A Comparative Study of Laparoscopic versus Open Pancreaticoduodenectomy for Ampulla of Vater Carcinoma
Abstract
1. Introduction
2. Methods
2.1. Patient Database
2.2. Operative Procedure
2.3. Statistical Analysis
3. Results
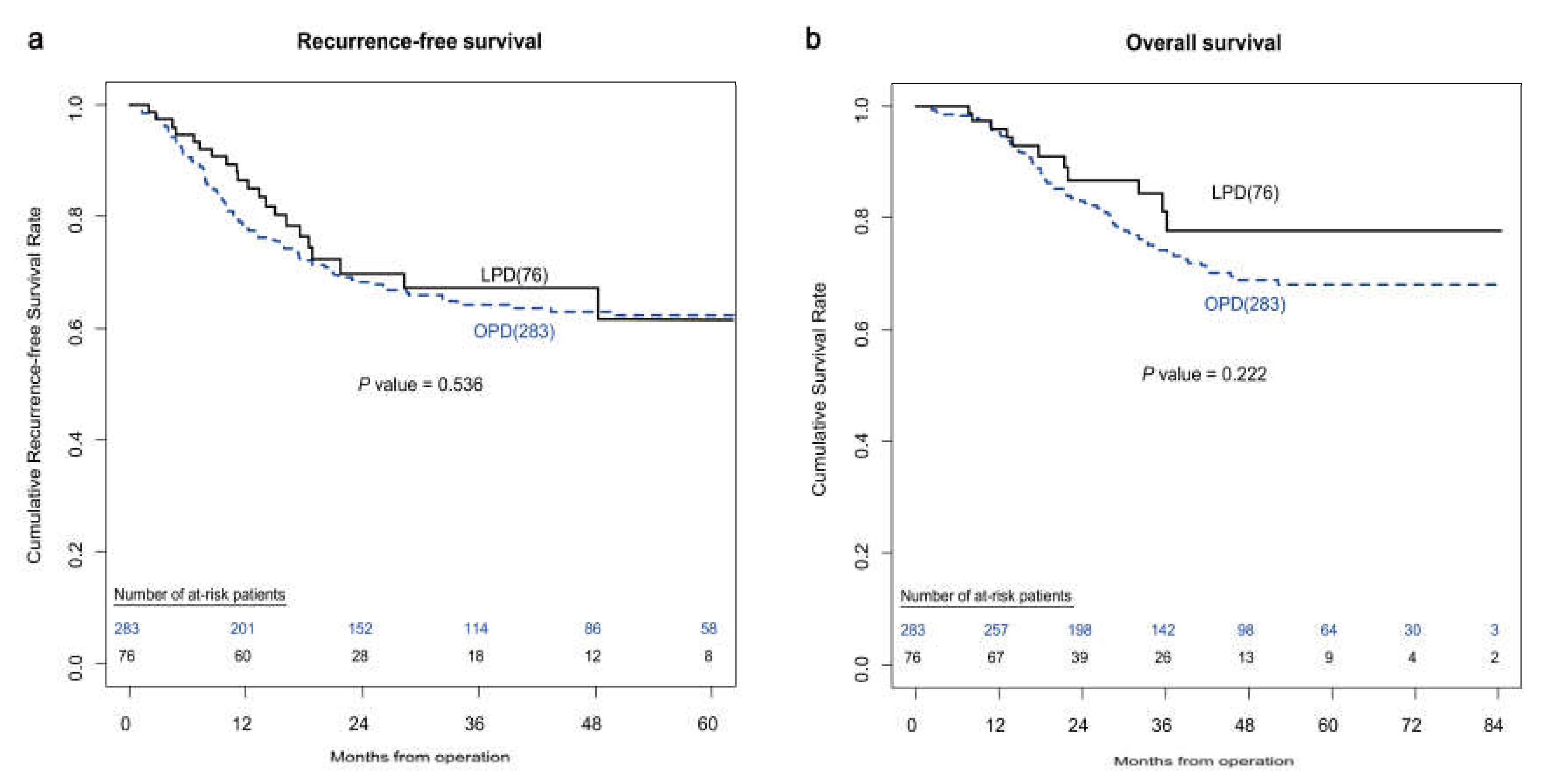
3.1. Comparative Analysis between the LPD and OPD Groups—Unmatched Patients
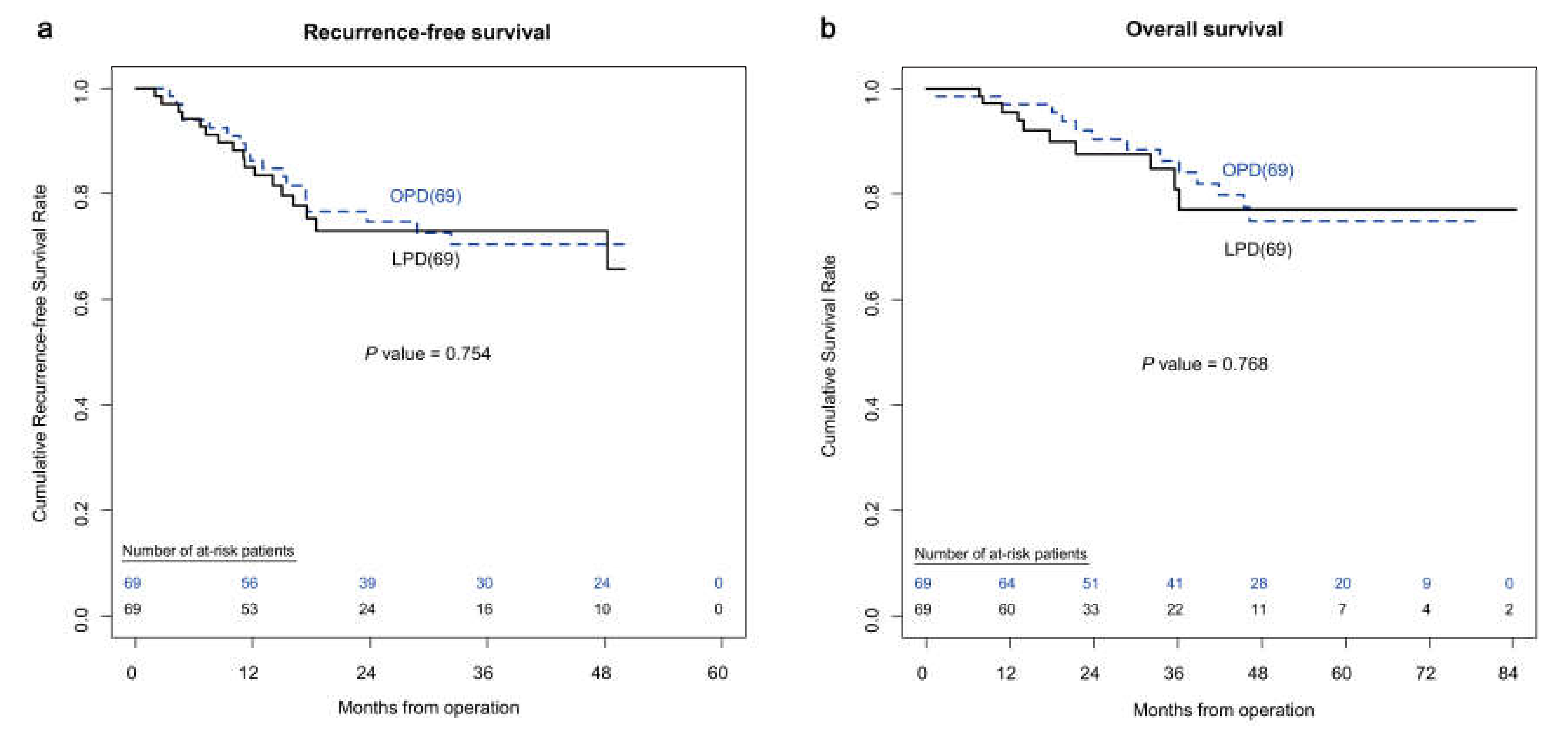
3.2. Comparative Analysis between the LPD and OPD Groups—Propensity-Score-Matched Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gaspar, B.; Beuran, M.; Paun, S.; Ganescu, R.; Hostiuc, S.; Negoi, I. Current strategies in the therapeutic approach for adenocarcinoma of the ampulla of Vater. J. Med. Life 2013, 6, 260–265. [Google Scholar] [PubMed]
- Senatore, F.J.; Ynson, M.L.; Dasanu, C.A. Adenocarcinoma of the ampulla of Vater: What treatment options are available? J. Oncol. Pharm. Pract. 2015, 21, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Gagner, M.; Pomp, A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg. Endosc. 1994, 8, 408–410. [Google Scholar] [CrossRef]
- Song, K.B.; Kim, S.C.; Hwang, D.W.; Lee, J.H.; Lee, D.J.; Lee, J.W.; Park, K.M.; Lee, Y.J. Matched case-control analysis comparing laparoscopic and open pylorus-preserving pancreaticoduodenectomy in patients with periampullary tumors. Ann. Surg. 2015, 262, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Song, K.B.; Jung, Y.S.; Kim, Y.H.; Park, D.H.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Park, K.M.; et al. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: Improvement with surgical experience. Surg. Endosc. 2013, 27, 95–103. [Google Scholar] [CrossRef]
- De Rooij, T.; Lu, M.Z.; Steen, M.W.; Gerhards, M.F.; Dijkgraaf, M.G.; Busch, O.R.; Lips, D.J.; Festen, S.; Besselink, M.G. Minimally invasive versus open pancreatoduodenectomy: Systematic review and meta-analysis of comparative cohort and registry studies. Ann. Surg. 2016, 264, 257–267. [Google Scholar] [CrossRef]
