Diagnostic Value of 18F-FDG-PET/CT in Patients with FUO
Abstract
1. Introduction
2. Experimental Section
2.1. Patient Population
2.2. 18F-FDG-PET/CT Imaging
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics and Final Diagnoses
3.2. 18F-FDG-PET/CT Results
3.3. Baseline Patient Characteristics in Contributory and Non-Contributory Scans
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petersdorf, R.G.; Beeson, P.B. Fever of unexplained origin: Report on 100 cases. Medicine 1961, 40, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Durack, D.T.; Street, A.C. Fever of unknown origin—Reexamined and redefined. Curr. Clin. Top. Infect. Dis. 1991, 11, 35–51. [Google Scholar] [PubMed]
- Petersdorf, R.G. Fever of unknown origin. An old friend revisited. Arch. Intern. Med. 1992, 152, 21–22. [Google Scholar] [CrossRef]
- de Kleijn, E.M.; Vandenbroucke, J.P.; van der Meer, J.W. Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group. Medicine 1997, 76, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Knockaert, D.C.; Vanderschueren, S.; Blockmans, D. Fever of unknown origin in adults: 40 years on. J. Intern. Med. 2003, 253, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, H.W. Fever of unknown origin or fever of too many origins? N. Eng. J. Med. 2013, 368, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Mourad, O.; Palda, V.; Detsky, A.S. A comprehensive evidence-based approach to fever of unknown origin. Arch. Intern. Med. 2003, 163, 545–551. [Google Scholar] [CrossRef]
- Mulders-Manders, C.; Simon, A.; Bleeker-Rovers, C. Fever of unknown origin. Clin. Med. 2015, 15, 280–284. [Google Scholar] [CrossRef]
- Kouijzer, I.J.E.; Bleeker-Rovers, C.P.; de Geus-Oei, L.-F. Nuclear medicine imaging of fever of unknown origin. In Nuclear Medicine in Infectious Diseases; Signore, A., Glaudemans, A.W.J.M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 199–214. [Google Scholar] [CrossRef]
- Meller, J.; Sahlmann, C.O.; Scheel, A.K. 18F-FDG PET and PET/CT in fever of unknown origin. J. Nucl. Med. 2007, 48, 35–45. [Google Scholar]
- Goldsmith, S.J.; Vallabhajosula, S. Clinically proven radiopharmaceuticals for infection imaging: Mechanisms and applications. Semin. Nucl. Med. 2009, 39, 2–10. [Google Scholar] [CrossRef]
