Plasma Metabolomic Profiling Associates Bicuspid Aortic Valve Disease and Ascending Aortic Dilation with a Decrease in Antioxidant Capacity †
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Blood Sampling and Analyses
2.3. Metabolomics Profiling
2.4. Random Forest Model Construction and Validation
2.5. Statistical Analysis
3. Results
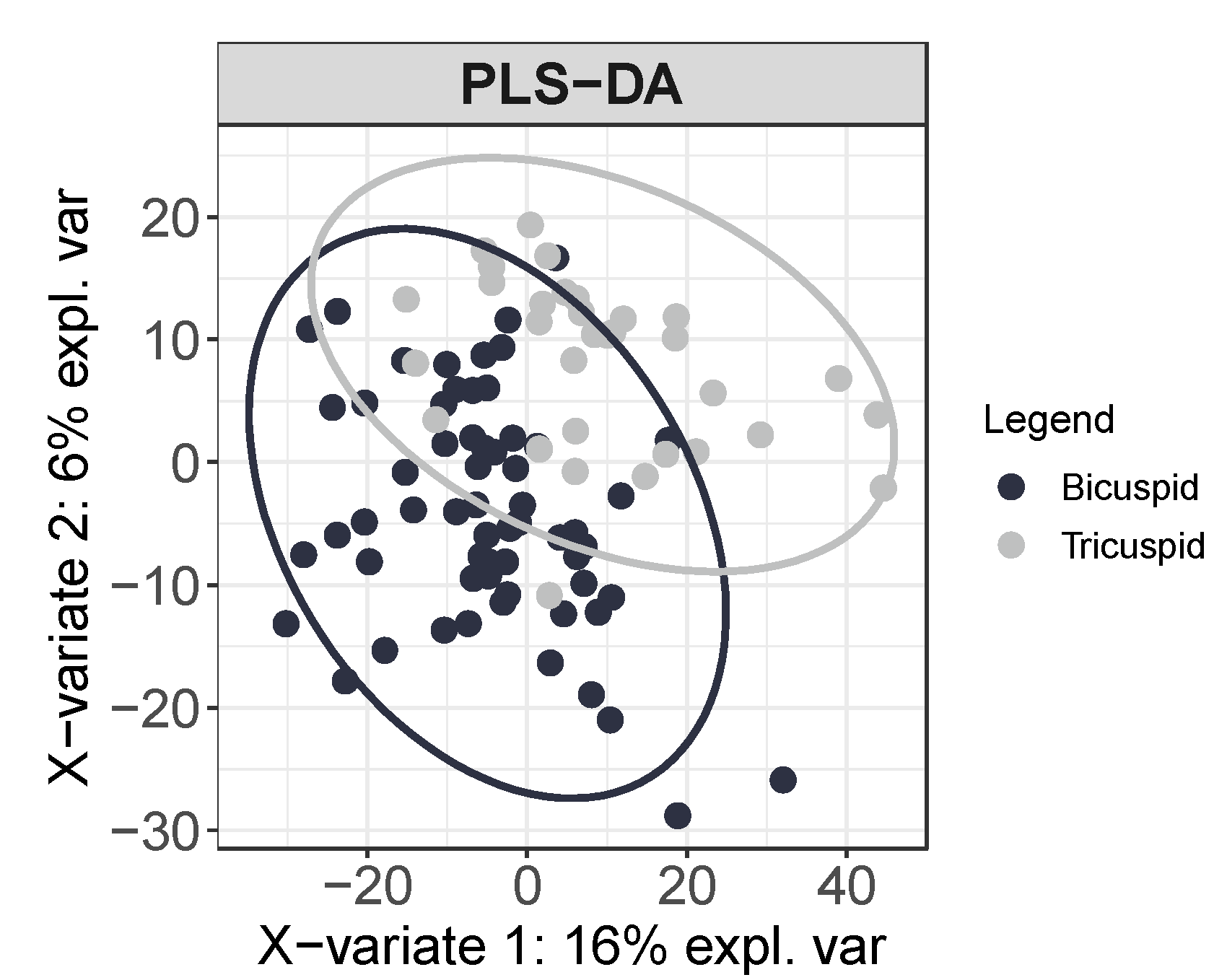
3.1. Circulating Profile of Metabolites Depends on the Morphology of the Aortic Valve
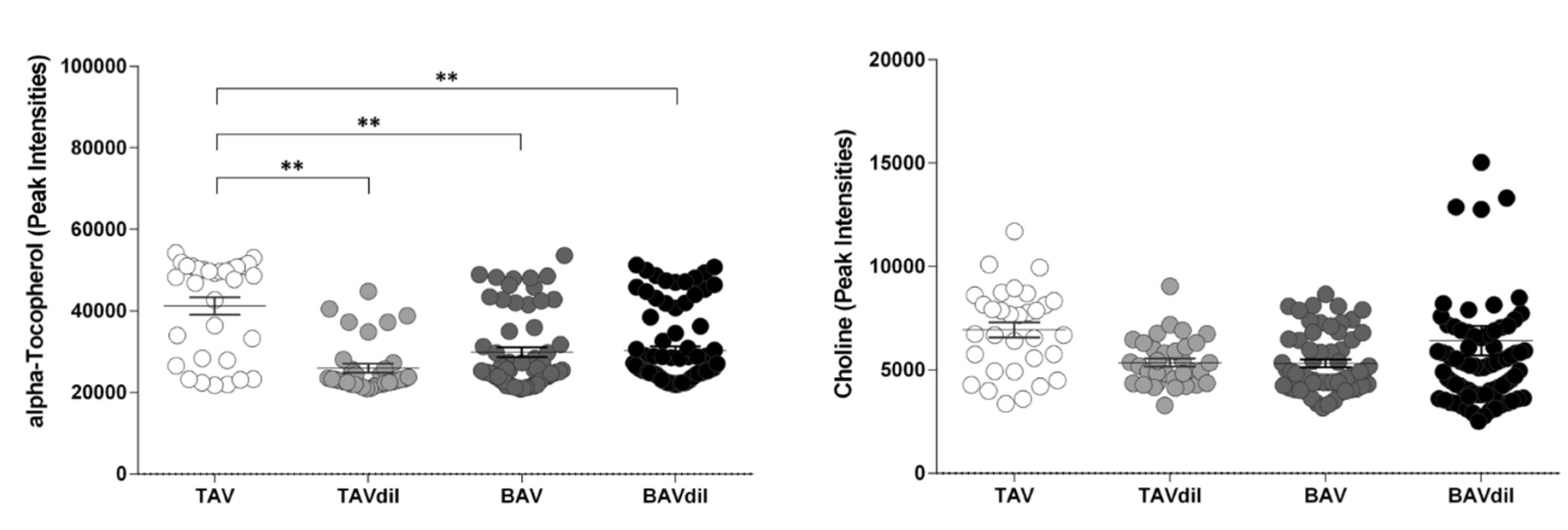
3.2. Functional Implication of the Identified Metabolites
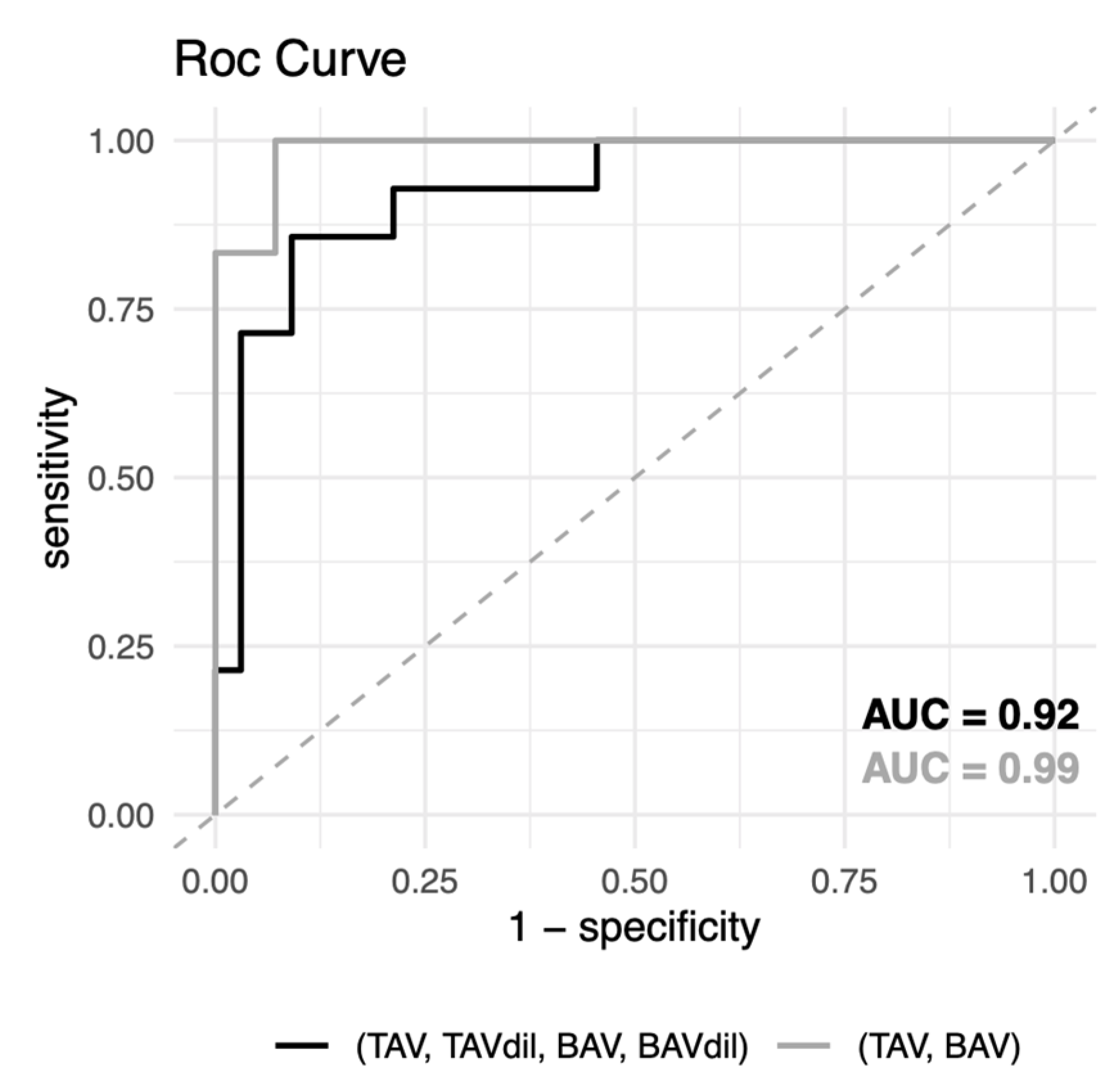
3.3. Prediction of the Morphology of the Aortic Valve or the Dilation of the Ascending Aorta in BAV Patients
4. Discussion
Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Tricuspid aortic valve | TAV |
| Tricuspid aortic valve with ascending aortic dilation | TAVdil |
| Bicuspid aortic valve | BAV |
| Bicuspid aortic valve with ascending aortic dilation | BAVdil |
| endothelial microparticles | EMPs |
| C-reactive protein | CRP |
| apolipoprotein A1 | ApoA1 |
| apolipoprotein B100 | ApoB100 |
References