- Delitto, D.; Luckhurst, C.M.; Black, B.S.; Beck, J.L.; George, T.J., Jr.; Sarosi, G.A.; Thomas, R.M.; Trevino, J.G.; Behrns, K.E.; Hughes, S.J. Oncologic and perioperative outcomes following selective application of laparoscopic pancreaticoduodenectomy for periampullary malignancies. J. Gastrointest. Surg. 2016, 20, 1343–1349. [Google Scholar] [CrossRef]
- Klompmaker, S.; van Hilst, J.; Wellner, U.F.; Busch, O.R.; Coratti, A.; D’Hondt, M.; Dokmak, S.; Festen, S.; Kerem, M.; Khatkov, I.; et al. Outcomes after minimally-invasive versus open pancreatoduodenectomy: A Pan-European propensity score matched study. Ann. Surg. 2020, 271, 356–363. [Google Scholar] [CrossRef]
- Liao, C.H.; Wu, Y.T.; Liu, Y.Y.; Wang, S.Y.; Kang, S.C.; Yeh, C.N.; Yeh, T.S. Systemic review of the feasibility and advantage of minimally invasive pancreaticoduodenectomy. World J. Surg. 2016, 40, 1218–1225. [Google Scholar] [CrossRef]
- Torphy, R.J.; Friedman, C.; Halpern, A.; Chapman, B.C.; Ahrendt, S.S.; McCarter, M.M.; Edil, B.H.; Schulick, R.D.; Gleisner, A. Comparing short-term and oncologic outcomes of minimally invasive versus open pancreaticoduodenectomy across low and high volume centers. Ann. Surg. 2019, 270, 1147–1155. [Google Scholar] [CrossRef]
- Asbun, H.J.; Stauffer, J.A. Laparoscopic vs open pancreaticoduodenectomy: Overall outcomes and severity of complications using the Accordion Severity Grading System. J. Am. Coll. Surg. 2012, 215, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, M.L.; Cusati, D. Total laparoscopic pancreaticoduodenectomy: Feasibility and outcome in an early experience. Arch. Surg. 2010, 145, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Farnell, M.B.; Que, F.G.; Reid-Lombardo, K.M.; Truty, M.J.; Nagorney, D.M.; Kendrick, M.L. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: Oncologic advantages over open approaches? Ann. Surg. 2014, 260, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.A.; Choudhury, K.; Dinan, M.A.; Reed, S.D.; Scheri, R.P.; Blazer, D.G., 3rd; Roman, S.A.; Sosa, J.A. Minimally invasive versus open pancreaticoduodenectomy for cancer: Practice patterns and short-term outcomes among 7061 patients. Ann. Surg. 2015, 262, 372–377. [Google Scholar] [CrossRef]
