Prognostic Implications of 18-FDG Positron Emission Tomography/Computed Tomography in Resectable Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Population
2.2. 18-FDG-PET/CT Imaging
2.3. Statistical Analyses
3. Results
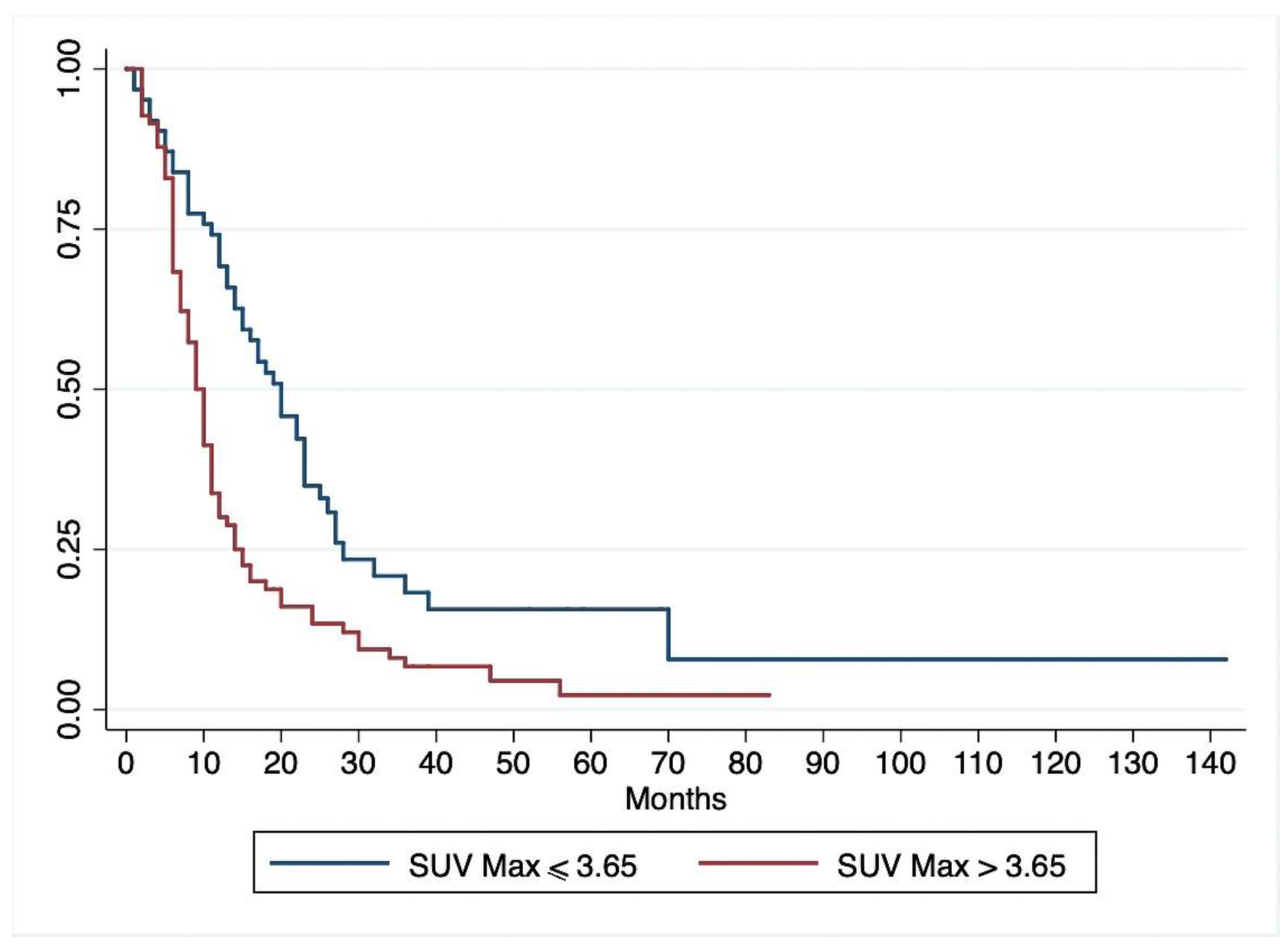
3.1. Disease-Free Survival
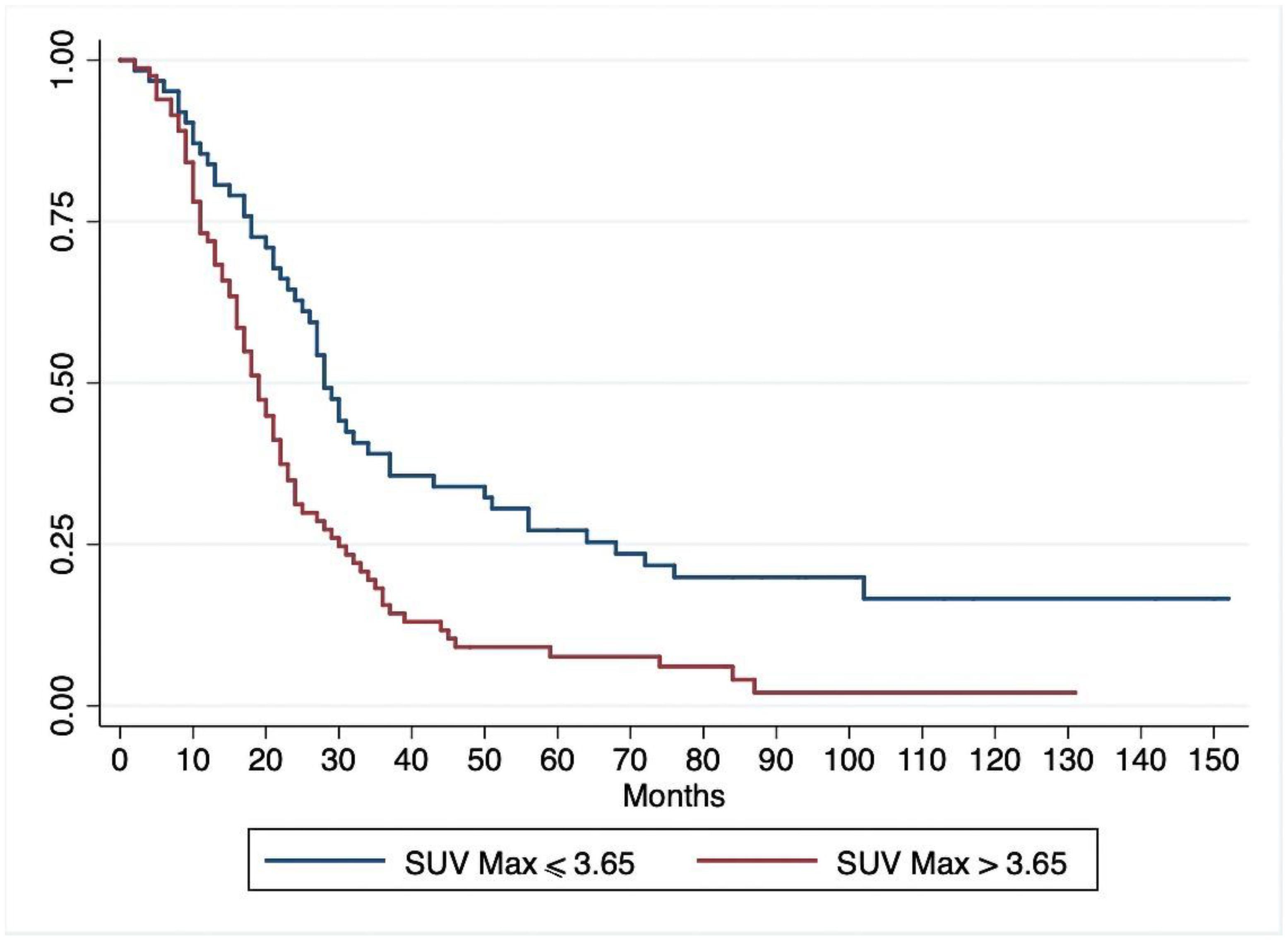
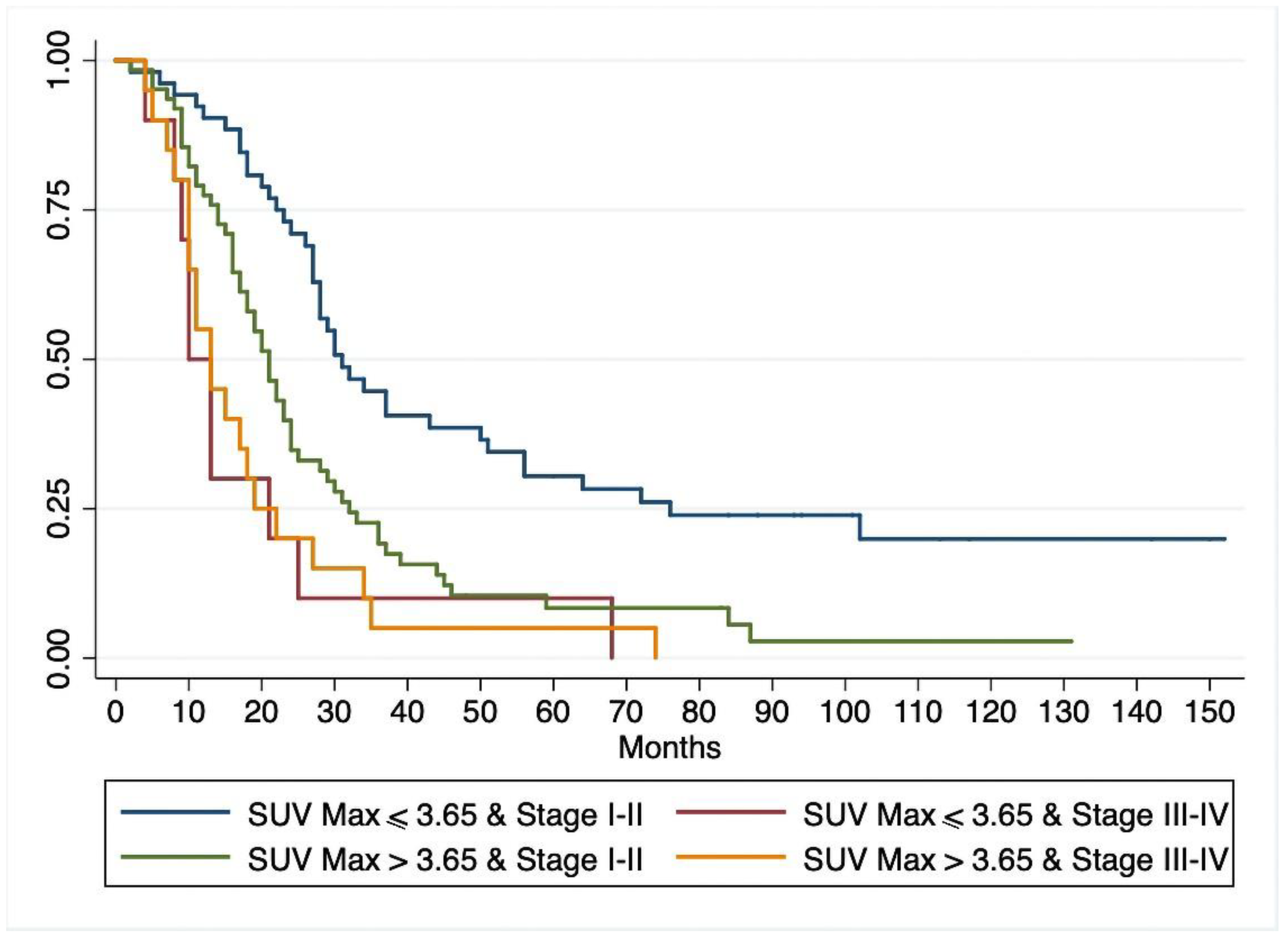
3.2. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020. [Google Scholar] [CrossRef] [PubMed]
- Vareedayah, A.A.; Alkaade, S.; Taylor, J.R. Pancreatic adenocarcinoma. Mo. Med. 2018, 115, 230–235. [Google Scholar]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta. Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef]
- Shaib, Y.; Davila, J.; Naumann, C.; El-Serag, H. The impact of curative intent surgery on the survival of pancreatic cancer patients: A US Population-based study. Am. J. Gastroenterol. 2007, 102, 1377–1382. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Heh, V.; Pawlik, T.M.; Ejaz, A.; Dillhoff, M.; Tsung, A.; Williams, T.; Abushahin, L.; Bridges, J.F.P.; Santry, H. Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: A meta-analysis of randomized controlled trials. J. Clin. Med. 2020, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, E.; Fulgenzi, C.A.M.; Minelli, A.; Citarella, F.; Stellato, M.; Pantano, F.; Russano, M.; Cursano, M.C.; Napolitano, A.; Zeppola, T.; et al. Prognostic and predictive factors in pancreatic cancer. Oncotarget 2020, 11, 924–941. [Google Scholar] [CrossRef]
- Lim, J.E.; Chien, M.W.; Earle, C.C. Prognostic factors following curative resection for pancreatic adenocarcinoma. A population-based, linked database analysis of 396 patients. Ann. Surg. 2003, 237, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Bilici, A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J. Gastroenterol. 2014, 20, 10802–10812. [Google Scholar] [CrossRef]