- Meller, J.; Altenvoerde, G.; Munzel, U.; Jauho, A.; Behe, M.; Gratz, S.; Luig, H.; Becker, W. Fever of unknown origin: Prospective comparison of [18F]FDG imaging with a double-head coincidence camera and gallium-67 citrate SPET. Eur. J. Nucl. Med. 2000, 27, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Blockmans, D.; Knockaert, D.; Maes, A.; De Caestecker, J.; Stroobants, S.; Bobbaers, H.; Mortelmans, L. Clinical value of [(18)F]fluoro-deoxyglucose positron emission tomography for patients with fever of unknown origin. Clin. Infect. Dis. 2001, 32, 191–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knockaert, D.C.; Mortelmans, L.A.; De Roo, M.C.; Bobbaers, H.J. Clinical value of gallium-67 scintigraphy in evaluation of fever of unknown origin. Clin. Infect. Dis. 1994, 18, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Jamar, F.; Israel, O.; Buscombe, J.; Martin-Comin, J.; Lazzeri, E. Clinical indications, image acquisition and data interpretation for white blood cells and anti-granulocyte monoclonal antibody scintigraphy: An EANM procedural guideline. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1816–1831. [Google Scholar] [CrossRef]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Israel, O.; Martin-Comin, J.; Signore, A. EANM/SNMMI Guideline for 18F-FDG Use in Inflammation and Infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef]
- Tahara, T.; Ichiya, Y.; Kuwabara, Y.; Otsuka, M.; Miyake, Y.; Gunasekera, R.; Masuda, K. High [18F]-fluorodeoxyglucose uptake in abdominal abscesses: A PET study. J. Comput. Assist. Tomogr. 1989, 13, 829–831. [Google Scholar] [CrossRef]
- Glaudemans, A.W.J.M.; de Vries, E.F.J.; Galli, F.; Dierckx, R.A.J.O.; Slart, R.H.J.A.; Signore, A. The use of 18F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef]
- Al-Zaghal, A.; Raynor, W.Y.; Seraj, S.M.; Werner, T.J.; Alavi, A. FDG-PET imaging to detect and characterize underlying causes of fever of unknown origin: An unavoidable path for the foreseeable future. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2–7. [Google Scholar] [CrossRef]
- Bleeker-Rovers, C.P.; de Kleijn, E.M.H.A.; Corstens, F.H.M.; van der Meer, J.W.M.; Oyen, W.J.G. Clinical value of FDG PET in patients with fever of unknown origin and patients suspected of focal infection or inflammation. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 29–37. [Google Scholar] [CrossRef]
