Association of Hypertensive Intracerebral Hemorrhage with Left Ventricular Hypertrophy on Transthoracic Echocardiography
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Echocardiographic Findings
2.3. Statistical Analysis
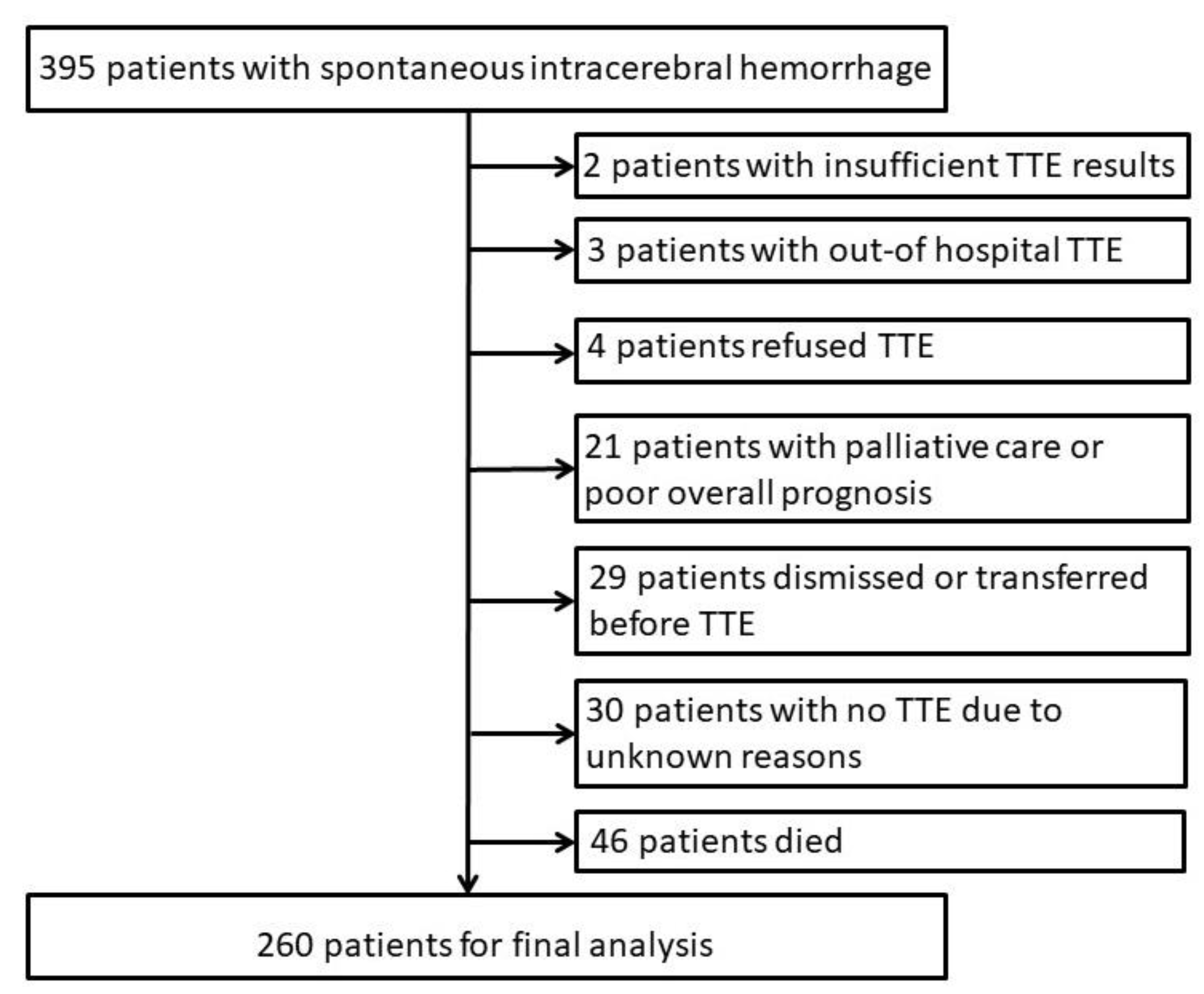
3. Results
3.1. Patients
3.2. Transthoracic Echocardiography (TTE) Findings
3.3. Association of TTE Findings with Spontaneous Intracerebral Hemorrhage (sICH) Etiology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Barker-Collo, S.L.; Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009, 8, 355–369. [Google Scholar] [CrossRef]
- Sennfalt, S.; Norrving, B.; Petersson, J.; Ullberg, T. Long-Term Survival and Function after Stroke. Stroke 2019, 50, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaled, M.; Awwad, S.; Bruning, T. Nontraumatic spontaneous intracerebral hemorrhage: Baseline characteristics and early outcomes. Brain Behav. 2020, 10, e01512. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Kim, T.J.; Yoon, B.W. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J. Stroke 2017, 19, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Gross, B.A.; Jankowitz, B.T.; Friedlander, R.M. Cerebral Intraparenchymal Hemorrhage: A Review. JAMA 2019, 321, 1295–1303. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwick, T.H.; Gillebert, T.C.; Aurigemma, G.; Chirinos, J.; Derumeaux, G.; Galderisi, M.; Gottdiener, J.; Haluska, B.; Ofili, E.; Segers, P.; et al. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J. Am. Soc. Echocardiogr. 2015, 28, 727–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, M.H.; Lavie, C.J.; Shah, S.; Englert, J.; Gilliland, Y.; Qamruddin, S.; Dinshaw, H.; Cash, M.; Ventura, H.; Milani, R. Prognostic Implications of Left Ventricular Hypertrophy. Prog. Cardiovasc. Dis. 2018, 61, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.C.; Rohan, S.; Dastidar, A.G.; Trickey, A.; Szantho, G.; Ratcliffe, L.E.; Burchell, A.E.; Hart, E.C.; Bucciarelli-Ducci, C.; Hamilton, M.C.; et al. The Relationship between Left Ventricular Wall Thickness, Myocardial Shortening, and Ejection Fraction in Hypertensive Heart Disease: Insights from Cardiac Magnetic Resonance Imaging. J. Clin. Hypertens. 2016, 18, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Albright, K.C.; Alexandrov, A.; Howard, G.; Martin-Schild, S. Is there a role for echocardiography in intracerebral haemorrhage? Int. J. Stroke 2010, 5, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G. ESC Scientific Document Group 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Kneissl, G.D.; Schweizer, P.; Lambertz, H.J.; Engberding, R. Qualitätsleitlinien in der Echokardiographie. Z. Kardiol. 1997, 86, 387–403. [Google Scholar]