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Cecconi, M.; Nistri, S.; Quarti, A.; Manfrin, M.; Colonna, P.L.; Molini, E.; Perna, G.P. Aortic dilatation in patients with bicuspid aortic valve. J. Cardiovasc. Med. 2006, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Gallego, P.; Calvo-Iglesias, F.; Bermejo, J.; Robledo-Carmona, J.; Sánchez, V.; Saura, D.; Arnold, R.; Carro, A.; Maldonado, G.; et al. Anatomical and clinical predictors of valve dysfunction and aortic dilation in bicuspid aortic valve disease. Heart 2018, 104, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Kale, R.; Maldonado, G.; Teixidó-Turà, G.; Galian, L.; Huguet, M.; Valente, F.; Gutiérrez, L.; et al. Aortic flow patterns and wall shear stress maps by 4D-flow cardiovascular magnetic resonance in the assessment of aortic dilatation in bicuspid aortic valve disease. J. Cardiovasc. Magn. Reson. 2018, 20, 28. [Google Scholar]
- van de Pol, V.; Kurakula, K.; DeRuiter, M.C.; Goumans, M.J. Thoracic aortic aneurysm development in patients with bicuspid aortic valve: What is the role of endothelial cells? Front. Physiol. 2017, 8, 938. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.A.; Chapman, M.; Nguyen, T.H.; Chirkov, Y.Y.; Heresztyn, T.; Mundisugih, J.; Horowitz, J.D. Interactions between inflammatory activation and endothelial dysfunction selectively modulate valve disease progression in patients with bicuspid aortic valve. Heart 2014, 100, 800–805. [Google Scholar] [CrossRef]