- Kim, K.S.; Kwon, J.; Kim, K.; Chie, E.K. Impact of resection margin distance on survival of pancreatic cancer: A systematic review and meta-analysis. Cancer Res. Treat. 2017, 49, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Pasquali, C.; Catalini, S.; Cappellazzo, F.; Bonadimani, B.; Behboo, R.; Pedrazzoli, S. CA 19-9 as a prognostic index after resection for pancreatic cancer. J. Surg. Oncol. 1993, 52, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Yeo, C.J.; Brody, J.R. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J. Surg. Oncol. 2013, 107, 15–22. [Google Scholar] [CrossRef]
- Vogel, I.; Kalthoff, H.; Henne-Bruns, D.; Kremer, B. Detection and prognostic impact of disseminated tumor cells in pancreatic carcinoma. Pancreatology 2002, 2, 79–88. [Google Scholar] [CrossRef]
- Delbeke, D.; Martin, W.H. Positron emission tomography imaging in oncology. Radiol. Clin. N. Am. 2001, 39, 883–917. [Google Scholar] [CrossRef]
- Hustinx, R.; Benard, F.; Alavi, A. Whole-body imaging in the management of patients with cancer. Semin. Nucl. Med. 2002, 32, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Delbeke, D.; Beauchamp, D.; Chapman, W.C.; Sandler, M.P.; Sharp, K.W.; Richards, W.O.; Write, J.K.; Frexes, M.E.; Pinson, C.W.; et al. 18-Fluorodeoxyglucose positron emission tomography in the management of patients with suspected pancreatic cancer. Ann. Surg. 1998, 229, 729–738. [Google Scholar] [CrossRef]
- Van Heertum, R.L.; Fawrwaz, R.A. The role of nuclear medicine in the evaluation of pancreatic disease. Surg. Clin. N. Am. 2001, 82, 345–348. [Google Scholar] [CrossRef]
- Nakata, B.; Nishimura, S.; Ishikawa, T.; Ohira, M.; Nishino, H.; Kawabe, J.; Ochi, H.; Hirakawa, K. Prognostic predictive value of 18-fluorodeoxyglucose positron emission tomography for patients with pancreatic cancer. Int. J. Oncol. 2001, 19, 53–58. [Google Scholar]
- Maisey, N.R.; Webb, A.; Flux, G.D.; Padhani, A.; Cunningham, D.C.; Ott, R.J.; Norman, A. FDG-PET in the prediction of survival of patients with cancer of the pancreas: A pilot study. Br. J. Cancer 2000, 83, 287–293. [Google Scholar] [CrossRef]
- Zimny, M.; Fass, J.; Bares, R.; Cremerius, U.; Sabri, O.; Buechin, P.; Schumpelick, V.; Buell, U. Fluorodeoxyglucose positron emission tomography and the prognosis of pancreatic carcinoma. Scand. J. Gastroenterol. 2000, 35, 883–888. [Google Scholar] [PubMed]