- Bleeker-Rovers, C.P.; Vos, F.J.; Mudde, A.H.; Dofferhoff, A.S.M.; de Geus-Oei, L.F.; Rijnders, A.J.; Krabbe, P.F.M.; Corstens, F.H.M.; van der Meer, J.W.M.; Oyen, W.J.G. A prospective multi-centre study of the value of FDG-PET as part of a structured diagnostic protocol in patients with fever of unknown origin. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 694–703. [Google Scholar] [CrossRef]
- Kjaer, A.; Lebech, A.-M.; Eigtved, A.; Hojgaard, L. Fever of unknown origin: Prospective comparison of diagnostic value of 18F-FDG PET and 111In-granulocyte scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.; Buchert, R.; Bohuslavizki, K.H. Value of FDG PET in patients with fever of unknown origin. Nucl. Med. Commun. 2001, 22, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Keidar, Z.; Gurman-Balbir, A.; Gaitini, D.; Israel, O. Fever of unknown origin: The role of 18F-FDG PET/CT. J. Nucl. Med. 2008, 49, 1980–1985. [Google Scholar] [CrossRef] [PubMed]
- Balink, H.; Collins, J.; Bruyn, G.A.; Gemmel, F. F-18 FDG PET/CT in the diagnosis of fever of unknown origin. Clin. Nucl. Med. 2009, 34, 862–868. [Google Scholar] [CrossRef]
- Buch-Olsen, K.M.; Andersen, R.V.; Hess, S.; Braad, P.E.; Schifter, S. 18F-FDG-PET/CT in of unknown origin: Clinical value. Nucl. Med. Commun. 2014, 35, 955–960. [Google Scholar] [CrossRef]
- Gafter-Gvili, A.; Raibman, S.; Grossman, A.; Anvi, T.; Paul, M.; Leibovici, L.; Tadmor, B.; Groshar, D.; Bernstine, H. [18F]FDG-PET/CT for the diagnosis of patients with fever of unknown origin. Q J. Med 2015, 108, 289–298. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, R.; Malhotra, A.; Bhalla, A.S.; Kumar, U.; Sood, R. Diagnostic utility of fluorodeoxyglucose positron emission tomography/computed tomography in pyrexia of unknown origin. Indian J. Nucl. Med. 2015, 30, 204–212. [Google Scholar] [CrossRef]
- Efstathiou, S.P.; Pefanis, A.V.; Tsiakou, A.G.; Skeva, I.I.; Tsioulos, D.I.; Achimastos, A.D.; Mountokalakis, T.D. Fever of unknown origin: Discrimination between infectious and non-infectious causes. Eur. J. Intern. Med. 2010, 21, 137–143. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Tsioutis, C.; Markaki, L.; Zafeiri, M.; Soundoulounaki, S.; Gikas, A. Fever of unknown origin caused by infectious diseases in the era of migrant and refugee crisis. Travel Med. Infect. Dis. 2020, 33, 101425. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Bleeker-Rovers, C.P.; Vos, F.J.; de Kleijn, E.M.H.A.; Mudde, A.H.; Dofferhoff, T.S.M.; Richter, C.; Smilde, T.J.; Krabbe, P.F.M.; Oyen, W.J.G.; van der Meer, J.W.M. A prospective multicenter study on fever of unknown origin: The yield of a structured diagnostic protocol. Medicine 2007, 86, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, J.; Boudousq, V.; Lechiche, C.; Pouget, J.P.; Kotzki, P.O.; Collombier, L.; Lavigne, J.P.; Sotto, A. Place of (18)F-FDG-PET with computed tomography in the diagnostic algorithm of patients with fever of unknown origin. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1727–1733. [Google Scholar] [CrossRef]
- Kouijzer, I.J.E.; Mulders-Manders, C.M.; Bleeker-Rovers, C.P.; Oyen, W.J.G. Fever of Unknown Origin: The Valueof FDG-PET/CT. Semin. Nucl. Med. 2018, 48, 100–107. [Google Scholar] [CrossRef]
- Jaruskova, M.; Belohlavek, O. Role of FDG-PET and PET/CT in the diagnosis of prolonged febrile states. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Cheng, Z.-T.; Zhu, J.-L.; Xing, M.-Y.; Zheng, C.-F.; Wang, S.-J.; Xie, N.-N.; XianYu, Z.-Q.; Song, J.-X. Combined clinical parameters improve the diagnostic efficacy of 18F-FDG PET/CT in patients with fever of unknown origin (FUO) and inflammation of unknown origin (IUO): A prospective study in China. Int. J. Infect. Dis. 2020, 93, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Nakamoto, Y.; Tamaki, N.; Kanegae, K.; Fukuda, H.; Kaneda, T.; Kitajima, K.; Tateishi, U.; Morooka, M.; Ito, K.; et al. FDG-PET for the diagnosis of fever of unknown origin: A Japanese multi-center study. Ann. Nucl. Med. 2011, 25, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Kim, S.-J.; Seo, Y.; Jeong, S.Y.; Ahn, B.-C.; Lee, J. F-18 FDG PET for assessment of disease activity of large vessel vasculitis: A systematic review and meta-analysis. J. Nucl. Cardiol. 2019, 26, 59–67. [Google Scholar] [CrossRef]
- Kan, Y.; Wang, W.; Liu, J.; Yang, J.; Wang, Z. Contribution of 18F-FDG PET/CT in a case-mix of fever of unknown origin and inflammation of unknown origin: A meta-analysis. Acta Radiol. 2018, 60, 716–725. [Google Scholar] [CrossRef]
- Besson, F.L.; Chaumet-Riffaud, P.; Playe, M.; Noel, N.; Lambotte, O.; Goujard, C.; Prigent, A.; Durand, E. Contribution of 18F-FDG PET in the diagnostic assessment of fever of unknown origin (FUO): A stratification-based meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1887–1895. [Google Scholar] [CrossRef]