- Girdauskas, E.; Kaemmerer, H.; von Kodolitsch, Y. Unravelling the Pathogenetic Mechanisms in Congenital Aortopathies: Need for an Integrative Translational Approach. J. Clin. Med. 2020, 9, 204. [Google Scholar] [CrossRef]
- Cheng, M.-L.; Wang, C.-H.; Shiao, M.-S.; Liu, M.-H.; Huang, Y.-Y.; Huang, C.-Y.; Mao, C.-T.; Lin, J.-F.; Ho, H.-Y.; Yang, N.-I. Metabolic Disturbances Identified in Plasma Are Associated With Outcomes in Patients With Heart Failure. J. Am. Coll. Cardiol. 2015, 65, 1509–1520. [Google Scholar] [CrossRef]
- Alegret, J.M.; Martínez-Micaelo, N.; Aragonès, G.; Beltrán-Debón, R. Circulating endothelial microparticles are elevated in bicuspid aortic valve disease and related to aortic dilation. Int. J. Cardiol. 2016, 217, 35–41. [Google Scholar] [CrossRef]
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Baiges, I.; Faiges, M.; Alegret, J.M. Specific circulating microRNA signature of bicuspid aortic valve disease. J. Transl. Med. 2017, 15, 76. [Google Scholar] [CrossRef]
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Aragonés, G.; Faiges, M.; Alegret, J.M. MicroRNAs Clustered within the 14q32 Locus Are Associated with Endothelial Damage and Microparticle Secretion in Bicuspid Aortic Valve Disease. Front. Physiol. 2017, 8, 648. [Google Scholar] [CrossRef]