- Okamoto, K.; Koyama, I.; Miyazawa, M.; Toshimitsu, Y.; Aikawa, M.; Okada, K.; Imabayashi, E.; Matsuda, H. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts early recurrence after pancreatic cancer resection. Int. J. Clin. Oncol. 2011, 16, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sugiura, T.; Mizuno, T.; Okamura, Y.; Aramaki, T.; Endo, M.; Uesaka, K. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann. Surg. Oncol. 2015, 22, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, P.; Wang, W.; Li, M.; Hu, W.; Liu, X.; Tian, B. Early recurrence detected by 18F-FDG PET/CT in patients with resected pancreatic ductal adenocarcinoma. Medicine 2020, 99, e19504. [Google Scholar] [CrossRef] [PubMed]
- Sperti, C.; Pasquali, C.; Chierichetti, F.; Ferronato, A.; Decet, G.; Pedrazzoli, S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J. Gastrointest. Surg. 2003, 7, 953–959. [Google Scholar] [CrossRef]
- Japan Pancreas Society. Classification of Pancreatic Carcinoma, 2nd English ed.; Kanehara & Co.: Tokyo, Japan, 2003. [Google Scholar]
- Menon, K.V.; Gomez, D.; Smith, A.M.; Anthoney, A.; Verbeke, C.S. Impact of margin status on survival following pancreatoduodenectomy for cancer: The Leeds Pathology Protocol (LEEPP). HPB 2009, 11, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumors, 8th ed.; UICC: Geneva, Switzerland, 2017. [Google Scholar]
- Pizzi, S.; Porzionato, A.; Pasquali, C.; Guidolin, D.; Sperti, C.; Fogar, P.; Macchi, V.; De Caro, R.; Pedrazzoli, S.; Parenti, A. Glucose transporter-1 expression and prognostic significance in pancreatic carcinogenesis. Histol. Histopathol. 2009, 24, 175–185. [Google Scholar]
- Schek, N.; Hall, B.L.; Finn, O.J. Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in human pancreatic adenocarcinoma. Cancer Res. 1988, 48, 6354–6359. [Google Scholar]
- Choi, M.; Heilbrun, L.K.; Venkatramanamoorthy, R.; Lawhorn-Crews, J.M.; Zalupski, M.M.; Shields, A.F. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am. J. Clin. Oncol. 2010, 33, 257–261. [Google Scholar] [CrossRef]
