation Exposure. Need of blood manipulation. Limited sensitivity for small lesions. Relative complex procedure. Low availability. Long acquisition time. | Absence of ionizing radiation. It can offer diagnostic images even without using contrast medium (can replace CCTA in patients with renal failure). It offers morphological and functional information (i.e., valve dysfunction, shunt quantification). | Sensitive to breath artifacts (good patient compliance required). Intermediate availability. Long acquisition time. |
| Left-sided IE | Good visualization of mitral and aortic valve. Valvular dysfuction assessment. Identification of complication (i.e., valvular regurgitation). | Difficult differential diagnosis in presence of marantic vegetations or high calcification. | Detection of vegetations and valve perforation. Assessment of perivalvular extent of disease (abscesses, pseudoaneursysm, fistula). | Inferior to TTE/TOE in detecting small vegetations (<2 mm). | Prognostic assessment: simultaneous detection of embolism, metastatic lesions and portal of entry. | Limited sensitivity for small vegetations. | Evaluation of distant emboli and portal of entry. | Limited role because of low sensitivity for small vegetations. | Capability to assess vegetations (inferior to TTE/TOE). Capability to assess local complications. Independent by acoustic window. May detect concomitant myocardial inflammation. | Not included in current guidelines for IE diagnosis. |
| Right-sided IE | TTE generally provides good visualization of tricuspid valve. TOE is useful in the assessment of IE related to CHD. | Pulmonary valve is difficult to assess. | ||||||||
| PVE | Routinely used for follow up; it allows sequential assessment of prosthesis function. TOE is often required to correctly assess the prosthesis. | Limited by prosthetic material artifacts (i.e., acoustic shadow). Early complication (i.e., abscess) can be difficult to identify. | Identification of complications (paravalvular leakage, abscesses, pseudoaneurysm, dehiscence, and extension to adjacent structures). Capability to visualize large vegetations (>10 mm). | Low image quality for beam hardening artifacts. Limited in assessing small vegetations (<4 mm). | High diagnostic accuracy. Good assessment of perivalvular/periprosthetic complications. Reduction of rate of misdiagnosed PVE. Role in prediction of MACEs. Prognostic significance. | Host reaction may reduce specificity (risk of false-positive studies until 3 months after surgery). | High specificity for infection. Reduction of rate of misdiagnosed PVE. Differential diagnosis between septic and sterile vegetations. | Limited sensitivity for small lesions. | Image quality severely hampered by susceptibility artifacts (especially from mechanical prostheses). | |
| CIED-IE | Useful to assess intracardiac lead segments. TTE can be integrated by ultrasound evaluation of device pocket, to assessing inflamation or fluid collection. | Limited role in the assessment of unexplorable lead segments. Differential diagnosis of vegetation vs. lead fibrosis/thrombi can be challenging. | Possibility to combine the CT assessment of generator pocket. | Blooming and beam hardening artefacts. Poor sensitivity in detecting vegetations on leads. | Very high sensitivity and specificity for generator/pocket and extracardiac or extravascular lead infection. | Low sensitivity for small vegetations along the leads. | Good sensitivity and specificity for generator/pocket and extracardiac or extravascular lead infection. | Limited diagnostic sensitivity for intracardiac and intravascular lead infection. | Image quality severely hampered by susceptibility artifacts from lead and device. Limited to patients with MRI conditional devices and with numerous precautions. | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galea, N.; Bandera, F.; Lauri, C.; Autore, C.; Laghi, A.; Erba, P.A. Multimodality Imaging in the Diagnostic Work-Up of Endocarditis and Cardiac Implantable Electronic Device (CIED) Infection. J. Clin. Med. 2020, 9, 2237. https://doi.org/10.3390/jcm9072237
Galea N, Bandera F, Lauri C, Autore C, Laghi A, Erba PA. Multimodality Imaging in the Diagnostic Work-Up of Endocarditis and Cardiac Implantable Electronic Device (CIED) Infection. Journal of Clinical Medicine. 2020; 9(7):2237. https://doi.org/10.3390/jcm9072237
Chicago/Turabian StyleGalea, Nicola, Francesco Bandera, Chiara Lauri, Camillo Autore, Andrea Laghi, and Paola Anna Erba. 2020. "Multimodality Imaging in the Diagnostic Work-Up of Endocarditis and Cardiac Implantable Electronic Device (CIED) Infection" Journal of Clinical Medicine 9, no. 7: 2237. https://doi.org/10.3390/jcm9072237
APA StyleGalea, N., Bandera, F., Lauri, C., Autore, C., Laghi, A., & Erba, P. A. (2020). Multimodality Imaging in the Diagnostic Work-Up of Endocarditis and Cardiac Implantable Electronic Device (CIED) Infection. Journal of Clinical Medicine, 9(7), 2237. https://doi.org/10.3390/jcm9072237