- Chapman, B.C.; Gleisner, A.; Ibrahim-Zada, I.; Overbey, D.M.; Paniccia, A.; Meguid, C.; Brauer, B.; Gajdos, C.; McCarter, M.D.; Schulick, R.D.; et al. Laparoscopic pancreaticoduodenectomy: Changing the management of ampullary neoplasms. Surg. Endosc. 2018, 32, 915–922. [Google Scholar] [CrossRef]
- Lu, B.; Cai, X.; Lu, W.; Huang, Y.; Jin, X. Laparoscopic pancreaticoduodenectomy to treat cancer of the ampulla of Vater. JSLS 2006, 10, 97–100. [Google Scholar]
- Owens, W.D.; Felts, J.A.; Spitznagel, E.L., Jr. ASA physical status classifications: A study of consistency of ratings. Anesthesiology 1978, 49, 239–243. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Dindo, D.; Clavien, P.A. What is a surgical complication? World J. Surg. 2008, 32, 939–941. [Google Scholar] [CrossRef]
- Bonjer, H.J.; Hop, W.C.; Nelson, H.; Sargent, D.J.; Lacy, A.M.; Castells, A.; Guillou, P.J.; Thorpe, H.; Brown, J.; Delgado, S.; et al. Laparoscopically assisted vs open colectomy for colon cancer: A meta-analysis. Arch. Surg. 2007, 142, 298–303. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, S.C.; Song, K.B.; Hwang, D.W.; Lee, J.H.; Lee, D.; Lee, J.W.; Jun, E.; Park, K.M.; Lee, Y.J. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: A propensity score-matched analysis. J. Am. Coll. Surg. 2015, 220, 177–185. [Google Scholar] [CrossRef]
- Veldkamp, R.; Gholghesaei, M.; Bonjer, H.J.; Meijer, D.W.; Buunen, M.; Jeekel, J.; Anderberg, B.; Cuesta, M.A.; Cuschierl, A.; Fingerhut, A.; et al. Laparoscopic resection of colon cancer: Consensus of the European Association of Endoscopic Surgery (EAES). Surg. Endosc. 2004, 18, 1163–1185. [Google Scholar] [CrossRef]