- Alkema, M.; Spitzer, E.; Soliman, O.I.; Loewe, C. Multimodality Imaging for Left Ventricular Hypertrophy Severity Grading: A Methodological Review. J. Cardiovasc. Ultrasound 2016, 24, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Katsanos, A.H.; Patsouras, D.; Tsivgoulis, G.; Kosmidou, M.; Katsanos, K.H.; Kyritsis, A.P.; Giannopoulos, S. The value of transesophageal echocardiography in the investigation and management of cryptogenic cerebral ischemia: A single-center experience. Neurol. Sci. 2016, 37, 629–632. [Google Scholar] [CrossRef]
- Pallesen, L.P.; Ragaller, M.; Kepplinger, J.; Barlinn, K.; Zerna, C.; Siepmann, T.; Wiedemann, B.; Braun, S.; Weise, M.; Bodechtel, U.; et al. Diagnostic Impact of Transesophageal Echocardiography in Patients with Acute Cerebral Ischemia. Echocardiography 2016, 33, 555–561. [Google Scholar] [CrossRef]
- Martin-Schild, S.; Albright, K.C.; Hallevi, H.; Barreto, A.D.; Philip, M.; Misra, V.; Grotta, J.C.; Savitz, S.I. Intracerebral hemorrhage in cocaine users. Stroke 2010, 41, 680–684. [Google Scholar] [CrossRef] [Green Version]
- Masugata, H.; Senda, S.; Goda, F.; Yamagami, A.; Okuyama, H.; Kohno, T.; Hosomi, N.; Imai, M.; Yukiiri, K.; Kohno, M. Differences in left ventricular hypertrophy and dysfunction between patients with cerebral hemorrhage and those with cerebral infarction. Tohoku J. Exp. Med. 2008, 215, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albright, K.C.; Burak, J.M.; Chang, T.R.; Aysenne, A.; Siegler, J.E.; Schluter, L.; Martini, S.R.; Boehme, A.K.; Martin-Schild, S. The Impact of Left Ventricular Hypertrophy and Diastolic Dysfunction on Outcome in Intracerebral Hemorrhage Patients. ISRN Stroke 2013, 2013, 898163. [Google Scholar] [CrossRef]
- Valentine, D.; Lord, A.S.; Torres, J.; Frontera, J.; Ishida, K.; Czeisler, B.M.; Lee, F.; Rosenthal, J.; Calahan, T.; Lewis, A. How Does Preexisting Hypertension Affect Patients with Intracerebral Hemorrhage? J. Stroke Cerebrovasc. Dis. 2019, 28, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Rescaldani, M.; Sala, C. Prevalence of echocardiographic left-atrial enlargement in hypertension: A systematic review of recent clinical studies. Am. J. Hypertens. 2013, 26, 456–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancusi, C.; Canciello, G.; Izzo, R.; Damiano, S.; Grimaldi, M.G.; de Luca, N.; de Simone, G.; Trimarco, B.; Losi, M.A. Left atrial dilatation: A target organ damage in young to middle-age hypertensive patients. The Campania Salute Network. Int. J. Cardiol. 2018, 265, 229–233. [Google Scholar] [CrossRef]