- Chang, J.S.; Choi, S.H.; Lee, Y.; Kim, K.H.; Park, J.Y.; Song, S.Y.; Cho, A.; Yun, M.; Lee, J.D.; Seong, J. Clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography in patients with locally advanced pancreatic cancer planned to undergo concurrent chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 126–133. [Google Scholar] [CrossRef]
- Nakata, B.; Chung, Y.S.; Nishimura, S.; Nishihara, T.; Sakurai, Y.; Sawada, T.; Okamura, T.; Kawabe, J.; Ochi, H.; Sowa, M. 18F-fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer 1997, 79, 695–699. [Google Scholar] [CrossRef]
- Zhao, J.G.; Hu, Y.; Liao, Q.; Niu, Z.Y.; Zhao, Y.P. Prognostic significance of SUVmax and serum carbohydrate antigen 19-9 in pancreatic cancer. World J. Gastroenterol. 2014, 20, 5875–5880. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhou, R.; Li, C.; Liu, R.; Zhao, Z.; Gao, Y.; Xu, Y. Preoperative maximum standardized uptake value and carbohydrate antigen 19-9 were independent predictors of pathological stages and overall survival in Chinese patients with pancreatic duct adenocarcinoma. BMC Cancer 2019, 19, 456. [Google Scholar] [CrossRef] [PubMed]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef]
- Hwang, J.P.; Lim, I.; Chang, K.J.; Kim, B.I.; Choi, C.W.; Lim, S.M. Prognostic value of SUVmax measured by fluorine-18 fluorodeoxyglucose positron emission tomography with computed tomography in patients with pancreatic cancer. Nucl. Med. Mol. Imaging 2012, 46, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kang, C.M.; Lee, W.J.; Song, S.Y.; Cho, A.; Yun, M.; Lee, J.D.; Kim, J.H.; Lee, J.H. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography in patients with resectable pancreatic cancer. Yonsei Med. J. 2013, 54, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Kitasato, Y.; Yasunaga, M.; Okuda, K.; Kinoshita, H.; Tanaka, H.; Okabe, Y.; Kawahara, A.; Kage, M.; Kaida, H.; Ishibashi, M. Maximum standardized uptake value on 18F-fluoro-2-deoxy-glucose positron emission tomography/computed tomography and glucose transporter-1 expression correlates with survival in invasive ductal carcinoma of the pancreas. Pancreas 2014, 43, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kang, C.M.; Choi, W.J.; Lee, W.J.; Song, S.Y.; Lee, J.H. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18FDG PET/CT in patients with pancreatic cancer. J. Nucl. Med. 2014, 55, 896–904. [Google Scholar] [CrossRef]
- Ariake, K.; Motoi, F.; Shimomura, H.; Mizuma, M.; Maeda, S.; Terao, C.; Tatewaki, Y.; Ohtsuka, H.; Fukase, K.; Masuda, K.; et al. 18-Fuorodeoxyglucose positron emission tomography predicts recurrence in resected pancreatic ductal adenocarcinoma. J. Gastrointest. Surg. 2018, 22, 279–287. [Google Scholar] [CrossRef]
- Nunna, P.; Sheikhbahaei, S.; Ahn, S.; Young, B.; Subramaniam, R.M. The role of positron emission tomography/computed tomography in management and prediction of survival in pancreatic cancer. J. Comput. Assist. Tomogr. 2016, 40, 142–151. [Google Scholar] [CrossRef]
- Parlak, C.; Topkan, E.; Onal, C.; Reyhan, M.; Selek, U. Prognostic value of gross tumor volume delineated by FDG-PET-CT based radiotherapy treatment planning in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Radiat. Oncol. 2012, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Chen, T.; Wang, W.Q.; Wu, C.T.; Liu, C.; Long, J.; Xu, J.; Zhang, Y.J.; Chen, R.H.; Liu, L.; et al. Metabolic tumor burden assessed by 18F-FDG PET/CT associated with serum CA 19-9 predicts pancreatic cancer outcome. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1093–1102. [Google Scholar] [CrossRef] [PubMed]


| All Patients | SUVmax ≤ 3.65 | SUVmax > 3.65 | p Value | |
|---|---|---|---|---|
| Patients, n (%) | 144 | 62 (43.1%) | 82 (56.9%) | |
| Age, yrs (mean ± SD) | 66.32 ± 11.40 | 66.48 ± 09.32. | 67.55 ± 10.31 | |
| Sex M | 70 | 32 | 38 | |
| F | 74 | 30 | 44 | |
| UICC | 0.158 | |||
| I–II, n (%) | 114 (79.2%) | 52 (45.6%) | 62 (54.4%) | |
| III–IV, n (%) | 30 (20.8%) | 10 (33.3%) | 20 (66.7%) | |
| Grade, n (%) | 0.023 | |||
| Well- or moderately differentiated (G1–G2) | 95 (66%) | 47 (49.5%) | 48 (50.5%) | |
| Poorly-differentiated (G3) | 49 (34%) | 15 (30.6%) | 34 (69.4%) | |