- Ferrone, C.R.; Finkelstein, D.M.; Thayer, S.P.; Muzikansky, A.; Fernandez-delCastillo, C.; Warshaw, A.L. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2006, 24, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Austin, P.C. Some methods of propensity-score matching had superior performance to others: Results of an empirical investigation and Monte Carlo simulations. Biom. J. 2009, 51, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, C.A.; Limongelli, P.; Pai, M.; Ahmad, R.; Stamp, G.; Habib, N.A.; Williamson, R.C.; Jiao, L.R. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: An analysis of clinicopathological factors. J. Surg. Oncol. 2009, 100, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, C.; Senthilnathan, P.; Sabnis, S.C.; Babu, N.S.; Srivatsan Gurumurthy, S.; Anand Vijai, N.; Nalankilli, V.P.; Praveen Raj, P.; Parthasarathy, R.; Rajapandian, S. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br. J. Surg. 2017, 104, 1443–1450. [Google Scholar] [CrossRef]
- Marjanovic, G.; Kuvendziska, J.; Holzner, P.A.; Glatz, T.; Sick, O.; Seifert, G.; Kulemann, B.; Kusters, S.; Fink, J.; Timme, S.; et al. A prospective clinical study evaluating the development of bowel wall edema during laparoscopic and open visceral surgery. J. Gastrointest. Surg. 2014, 18, 2149–2154. [Google Scholar] [CrossRef]
- Hiki, N.; Shimizu, N.; Yamaguchi, H.; Imamura, K.; Kami, K.; Kubota, K.; Kaminishi, M. Manipulation of the small intestine as a cause of the increased inflammatory response after open compared with laparoscopic surgery. Br. J. Surg. 2006, 93, 195–204. [Google Scholar] [CrossRef]
| Characteristics | LPD | OPD | p-Value |
|---|---|---|---|
| (n = 76) | (n = 283) | ||
| Age, years (mean ± SD) | 62.5 ± 10.3 | 63.7 ± 8.8 | 0.295 |
| Sex, n (%) | 0.336 | ||
| Female | 38 (50.0) | 124 (43.8) | |
| Male | 38 (50.0) | 159 (56.2) | |
| BMI, kg/m2 (mean ± SD) | 23.0 ± 2.8 | 23.6 ± 2.8 | 0.086 |
| ASA score, n (%) | 0.398 | ||
| Class I | 7 (9.2) | 28 (9.9) | |
| Class II | 60 (78.9) | 235 (83.0) | |
| Class ≥ III | 9 (11.8) | 20 (7.1) | |