- Alegret, J.M.; Palazón, O.; Duran, I.; Vernis, J.M. Aortic valve morphology definition with transthoracic combined with transesophageal echocardiography in a population with high prevalence of bicuspid aortic valve. Int. J. Cardiovasc. Imaging 2005, 21, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, T.G.; Mwangi, I.; Horgan, S.J.; Cradock, A.; Fitzpatrick, P.; Murray, J.G. Steady-state free-precession (SSFP) cine MRI in distinguishing normal and bicuspid aortic valves. J. Magn. Reson. Imaging 2008, 28, 873–878. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Vinaixa, M.; Samino, S.; Saez, I.; Duran, J.; Guinovart, J.J.; Yanes, O. A Guideline to Univariate Statistical Analysis for LC/MS-Based Untargeted Metabolomics-Derived Data. Metabolites 2012, 2, 775–795. [Google Scholar] [CrossRef]
- Costa, C.; Maraschin, M.; Rocha, M. An R package for the integrated analysis of metabolomics and spectral data. Comput. Methods Programs Biomed. 2016, 129, 117–124. [Google Scholar] [CrossRef]
- Wiener AL and, M. Classification and Regression by randomForest. R News 2. 2003. Available online: http://www.stat.berkeley.edu/ (accessed on 2 July 2020).