| Resection margins | 0.232 | |||
| R0, n (%) | 106 (73.6%) | 48 (45.3%) | 58 (54.7%) | |
| R1, n (%) | 38 (26.4%) | 14 (36.8%) | 24 (63.2%) | |
| Lymph nodes | 0.036 | |||
| Negative, n (%) | 41 (28.5%) | 23 (56.1%) | 18 (43.9%) | |
| Positive, n (%) | 103 (71.5%) | 39 (37.9%) | 64 (62.1%) | |
| Diabetes | 0.170 | |||
| No, n (%) | 90 (62.5%) | 42(46.7%) | 48(53.3%) | |
| Yes, n (%) | 54 (37.5%) | 20 (37%) | 34 (63%) | |
| SUVmax, mean (±SD) | 5 (±3.2) | 2.6 (±1.2) | 6.9 (±3.1) | |
| Serum CA 19-9, mean (±SD) | 524.5 (±1123) | 392.9 (±1051.9) | 623.9 (±1172.1) | 0.88 |
| Serum CA 19-9, median (IQR), range | 114 (IQR 23–382) range 1–6637 | 52.9 (IQR 18–256) range 1–6637 | 154.35 (IQR 27–470) range 1–5460 | 0.032 |
| CA 19-9 < 114 kU/L | 81 (56.3%) | 41 (50.6%) | 40 (49.4%) | 0.028 |
| CA 19-9 > 114 kU/L | 63 (43.7%) | 21 (33.3%) | 42 (66.7%) | |
| OS, median (95%CI) | 22 (19–27) | 28 (24–37) | 19 (16–22) | 0.002 |
| DFS, median (95%CI) | 12 (10–14) | 20 (14–23) | 9 (8–11) | 0.001 |
| Variables | HR a | 95%CI a | P Value a | HR b | 95%CI b | P Value b |
|---|---|---|---|---|---|---|
| Lymph node metastases | 2.33 | 1.511–3.596 | <0.0001 | 1.779 | 1.130–2.800 | 0.013 |
| Pathological grade | 1.581 | 1.090–2.293 | 0.016 | 1.661 | 1.137–2.426 | 0.009 |
| Radicality | 2.047 | 1.377–3.044 | <0.0001 | 1.840 | 1.223–2.769 | 0.003 |
| Stage | 2.181 | 1.429–3.330 | <0.0001 | 1.787 | 1.144–2.794 | 0.011 |
| Diabetes | 1.352 | 0.942–1.941 | 0.102 | - | - | - |
| SUVmax | 1.106 | 1.051–1.165 | <0.0001 | 1.085 | 1.025–1.148 | 0.004 |
| CA 19-9 | 1.001 | 0.999–1.001 | 0.312 | - | - | - |
| Variables | HR a | 95%CI a | P Value a | HR b | 95%CI b | P Value b |
|---|---|---|---|---|---|---|
| Lymph node metastases | 2.433 | 1.588–3.721 | <0.0001 | 1.730 | 1.101–2.719 | 0.017 |
| Pathological grade | 1.493 | 1.030–2.165 | 0.034 | 1.484 | 1.017–2.163 | 0.040 |
| Radicality | 2.352 | 1.583–3.495 | <0.0001 | 2.079 | 1.374–3.147 | 0.001 |
| Tumor stage | 2.489 | 1.637–3.784 | <0.0001 | 2.127 | 1.369–3.305 | 0.001 |
| Diabetes | 1.222 | 0.851–1.756 | 0.278 | - | - | - |