| Charlson comorbidity index (mean ± SD) | 2.2 ± 1.3 | 2.3 ± 1.3 | 0.447 |
| Preoperative biliary/pancreatic drainage, n (%) | 0.827 | ||
| No | 20 (26.3) | 71 (25.1) | |
| Yes | 56 (73.7) | 212 (74.9) |
| Characteristics | LPD | OPD | p-Value |
|---|---|---|---|
| (n = 76) | (n = 283) | ||
| Tumor size, cm (mean ± SD) | 1.8 ± 1.0 | 2.0 ± 0.9 | 0.295 |
| Differentiation, n (%) | 0.189 | ||
| Well | 27 (35.5) | 79 (28.2) | |
| Moderate | 45 (59.2) | 169 (60.4) | |
| Poor | 4 (5.3) | 32 (11.4) | |
| Perineural invasion, n (%) | 0.007 | ||
| No | 67 (88.2) | 208 (73.5) | |
| Yes | 9 (11.8) | 75 (26.5) | |
| Lymphovascular invasion, n (%) | 0.756 | ||
| No | 41 (53.9) | 147 (51.9) | |
| Yes | 35 (46.1) | 136 (48.1) | |
| AJCC 7th T stage, n (%) | 0.038 | ||
| T1 | 29 (38.2) | 63 (22.3) | |
| T2 | 26 (34.2) | 129 (45.6) | |
| T3 | 18 (23.7) | 82 (29.0) | |
| T4 | 3 (3.9) | 9 (3.2) | |
| AJCC 7th N stage, n (%) | 0.326 | ||
| N0 | 55 (72.4) | 187 (66.3) | |
| N1 | 21 (27.6) | 95 (33.7) |
| Characteristics | LPD | OPD | p-Value |
|---|---|---|---|
| (n = 76) | (n = 283) | ||
| Operative time, min (mean ± SD) | 371.0 ± 90.6 | 317.0 ± 82.4 | <0.001 |
| Transfusion, n (%) | 0.343 | ||
| No | 67 (88.2) | 237 (83.7) | |
| Yes | 9 (11.8) | 46 (16.3) | |
| Number of harvested LN, n (mean ± SD) | 14.0 ± 6.1 | 16.6 ± 8.0 | 0.003 |
| Resection margin status, n (%) | >0.999 | ||
| Negative (R0) | 75 (98.7) | 279 (98.6) | |
| Positive (R1) | 1 (1.3) | 4 (1.4) | |
| Length of hospital stay, days (mean ± SD) | 13.6 ± 10.3 | 18.8 ± 18.4 | 0.02 |
| CR-POPF, n (%) | 0.027 | ||
| No | 69 (90.8) | 226 (79.9) | |
| Yes | 7 (9.2) | 57 (20.1) | |
| Delayed gastric emptying, n (%) | 0.453 | ||
| No | 72 (94.7) | 261 (92.2) | |
| Yes | 4 (5.3) | 22 (7.8) | |
| Complications, n (%) | 0.013 | ||
| Grade 0-I | 64 (84.2) | 198 (70.0) | |
| ≥Grade II | 12 (15.8) | 85 (30.0) | |
| Readmission due to complication, n (%) | 0.093 | ||
| No | 65 (85.5) | 260 (91.9) | |
| Yes | 11 (14.5) | 23 (8.1) |
| Characteristics | Matched LPD | Matched OPD | p-Value |