- Steiner, T.; Salman, R.A.; Beer, R.; Christensen, H.; Cordonnier, C.; Csiba, L.; Forsting, M.; Harnof, S.; Klijn, C.J.M.; Krieger, D.; et al. European Stroke Organisation (ESO) Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Int. J. Stroke 2014, 9, 840–855. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwabuo, C.C.; Vasan, R.S. Pathophysiology of Hypertensive Heart Disease: Beyond Left Ventricular Hypertrophy. Curr. Hypertens. Rep. 2020, 22, 11. [Google Scholar] [CrossRef]
- Schumann, C.L.; Jaeger, N.R.; Kramer, C.M. Recent Advances in Imaging of Hypertensive Heart Disease. Curr. Hypertens. Rep. 2019, 21, 3. [Google Scholar] [CrossRef]
- Janardhanan, R.; Kramer, C.M. Imaging in hyptertensive heart disease. Expert Rev. Cardiovasc. Ther. 2011, 9, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Wachtell, K.; Bella, J.N.; Rokkedal, J.; Palmieri, V.; Papademetriou, V.; Dahlöf, B.; Aalto, T.; Gerdts, E.; Devereux, R.B. Change in diastolic left ventricular filling after one year of antihypertensive treatment: The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study. Circulation 2002, 105, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Okin, P.M.; Devereux, R.B.; Jern, S.; Kjeldsen, S.E.; Julius, S.; Nieminen, M.S.; Snapinn, S.; Harris, K.E.; Peter, A.; Edelman, J.M.; et al. LIFE Study Investigators: Regression of Electrocardiographic Left Ventricular Hypertrophy During Antihypertensive Treatment and the Prediction of Major Cardiovascular Events. JAMA 2004, 292, 2343–2349. [Google Scholar] [CrossRef] [Green Version]
- Tadic, M.; Cuspidi, C.; Plein, S.; Milivojevic, I.G.; Wang, D.W.; Grassi, G.; Mancia, G. Comprehensive assessment of hypertensive heart disease: Cardiac magnetic resonance in focus. Heart Fail. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, A.; Bursi, F.; Mantovani, F.; Valenti, C.; Quaglia, M.; Berti, E.; Marino, M.; Modena, M.G. Left ventricular hypertrophy reclassification and death: Application of the Recommendation of the American Society of Echocardiography/European Association of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, E.-M.; Dudley, S.C. Diastolic Dysfunction: Potential New Diagnostics and Therapy. Circ. J. 2015, 79, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Paulus, W.J.; Tschöpe, C.; Sanderson, J.E.; Rusconi, C.; Flachskampf, F.A.; Rademakers, F.E.; Marino, P.; Smiseth, O.A.; De Keulenaer, G.; Leite-Moreira, A.F. How to Diagnose Diastolic Heart Failure: A Consensus Statement on the Diagnosis of Heart Failure with Normal Left Ventricular Ejection Fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007, 28, 2539–2550. [Google Scholar] [CrossRef] [Green Version]
- Dal Canto, E.; Remmelzwaal, S.; van Ballegooijen, A.J.; Handoko, M.L.; Heymans, S.; van Empel, V.; Paulus, W.J.; Nijpels, G.; Elders, P.; Beulens, J.W. Diagnostic Value of Echocardiographic Markers for Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction. Heart Fail. Rev. 2020. [Google Scholar] [CrossRef]