- Balink, H.; Tan, S.S.; Veeger, N.J.G.M.; Holleman, F.; van Eck-Smit, B.L.F.; Bennink, R.J.; Verberne, H.J. 18F-FDG PET/CT in inflammation of unknown origin: A cost-effectiveness pilot-study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1408–1413. [Google Scholar] [CrossRef]
- Knockaert, D.C.; Dujardin, K.S.; Bobbaers, H.J. Long-term follow-up of patients with undiagnosed fever of unknown origin. Arch. Intern. Med. 1996, 156, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Nihashi, T.; Gafter-Gvili, A.; García-Gómez, J.M.; Andres, E.; Blockmans, D.; Iwata, M.; Terasawa, T. Association of 18F-FDG PET or PET/CT results with spontaneous remission in classic fever of unknown origin: A systematic review and meta-analysis. Medicine 2018, 97, e12909. [Google Scholar] [CrossRef] [PubMed]
- Suhrcke, M.; Stuckler, D.; Suk, J.E.; Desai, M.; Senek, M.; McKee, M.; Tsolova, S.; Basu, S.; Abubakar, I.; Hunter, P.; et al. Impact of Economic Crises on Communicable Disease Transmission and Control: A Systematic Review of the Evidence. PLoS ONE 2011, 6, e20724. [Google Scholar] [CrossRef]
- Takeuchi, Μ.; Dahabreh, I.J.; Nihashi, T.; Iwata, M.; Varghese, G.M.; Terasawa, T. Nuclear Imaging for Classic Fever of Unknown Origin: Meta-Analysis. J. Nucl. Med. 2016, 57, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Balink, H.; Veeger, N.J.G.M.; Bennink, R.J.; Slart, R.H.J.A.; Holleman, F.; van Eck-Smit, B.L.F.; Verberne, H.J. The predictive value of C-reactive protein and erythrocyte sedimentation rate for 18F-FDG PET/CT outcome in patients with fever and inflammation of unknown origin. Nucl. Med. Commun. 2015, 36, 604–609. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.-M.; Li, Y.; Hua, F.-C.; Wang, Q.-S.; Zhang, X.-L.; Cheng, C.; Wu, H.; Yao, Z.-M.; Zhang, W.-F.; et al. 18F-FDGPET/CT in fever of unknown origin and inflammation of unknown origin: A Chinese multi-center study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 159–165. [Google Scholar] [CrossRef]
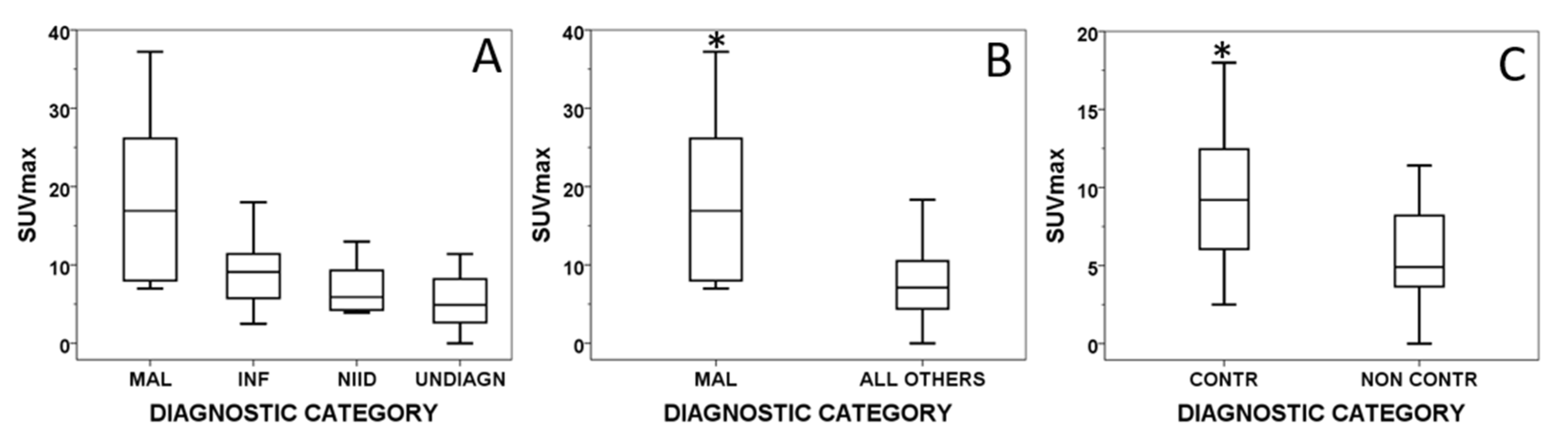
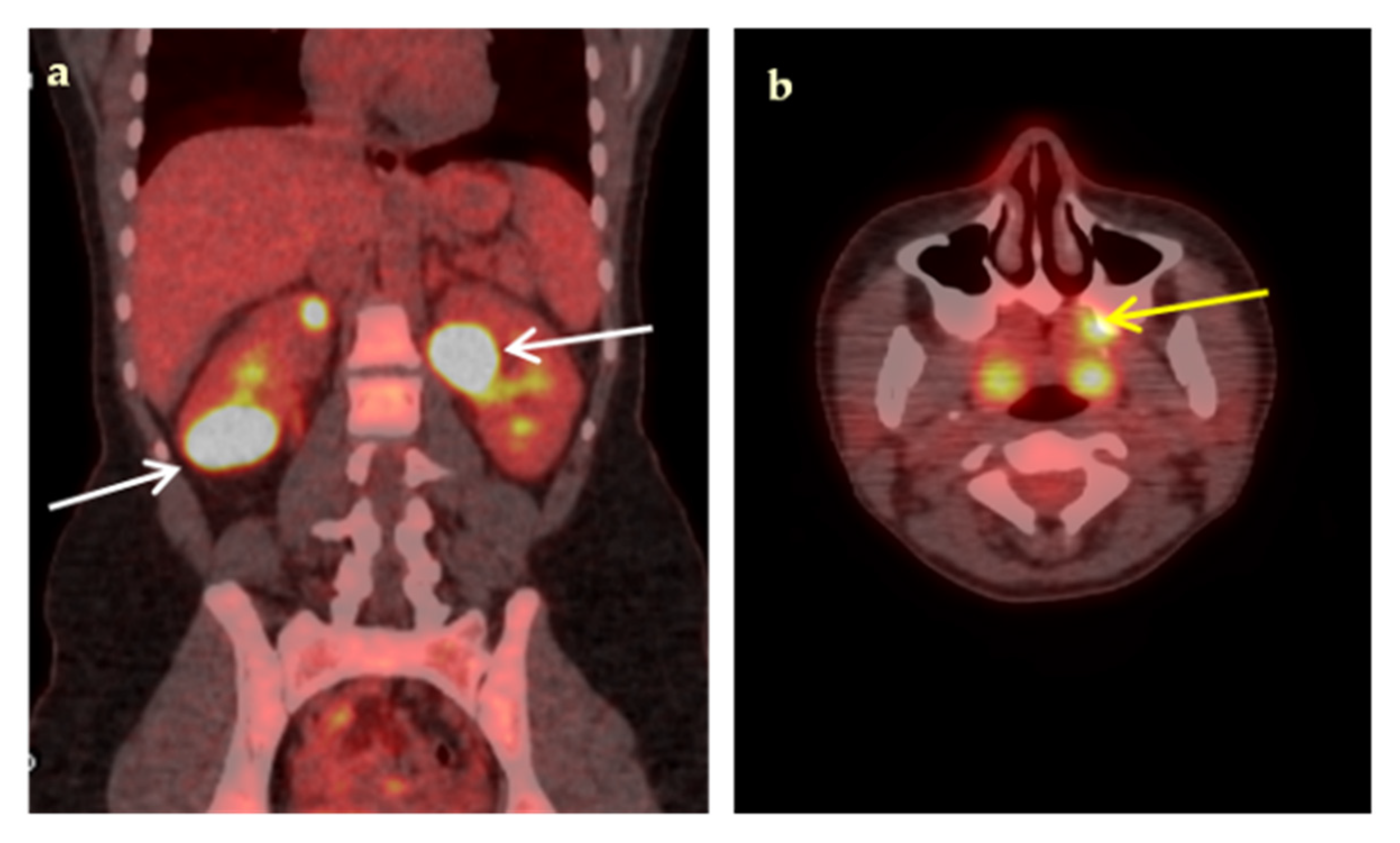
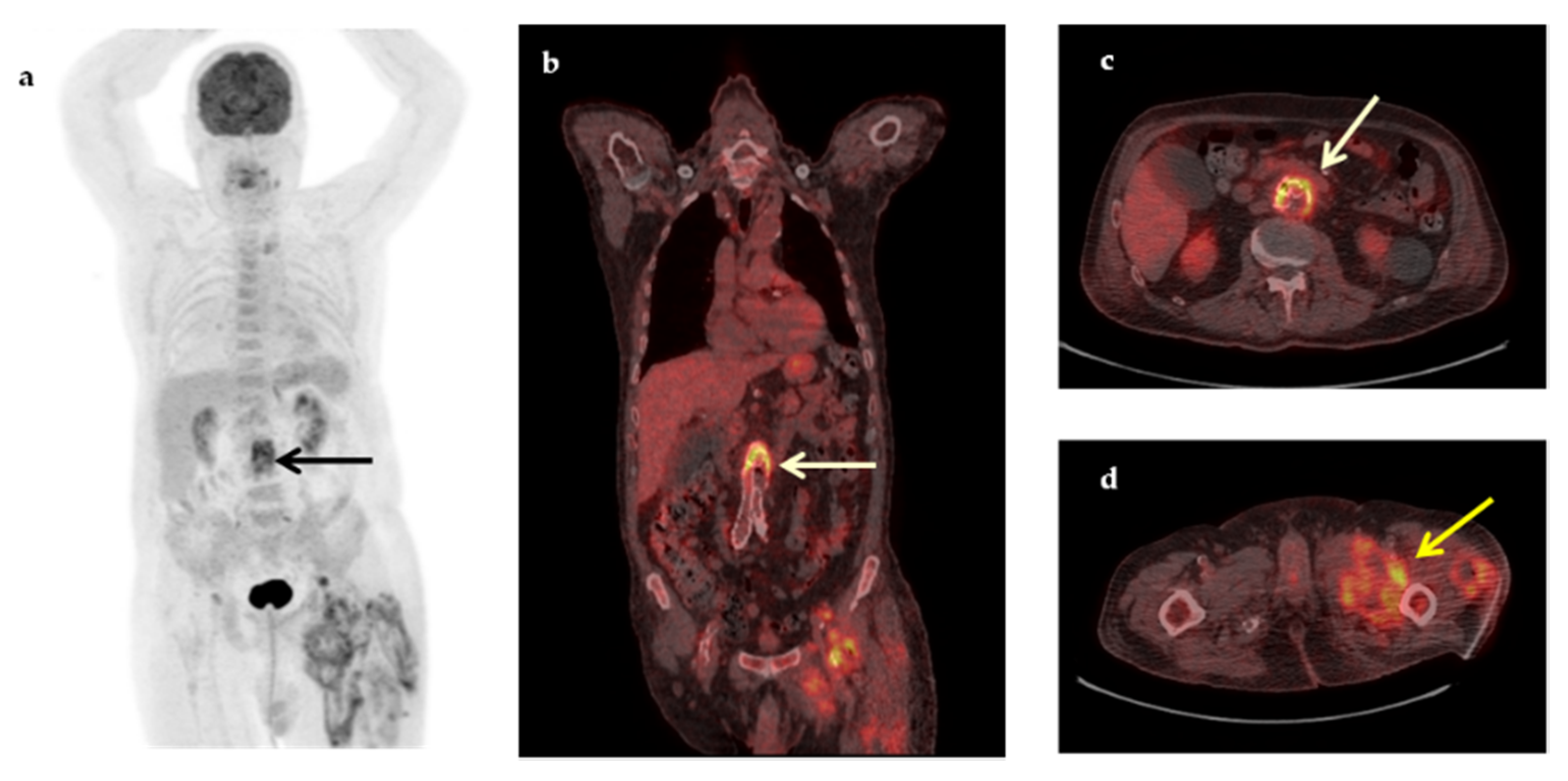
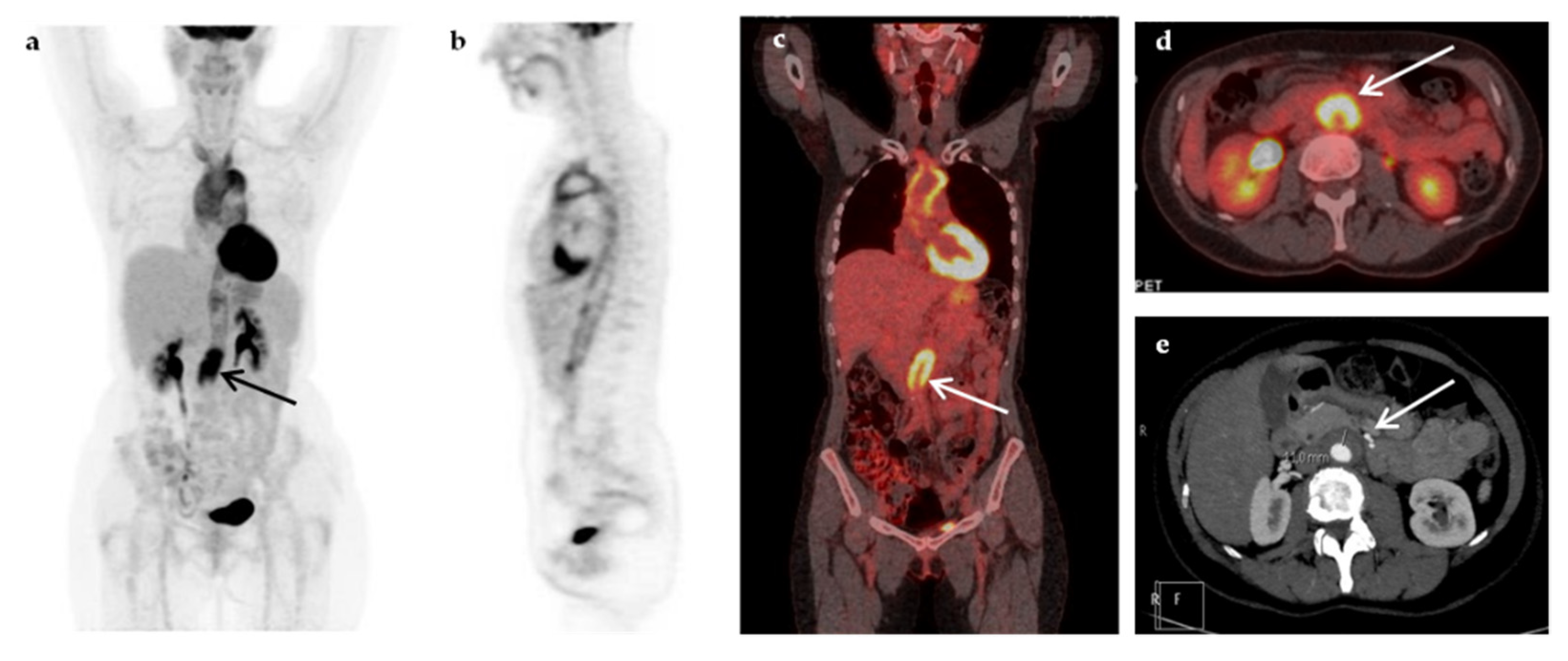
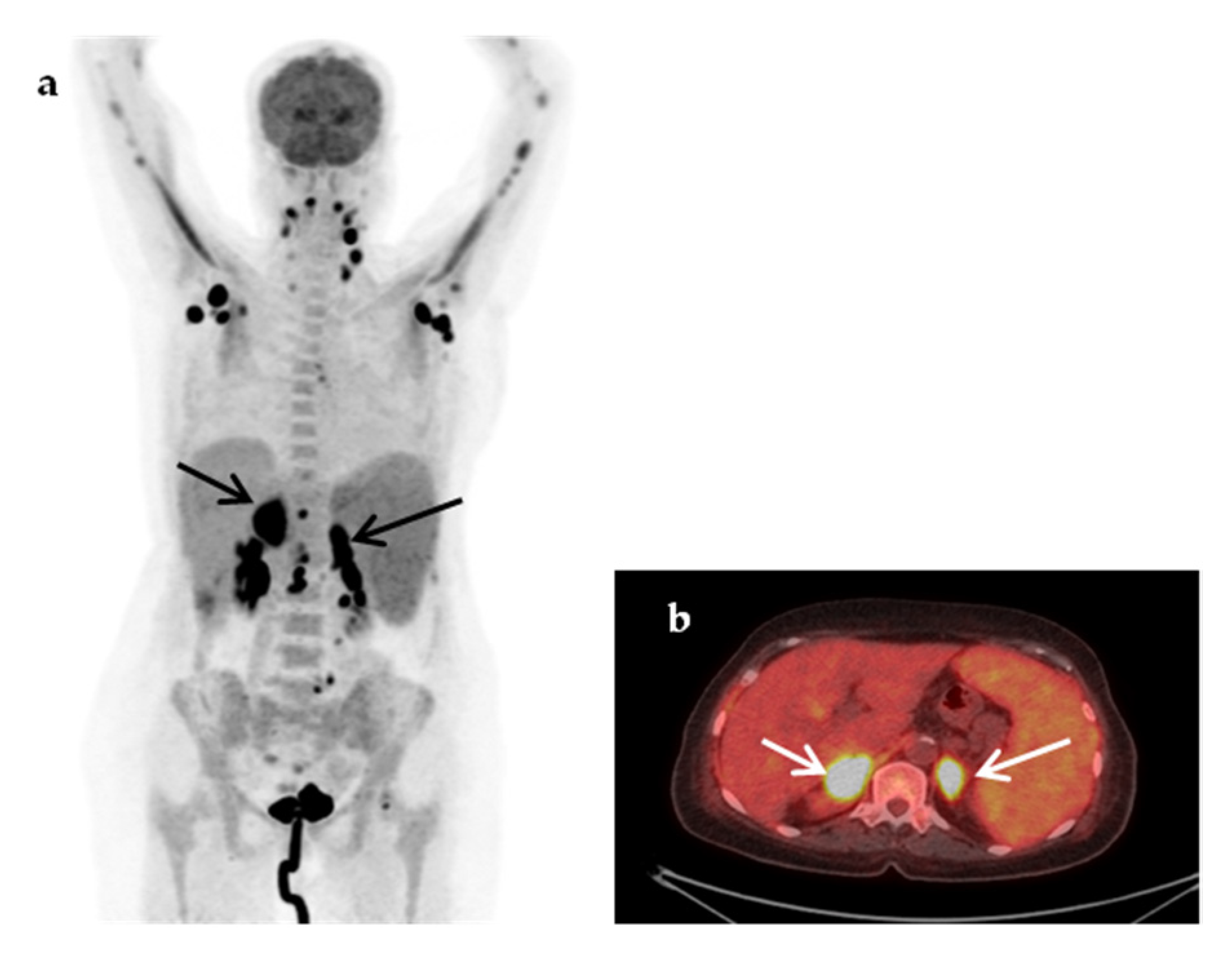
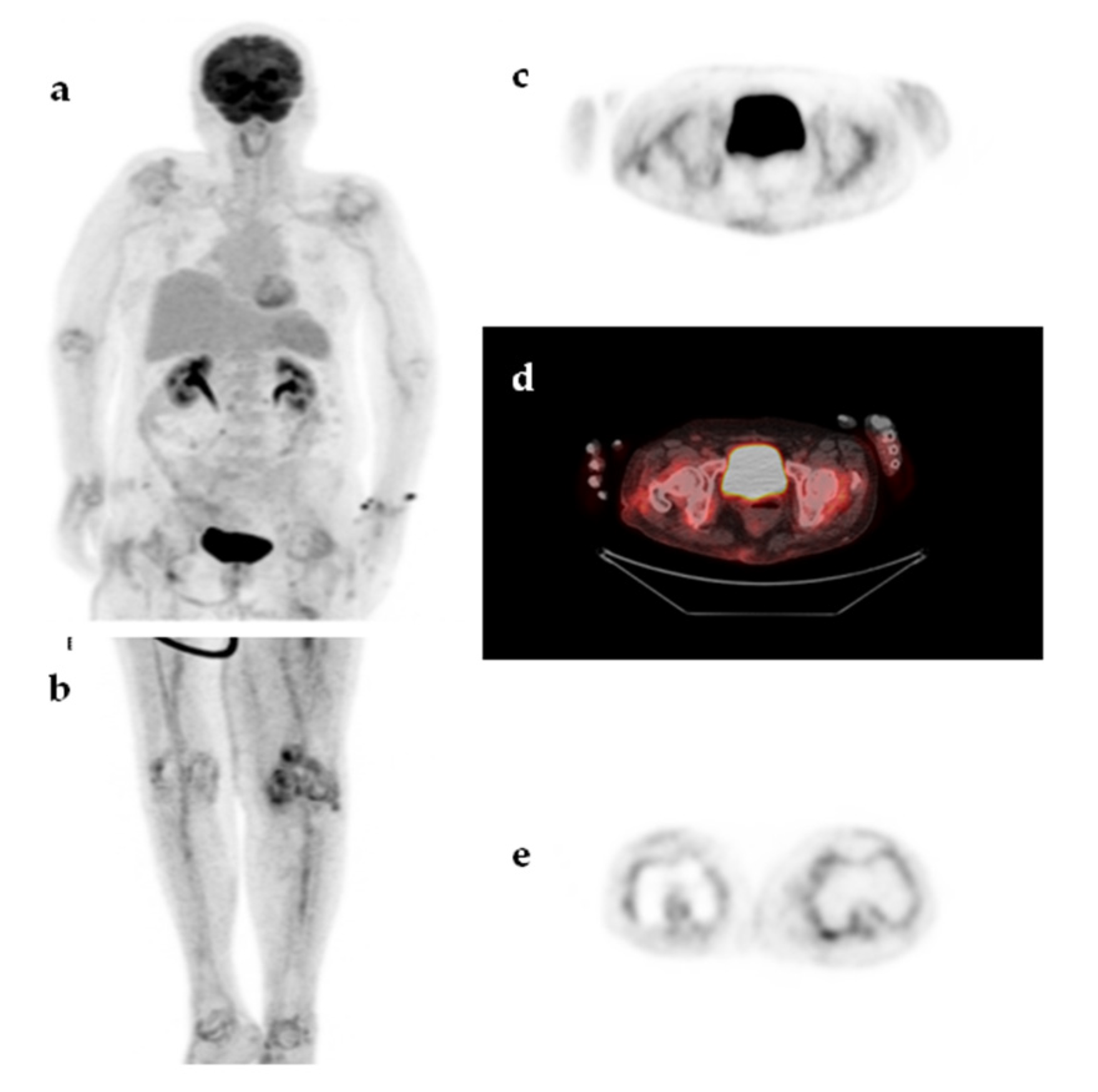