|---|---|---|---|
| (n = 69) | (n = 69) | ||
| Age, years (mean ± SD) | 62.8 ± 10.1 | 63.2 ± 8.6 | 0.806 |
| Sex, n (%) | 0.48 | ||
| Female | 35 (50.7) | 31 (44.9) | |
| Male | 34 (49.3) | 38 (55.1) | |
| BMI, kg/m2 (mean ± SD) | 23.1 ± 2.7 | 23.5 ± 3.3 | 0.367 |
| ASA score, n (%) | 0.8 | ||
| Class I | 5 (7.3) | 7 (10.1) | |
| Class II | 56 (81.2) | 55 (79.7) | |
| Class ≥ III | 8 (11.6) | 7 (10.1) | |
| Charlson comorbidity index (mean ± SD) | 2.2 ± 1.3 | 2.3 ± 1.1 | 0.667 |
| Preoperative biliary / pancreatic drainage, n (%) | >0.999 | ||
| No | 18 (26.1) | 18 (26.1) | |
| Yes | 51 (73.9) | 51 (73.9) | |
| Tumor size, cm (mean ± SD) | 1.9 ± 1.0 | 1.8 ± 1.0 | 0.551 |
| Differentiation, n (%) | 0.69 | ||
| Well | 26 (37.7) | 29 (42.0) | |
| Moderate | 39(56.5) | 38 (55.1) | |
| Poor | 4 (5.8) | 2 (2.9) | |
| Perineural invasion, n (%) | 0.739 | ||
| No | 62 (89.9) | 61 (88.4) | |
| Yes | 7 (10.1) | 8 (11.6) | |
| Lymphovascular invasion, n (%) | 0.369 | ||
| No | 39 (56.5) | 44 (63.8) | |
| Yes | 30 (43.5) | 25 (36.2) | |
| AJCC 7th T stage, n (%) | 0.836 | ||
| T1 | 26 (37.7) | 31 (44.9) | |
| T2 | 25 (32.2) | 22 (31.9) | |
| T3 | 16 (23.2) | 14 (20.3) | |
| T4 | 2 (2.9) | 2 (2.9) | |
| AJCC 7th N stage, n (%) | 0.532 | ||
| N0 | 51 (73.9) | 54 (78.3) | |
| N1 | 18 (26.1) | 15 (21.7) | |
| CA 19-9, U/ml | 0.647 | ||
| ≤35 | 52 (75.4) | 53 (76.8) | |
| 35~200 | 13 (18.8) | 13 (18.8) | |
| 200~1000 | 3 (4.4) | 2 (2.9) | |
| >1000 | 1 (1.4) | 1 (1.5) | |
| CEA, ng/mL | 2.3 ± 1.4 | 2.1 ± 1.7 | 0.61 |
| Characteristics | Matched LPD | Matched OPD | p-Value |
|---|---|---|---|
| (n = 69) | (n = 69) | ||
| Operative time, min (mean ± SD) | 400.2 ± 91.2 | 344.6 ± 80.9 | <0.001 |
| Transfusion, n (%) | 0.796 | ||
| No | 60 (87.0) | 59 (85.5) | |
| Yes | 9 (13.0) | 10 (14.5) | |
| Number of harvested LN, n (mean ± SD) | 14.2 ± 5.9 | 15.5 ± 7.4 | 0.261 |
| Resection margin status, n (%) | >0.999 | ||
| Negative (R0) | 68 (98.5) | 68 (98.5) | |