- Sophocleous, F.; Milano, E.G.; Pontecorboli, G.; Chivasso, P.; Caputo, M.; Rajakaruna, C.; Bucciarelli-Ducci, C.; Emanueli, C.; Biglino, G. Enlightening the Association between Bicuspid Aortic Valve and Aortopathy. J. Cardiovasc. Dev. Dis. 2018, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Braverman, A.C.; Güven, H.; Beardslee, M.A.; Makan, M.; Kates, A.M.; Moon, M.R. The bicuspid aortic valve. Curr. Probl. Cardiol. 2005, 30, 470–522. [Google Scholar] [CrossRef]
- Liu, T.; Xie, M.; Lv, Q.; Li, Y.; Fang, L.; Zhang, L.; Deng, W.; Wang, J. (2019) Bicuspid aortic valve: An update in morphology, genetics, biomarker, complications, imaging diagnosis and treatment. Front. Physiol. 2019, 9, 1921. [Google Scholar] [CrossRef]
- Della Corte, A.; Michelena, H.I.; Citarella, A.; Votta, E.; Piatti, F.; Lo Presti, F.; Ashurov, R.; Cipollaro, M.; Forte, A. Risk Stratification in Bicuspid Aortic Valve Aortopathy: Emerging Evidence and Future Perspectives. Curr. Probl. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gertsman, I.; Barshop, B.A. Promises and pitfalls of untargeted metabolomics. J. Inherit. Metab. Dis. 2018, 41, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Behrens, W.A.; Thompson, J.N.; Madère, R. Distribution of α-tocopherol in human plasma lipoproteins. Am. J. Clin. Nutr. 1982, 35, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; Fasano, M.; Ascenzi, P.; Zingg, J.M.; Azzi, A. α-Tocopherol binding to human serum albumin. BioFactors 2013, 39, 294–303. [Google Scholar] [CrossRef]
- Efrat, M.; Aviram, M. Paraoxonase 1 interactions with HDL, antioxidants and macrophages regulate atherogenesis—A protective role for HDL phospholipids. Adv. Exp. Med. Biol. 2010, 660, 153–166. [Google Scholar] [PubMed]
- Wallert, M.; Schmölz, L.; Galli, F.; Birringer, M.; Lorkowski, S. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. 2014, 2, 495–503. [Google Scholar] [CrossRef]
- Wang, W.; Maimaiti, A.; Zhao, Y.; Zhang, L.; Tao, H.; Nian, H.; Xia, L.; Kong, B.; Wang, C.; Liu, M.; et al. Analysis of Serum Metabolites to Diagnose Bicuspid Aortic Valve. Sci. Rep. 2016, 6, 37023. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Y.; Tang, Q.; Leng, Y.; Cai, W. The effects of choline on hepatic lipid metabolism, mitochondrial function and antioxidative status in human hepatic C3A cells exposed to excessive energy substrates. Nutrients 2014, 6, 2552–2571. [Google Scholar] [CrossRef]
- Zeisel, S. Choline, Other Methyl-Donors and Epigenetics. Nutrients 2017, 9, 445. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–166. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Y.; Bi, X.; Xu, M.; Yu, X.; Xue, R. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Keane, M.G.; Wiegers, S.E.; Plappert, T.; Pochettino, A.; Bavaria, J.E.; St John Sutton, M.G. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation 2000, 102, Iii-35–Iii-39. [Google Scholar] [CrossRef]