| SUVmax | 1.074 | 1.025–1.124 | 0.002 | 1.055 | 1.001–1.111 | 0.044 |
| CA 19-9 | 1.001 | 0.999–1.001 | 0.196 | - | - | - |
| Author | Year | Design | n | SUVmax | OS (mo) | p | DFS (mo) | p |
|---|---|---|---|---|---|---|---|---|
| Okamoto et al. [22] | 2011 | R | 56 | <5.5 >5.5 | NA | - | NA | 0.025 |
| Choi et al. [38] | 2013 | R | 64 | ≤3.5 >3.5 | 45.4 vs. 23.5 | 0.011 | 26.1 vs. 9.2 | 0.002 |
| Lee et al. [40] | 2014 | R | 87 | ≤4.7 >4.7 | 34.4 vs. 20.6 | 0.03 | 12.9 vs. 9.9 | 0.03 |
| Kitasato et al. [39] | 2014 | R | 41 | ≤3.4 >3.4 | NR | - | 610 vs. 354 days | 0.04 |
| Yamamoto et al. [23] | 2015 | R | 128 | <6.0 ≥6.0 | 37 vs. 18 | <0.001 | 23 vs. 6 | <0.001 |
| Ariake et al. [41] | 2018 | R | 138 | <4.85 ≥4.85 | 50.4 vs. 21.5 | <0.001 | 24.3 vs. 10.3 | <0.001 |
| Present series | 2020 | R | 144 | ≤3.65 >3.65 | <0.001 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperti, C.; Friziero, A.; Serafini, S.; Bissoli, S.; Ponzoni, A.; Grego, A.; Grego, E.; Moletta, L. Prognostic Implications of 18-FDG Positron Emission Tomography/Computed Tomography in Resectable Pancreatic Cancer. J. Clin. Med. 2020, 9, 2169. https://doi.org/10.3390/jcm9072169
Sperti C, Friziero A, Serafini S, Bissoli S, Ponzoni A, Grego A, Grego E, Moletta L. Prognostic Implications of 18-FDG Positron Emission Tomography/Computed Tomography in Resectable Pancreatic Cancer. Journal of Clinical Medicine. 2020; 9(7):2169. https://doi.org/10.3390/jcm9072169
Chicago/Turabian StyleSperti, Cosimo, Alberto Friziero, Simone Serafini, Sergio Bissoli, Alberto Ponzoni, Andrea Grego, Emanuele Grego, and Lucia Moletta. 2020. "Prognostic Implications of 18-FDG Positron Emission Tomography/Computed Tomography in Resectable Pancreatic Cancer" Journal of Clinical Medicine 9, no. 7: 2169. https://doi.org/10.3390/jcm9072169
APA StyleSperti, C., Friziero, A., Serafini, S., Bissoli, S., Ponzoni, A., Grego, A., Grego, E., & Moletta, L. (2020). Prognostic Implications of 18-FDG Positron Emission Tomography/Computed Tomography in Resectable Pancreatic Cancer. Journal of Clinical Medicine, 9(7), 2169. https://doi.org/10.3390/jcm9072169