| Positive (R1) | 1 (1.5) | 1 (1.5) | |
| Length of hospital stay, days (mean ± SD) | 13.7 ± 10.8 | 17.3 ± 9.4 | 0.048 |
| Number of postoperative painkiller administrations (IV or IM), n (mean ± SD) | 8.3 ± 7.9 | 11.1 ± 7.5 | 0.049 |
| CR-POPF, n (%) | 0.029 | ||
| No | 63 (91.3) | 54 (78.3) | |
| Yes | 6 (8.7) | 15 (21.7) | |
| Delayed gastric emptying, n (%) | 0.109 | ||
| No | 65 (94.2) | 59 (85.5) | |
| Yes | 4 (5.8) | 10 (14.5) | |
| Complications, n (%) | 0.012 | ||
| Grade 0-I | 58 (84.1) | 45 (65.2) | |
| ≥Grade II | 11 (15.9) | 24 (34.8) | |
| Readmission due to complication, n (%) | 0.808 | ||
| No | 60 (87.0) | 61 (88.4) | |
| Yes | 9 (13.0) | 8 (11.6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, D.; Song, K.B.; Lee, J.W.; Hwang, K.; Hong, S.; Shin, D.; Hwang, D.W.; Lee, J.H.; Lee, W.; Kwon, J.; et al. A Comparative Study of Laparoscopic versus Open Pancreaticoduodenectomy for Ampulla of Vater Carcinoma. J. Clin. Med. 2020, 9, 2214. https://doi.org/10.3390/jcm9072214
Yoo D, Song KB, Lee JW, Hwang K, Hong S, Shin D, Hwang DW, Lee JH, Lee W, Kwon J, et al. A Comparative Study of Laparoscopic versus Open Pancreaticoduodenectomy for Ampulla of Vater Carcinoma. Journal of Clinical Medicine. 2020; 9(7):2214. https://doi.org/10.3390/jcm9072214
Chicago/Turabian StyleYoo, Daegwang, Ki Byung Song, Jong Woo Lee, Kyungyeon Hwang, Sarang Hong, Dakyum Shin, Dae Wook Hwang, Jae Hoon Lee, Woohyung Lee, Jaewoo Kwon, and et al. 2020. "A Comparative Study of Laparoscopic versus Open Pancreaticoduodenectomy for Ampulla of Vater Carcinoma" Journal of Clinical Medicine 9, no. 7: 2214. https://doi.org/10.3390/jcm9072214
APA StyleYoo, D., Song, K. B., Lee, J. W., Hwang, K., Hong, S., Shin, D., Hwang, D. W., Lee, J. H., Lee, W., Kwon, J., Park, Y., Jun, E., & Kim, S. C. (2020). A Comparative Study of Laparoscopic versus Open Pancreaticoduodenectomy for Ampulla of Vater Carcinoma. Journal of Clinical Medicine, 9(7), 2214. https://doi.org/10.3390/jcm9072214