- Meierhofer, C.; Schneider, E.P.; Lyko, C.; Hutter, A.; Martinoff, S.; Markl, M.; Hager, A.; Hess, J.; Stern, H.; Fratz, S. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: A prospective study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Pasta, S.; Rinaudo, A.; Luca, A.; Pilato, M.; Scardulla, C.; Gleason, T.G.; Vorp, D.A. Difference in hemodynamic and wall stress of ascending thoracic aortic aneurysms with bicuspid and tricuspid aortic valve. J. Biomech. 2013, 46, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Magni, P. Bicuspid aortic valve, atherosclerosis and changes of lipid metabolism: Are there pathological molecular links? J. Mol. Cell. Cardiol. 2019, 129, 231–235. [Google Scholar] [CrossRef] [PubMed]
| TAV | BAV | TAVdil | BAVdil | p-Value | |
|---|---|---|---|---|---|
| n | 33 | 62 | 35 | 82 | |
| Age (years) | 47 ± 2 | 41 ± 2 | 63 ± 2 | 52 ± 2 | 3.9 × 10−11 **, b,c,d |
| Sex (male/female) | (25/8) | (45/17) | (22/13) | (58/24) | 0.670 |
| Body weight (kg) | 71.32 ± 2.4 | 71.00 ± 2.0 | 80.50 ± 11.5 | 76.52 ± 1.8 | 0.183 |
| Severe aortic stenosis (mean gradient ≥ 40 mm Hg) | 0 (0%) | 3 (5%) | 0 (0%) | 19 (23%) | 2.1 × 10−5 ** |
| Aortic valve gradient (mean, mm Hg) | 3.57 ± 1.0 | 12.04 ± 1.8 | 2.85 ± 0.8 | 24.75 ± 2.6 | 5.5 × 10−12 **, b,d |
| Left ventricle diastolic diameter (mm) | 50.22 ± 0.9 | 52.20 ± 0.8 | 53.48 ± 1.0 | 53.79 ± 1.0 | 0.100 |
| Left ventricle systolic diameter (mm) | 30.35 ± 1.2 | 32.56 ± 0.8 | 30.01 ± 0.9 | 33.36 ± 0.9 | 0.175 |
| Left ventricular ejection fraction (%) | 71.92 ± 1.9 | 70.72 ± 1.2 | 70.86 ± 1.8 | 69.18 ± 1.1 | 0.568 |
| Aortic root diameter (mm) | 33.06 ± 0.8 | 33.93 ± 0.8 | 41.94 ± 1.2 | 39.32 ± 0.6 | 2.4 × 10−13 **, b,c |
| Ascending aorta diameter (mm) | 30.81 ± 0.6 | 32.56 ± 0.4 | 44.88 ± 0.7 | 44.36 ± 0.6 | 5.5 × 10−47 **, b,c |
| Coefficient | Std-Coefficient | p-Value | |
|---|---|---|---|
| alpha-Tocopherol | |||
| Age (years) | −47.99 (−157.20 to 61.31) | −0.75 | 0.388 |
| Aortic root (mm) | 120.60 (−207.20 to 448.39) | 0.75 | 0.469 |
| Ascending aorta (mm) | −370.60 (−643.81 to −97.39) | −2.68 | 8.13 × 10−3 ** |
| Mean transaortic pressure gradient (mm Hg) | 25.91 (−58.78 to 110.61) | 0.046 | 0.547 |
| Left ventricle diastolic diameter (mm) | 29.72 (−316.57 to 376.01) | 0.021 | 0.169 |
| Left ventricle systolic diameter (mm) | 133.85 (−418.85 to 686.54) | 0.071 | 0.633 |
| Left ventricular ejection fraction (%) | 165.13 (−76.42 to 406.68) | 0.157 | 0.179 |
| TAV | BAV | TAVdil | BAVdil | p-Value | |
|---|---|---|---|---|---|
| Total cholesterol (mmol/L) | 5.61 ± 0.3 (5.04–6.18) | 4.77 ± 0.2 (4.43–5.01) | 5.20 ± 0.3 (4.60–5.79) | 5.07 ± 0.1 (4.79–5.33) | 0.171 |
| Triglycerides (mmol/L) | 1.36 ± 0.2 (0.96–1.76) | 0.97 ± 0.7 (0.82–1.11) | 1.48 ± 0.2 (1.13–1.84) | 1.33 ± 0.1 (1.06–1.60) | 0.139 |
| LDL (mmol/L) | 3.45 ± 0.2 (3.00–3.90) | 2.87 ± 0.1 (2.58–3.15) | 3.26 ± 0.2 (2.80–3.72) | 2.97 ± 0.1 (2.74–3.20) | 0.172 |
| HDL (mmol/L) | 1.55 ± 0.1 (1.32–1.77) | 1.47 ± 0.5 (1.37–1.57) | 1.26 ± 0.1 (1.14–1.39) | 1.49 ± 0.5 (1.39–1.59) | 0.049 * |
| ApoA1 (mg/dL) | 160.67 ± 7.3 (144.67–176.66) | 133.47 ± 4.4 (124.60–142.35) | 139.53 ± 4.5 (130.02–149.03) | 140.61 ± 3.7 (133.22–148.00) | 0.007 ** a, e |