| Characteristic | n (%) | Median (IQR, Min–Max) |
|---|---|---|
| Number of patients | 50 | |
| Gender (male/female) | 28/22 (56%/44%) | |
| Age (years) | 59 (25, 17–85) | |
| Concomitant Diseases/Conditions | 50 (100%) | |
| Malignancies | 8 (16%) | |
| Breast/ AML/H&N/URO/CR/WM | 2/2/1/1/1/1 | |
| Diabetes Mellitus | 7 (14%) | |
| Chronic kidney desease | 7 (14%) | |
| Cardiovascular devices | 6 (12%) | |
| Vascular grafts/Prosthetic valves/CIED | 4/1/1 | |
| Bowel diversions | 4 (8%) | |
| Thyroid diseases | 3 (6%) | |
| Multinodular goiter, Hashimoto thyroiditis | 2/1 | |
| Prosthetic joints | 3 (6%) | |
| Spinal surgery | 2 (4%) | |
| Miscellaneous | 3 (6%) | |
| SLE/AS/Meningioma | 1/1/1 | |
| Duration of fever (days) | 40 (60, 21–365) | |
| Common clinical/radiological findings | ||
| Lymphadenopathy | 16 (32%) | |
| Splenomegaly | 10 (20%) | |
| Elevated blood inflammatory markers (ESR, CRP, PCT) | 31 (62%) | |
| Medications | 17 (34%) | |
| Antibiotics | 13 (26%) | |
| Corticosteroids | 1 (2%) | |
| Chemotherapy (last dose 4 months ago) | 3 (6%) |
| Investigation | n (%) |
|---|---|
| Echocardiography | 20 (40%) |
| Computed tomography (CT) | |
| Thoracic CT | 39 (78%) |
| Abdominal CT | 36 (72%) |
| Cervical CT | 8 (16%) |
| Cerebral CT | 7 (14%) |
| Magnetic Resonance Imaging (MRI) | |
| Abdominal MRI | 7 (14%) |
| Lumbar spine MRI | 4 (8%) |
| Cerebral MRI | 3 (6%) |
| Cervical spine MRI | 1 (2%) |
| Endoscopy | |
| Gastroscopy | 4 (8%) |
| Colonoscopy | 4 (8%) |
| Bronchoscopy | 3 (6%) |
| Nuclear medicine procedures | |
| 99mTc-MDP bone scan | 2 (4%) |
| 99mTc-HMPAO-labeled leucocyte scan | 1 (2%) |
| 99mTc-pertechnetate thyroid scan | 1 (2%) |
| 99mTc-MAG-3 Renogram | 1 (2%) |
| Biopsies | |
| Lymph node or parenchymal organ biopsy | 7 (14%) |
| Bone marrow biopsy | 9 (18%) |
| Temporal artery biopsy | 3 (6%) |
| Diagnostic Categories | n (%) |
|---|---|
| Infections | 20 (40%) |
| Abdominal abscesses | 4 |
| Infectious cyst in polycystic renal disease | 3 |
| Pneumonia/inflammation of bronchiectasis cysts | 3 |
| Vascular graft infection | 3 |
| Tuberculous spondylitis | 1 |
| Bacterial spondylodiscitis | 1 |
| Pulmonary tuberculosis | 1 |
| CIED-associated infection | 1 |
| Infectious lymphadenopathy | 1 |
| Cryptococcosis | 1 |
| Leishmaniasis | 1 |
| Malignancy | 8 (16%) |