| ApoB100 (mg/dL) | 118.50 ± 6.9 (103.27–133.73) | 88.67 ± 4.1 (80.38–96.95) | 112.88 ± 6.2 (99.65–126.12) | 99.22 ± 3.7 (91.79–106.64) | 0.005 ** a, c |
| C-reactive protein (mg/dL) | 1.06 ± 0.3 (0.38–1.72) | 1.71 ± 0.3 (1.14–2.29) | 1.84 ± 0.5 (0.86–2.82) | 2.42 ± 0.4 (1.70–3.13) | 0.046 * e |
| Paraoxonase 1 (ng/mL) | 85.23 ± 6.5 (70.94–99.94) | 94.61 ± 8.8 (76.67–112.55) | 78.12 ± 8.0 (61.25–94.99) | 87.12 ± 7.1 (72.86–101.37) | 0.647 |
| Endothelial microparticles (log part/μL) | 2.49 ± 0.4 (1.55–3.43) | 3.68 ± 0.3 (3.05–4.27) | 3.24 ± 0.3 (2.55–3.92) | 3.78 ± 0.2 (3.35–4.20) | 0.020 * e |
| Albumin (g/dL) | 4.64 ± 0.3 (4.55–4.73) | 4.53 ± 0.1 (4.53–4.65) | 4.39 ± 0.1 (4.27–4.52) | 4.52 ± 0.1 (4.46–4.58) | 0.010 * c |
| Alpha-Tocopherol | ||
|---|---|---|
| R | p-Value | |
| Aortic root (mm) | −0.172 | 0.012 * |
| Ascending aorta (mm) | −0.271 | 7.20 × 10−5 ** |
| Mean transaortic pressure gradient (mm Hg) | −0.083 | 0.230 |
| Age (y) | −0.179 | 0.009 ** |
| Lipid Metabolism | ||
| Total cholesterol (mmol/L) | 0.052 | 0.514 |
| Triglycerides (mmol/L) | 0.023 | 0.770 |
| ApoA1 (mg/dL) | 0.209 | 0.008 ** |
| ApoB100 (mg/dL) | 0.082 | 0.305 |
| Inflammation | ||
| C-reactive protein (mg/dL) | 0.042 | 0.607 |
| Oxidative stress | ||
| Paraoxonase 1 | 0.195 | 0.030 * |
| Endothelial damage | ||
| Endothelial microparticles (log part/μL) | 0.097 | 0.263 |
| Liver damage | ||
| Albumin (g/dL) | 0.248 | 0.002 ** |
| p-Value | OOB Error | Accuracy (95% CI) | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| TAV + TAVdil + BAV + BAVdil | |||||
| 3.9 × 10−3 ** | 21.7% | 0.89 (0.77–0.96) | 0.91 | 0.86 | 0.92 |
| TAV + BAV | |||||
| 7.6 × 10−3 ** | 17.5% | 0.95 (0.75–0.99) | 0.93 | 1.00 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Micaelo, N.; Ligero, C.; Antequera-González, B.; Junza, A.; Yanes, O.; Alegret, J.M. Plasma Metabolomic Profiling Associates Bicuspid Aortic Valve Disease and Ascending Aortic Dilation with a Decrease in Antioxidant Capacity. J. Clin. Med. 2020, 9, 2215. https://doi.org/10.3390/jcm9072215
Martínez-Micaelo N, Ligero C, Antequera-González B, Junza A, Yanes O, Alegret JM. Plasma Metabolomic Profiling Associates Bicuspid Aortic Valve Disease and Ascending Aortic Dilation with a Decrease in Antioxidant Capacity. Journal of Clinical Medicine. 2020; 9(7):2215. https://doi.org/10.3390/jcm9072215
Chicago/Turabian StyleMartínez-Micaelo, Neus, Carme Ligero, Borja Antequera-González, Alexandra Junza, Oscar Yanes, and Josep M. Alegret. 2020. "Plasma Metabolomic Profiling Associates Bicuspid Aortic Valve Disease and Ascending Aortic Dilation with a Decrease in Antioxidant Capacity" Journal of Clinical Medicine 9, no. 7: 2215. https://doi.org/10.3390/jcm9072215
APA StyleMartínez-Micaelo, N., Ligero, C., Antequera-González, B., Junza, A., Yanes, O., & Alegret, J. M. (2020). Plasma Metabolomic Profiling Associates Bicuspid Aortic Valve Disease and Ascending Aortic Dilation with a Decrease in Antioxidant Capacity. Journal of Clinical Medicine, 9(7), 2215. https://doi.org/10.3390/jcm9072215