| Non-Hodgkin’s lymphoma | 5 |
| Hodgkin’s disease | 1 |
| Lung cancer | 1 |
| Relapse of urinary tract carcinoma | 1 |
| Non-infectious Inflammatory diseases (NIID) | 11 (22%) |
| Large vessel vasculitis/Takayasu’s arteritis | 3 |
| Adult-onset Still’s disease | 2 |
| Sarcoidosis | 1 |
| Polymyalgia rheumatica | 1 |
| Inflammatory bowel disease | 1 |
| Familial Mediterranean fever | 1 |
| Neo-esophagus inflammation from gastroesophageal reflux | 1 |
| Subacute thyroiditis | 1 |
| Undiagnosed fever | 11 (22%) |
| Spontaneous recovery of fever | 7 |
| Recovery of fever with corticosteroids or NSAIDs | 3 |
| Recurrent fever until death | 1 |
| Categories | TP | FP | TN | FN | N |
|---|---|---|---|---|---|
| Infections | 19 | 0 | 0 | 1 | 20 |
| Malignancies | 8 | 0 | 0 | 0 | 8 |
| Non-infectious inflammatory diseases | 9 | 2 | 0 | 0 | 11 |
| Undiagnosed fever | 0 | 4 | 6 | 1 | 11 |
| Total (%) | 36 (72%) | 6 (12%) | 6 (12%) | 2 (4%) | 50 |
| Gender, Age | Underlying Conditions | 18F-FDG-PET/CT Result | Final Diagnosis | Outcome |
|---|---|---|---|---|
| M, 81 years | COPD, recurrent respiratory infections, prosthetic AoV | FN | No diagnosis; possible viral encephalitis | Death |
| M, 78 years | CIED | FN | CIED-associated infection | Fever remission after CIED removal |
| M, 70 years | Recurrent episodes of aspiration pneumonia; neo-esophagus due to gastrointestinal bleeding | FP: possible pulmonary aspergillosis; diffuse hypermetabolic activity along neo-esophagus | Inflammation of the neo-esophagus from gastroesophageal reflux | Spontaneous recovery |
| F, 57 years | THA, SLE | FP: active axillary and subclavicular lymphadenopathy (d.d. lymphoma, sarcoidosis, non-specific inflammation) | No diagnosis | Lymph node biopsy without pathological findings; spontaneous recovery |
| M, 49 years | Lymphadenopathy | FP: extensive (intrapelvic, inguinal, axillary), moderately active lymphadenopathy and splenic involvement; overall impression in favor of lymphoproliferative disease, (d.d. inflammatory/granulomatous etiology) | No diagnosis | Lymph node biopsy without pathological findings; recovery after antibiotics |
| M, 54 years | AML | FP: multiple, diffuse hypermetabolic liver lesions (d.d. infectious lesions/infiltration from hematological disease) | No diagnosis | Spontaneous recovery |
| M, 56 years | L3–L4 spondylodiscitis | FP: increased prostate uptake, possible prostate abscess | Adult-onset Still’s disease | Recovery with steroids |
| F, 63 years | Multinodular goiter, TB | FP: finding compatible with moderately active pericarditis, inactive granulomatous lung disease | No diagnosis | Recovery with steroids |
| Characteristic | Contributory Scans (=35) n (%) Mean ± SD (median) | Non-Contributory Scans (=15) n (%) Mean ± SD (median) | p-Value |
|---|---|---|---|
| Age | 54.7 ± 18.6 (57.0) | 62.6 ± 17.1 (63.0) | 0.162 |
| Male Female | 18 (51.4) 17 (48.6) | 10 (66.7) 5 (33.3) | 0.609 |
| Duration of fever (days) | 72.1 ± 87.4 (30.0) | 94.6 ± 84.7 (60.0) | 0.080 |
| Prior antibiotic administration | 10 (28.6) | 3 (20) | 0.531 |
| Lymphadenopathy | 12 (34.3) | 4 (26.7) | 0.600 |
| Splenomegaly | 7 (20) | 3 (20) | 0.957 |
| Diabetes mellitus | 4 (11.4) | 3 (20) | 0.428 |
| Increased CRP (mg/dL) | 19 (54.3) 11.4 ± 10.0 (9.3) | 3 (20.0) 7.7 ± 9.0 (3.4) | 0.629 0.651 |
| Increased ESR (mm/h) | 12 (34.3) 72.7 ± 39.2 (76.5) | 4 (26.7) 109.5 ± 16.7 (115.0) | 0.133 0.114 |
| Number of advanced diagnostic tests performed | 3.2 ± 1.5 (3) | 3.5 ± 2.0 (4) | 0.463 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georga, S.; Exadaktylou, P.; Petrou, I.; Katsampoukas, D.; Mpalaris, V.; Moralidis, E.-I.; Arvaniti, K.; Papastergiou, C.; Arsos, G. Diagnostic Value of 18F-FDG-PET/CT in Patients with FUO. J. Clin. Med. 2020, 9, 2112. https://doi.org/10.3390/jcm9072112
Georga S, Exadaktylou P, Petrou I, Katsampoukas D, Mpalaris V, Moralidis E-I, Arvaniti K, Papastergiou C, Arsos G. Diagnostic Value of 18F-FDG-PET/CT in Patients with FUO. Journal of Clinical Medicine. 2020; 9(7):2112. https://doi.org/10.3390/jcm9072112
Chicago/Turabian StyleGeorga, Stamata, Paraskevi Exadaktylou, Ioannis Petrou, Dimitrios Katsampoukas, Vasilios Mpalaris, Efstratios-Iordanis Moralidis, Kostoula Arvaniti, Christos Papastergiou, and Georgios Arsos. 2020. "Diagnostic Value of 18F-FDG-PET/CT in Patients with FUO" Journal of Clinical Medicine 9, no. 7: 2112. https://doi.org/10.3390/jcm9072112
APA StyleGeorga, S., Exadaktylou, P., Petrou, I., Katsampoukas, D., Mpalaris, V., Moralidis, E.-I., Arvaniti, K., Papastergiou, C., & Arsos, G. (2020). Diagnostic Value of 18F-FDG-PET/CT in Patients with FUO. Journal of Clinical Medicine, 9(7), 2112. https://doi.org/10.3390/jcm9072112