- Jia, G.; Whaley-Connell, A.; Sowers, J.R. Diabetic Cardiomyopathy: A Hyperglycaemia- and Insulin-Resistance-Induced Heart Disease. Diabetologica 2018, 61, 21–28. [Google Scholar] [CrossRef] [PubMed]
| Study Population (N = 260) | Hypertensive sICH (N = 159) | Non-Hypertensive sICH (N = 101) | OR | CI95% | |
|---|---|---|---|---|---|
| Age, median (IQR) | 71.0 (17.0) | 71.0 (18.5) | 72.0 (11.0) | 0.98 | 0.96–1.01 |
| Sex, male, n/N (%) | 160/260 (61.5) | 102/159 (64.2) | 58 /101 (57.4) | 1.33 | 0.80–2.21 |
| NIHSS on admission, median (IQR) | 8.0 (13.0) | 9.0 (11.0) | 7.0 (14.0) | 1.01 | 0.99–1.04 |
| Systolic blood pressure on admission, mmHg, median (IQR) | 165.0 (31.2) | 169.0 (35.0) | 160.0 (32.5) | 1.01 | 1.00–1.02 * |
| Pre-existing ischemic stroke, n/N (%) | 60/260 (23.1) | 32/159 (20.1) | 28/101 (27.7) | 0.66 | 0.37–1.18 |
| Arterial hypertension, n/N (%) | 257/260 (98.8) | 159/159 (100.0) | 98/101 (97.0) | NA | NA–NA |
| Hyperlipidemia, n/N (%) | 131/260 (50.4) | 72/159 (45.3) | 59/101 (58.4) | 0.59 | 0.35–0.97 * |
| Diabetes, n/N (%) | 62/260 (23.8) | 45/159 (28.3) | 17/101 (16.8) | 1.95 | 1.06–3.72 * |
| Peripheral vascular disease, n/N (%) | 12/260 (4.6) | 9/159 (5.7) | 3/101 (3.0) | 1.96 | 0.57–9.00 |
| Coronary artery disease, n/N (%) | 36/260 (13.8) | 21/159 (13.2) | 15/101 (14.9) | 0.87 | 0.43–1.81 |
| Smoking, n/N (%) | 37/260 (14.2) | 23/159 (14.5) | 14/101 (13.9) | 1.05 | 0.52–2.20 |
| Atrial fibrillation, n/N (%) | 22/260 (8.5) | 11/159 (6.9) | 11/101 (10.9) | 0.61 | 0.25–1.48 |
| Antihypertensive drugs on admission | |||||
| one, n/N (%) | 53/224 (23.7) | 27/137 (19.7) | 26/87 (29.9) | 0.55 | 0.27–1.12 |
| more than one, n/N (%) | 93/224 (41.5) | 59/137 (43.1) | 34/87 (39.1) | 0.92 | 0.49–1.72 |
| Hypertrophy on ECG, n/N (%) | 15/245 (6.1) | 8/149 (5.4) | 7/96 (7.3) | 0.72 | 0.25–2.12 |
| Aspirin on admission, n/N (%) | 60/246 (24.4) | 32/151 (21.2) | 28/95 (29.5) | 0.73 | 0.40–1.35 |
| Anticoagulation on admission, n/N (%) | 36/246 (14.6) | 28/151 (18.5) | 8/95 (8.4) | 2.24 | 0.99–5.58 |
| Treatment on ICU, n/N (%) | 83/260 (31.9) | 49/159 (30.8) | 34/101 (33.7) | 0.88 | 0.52–1.50 |
| Surgery, n/N (%) | 33/260 (12.7) | 21/159 (13.2) | 12/101 (11.9) | 1.13 | 0.54–2.47 |
| Intraventricular bleeding, n/N (%) | 78/260 (30.0) | 51/159 (32.1) | 27/101 (26.7) | 1.29 | 0.75–2.27 |
| Length of hospital treatment, days, median (IQR) | 11.0 (8.0) | 11.0 (9.0) | 11.0 (6.0) | 1.03 | 0.99–1.07 |
| Length of ICU treatment, days, median (IQR) | 5.0 (11.0) | 5.0 (11.0) | 5.0 (10.0) | 1.02 | 0.99–1.06 |
| Discharge to rehabilitation, n/N (%) | 235/260 (90.4) | 146/159 (91.8) | 89/101 (88.1) | 1.51 | 0.65–3.48 |
| mRS 4-6 at discharge, n/N (%) | 139/254 (54.7) | 96/156 (61.5) | 43/98 (43.9) | 2.05 | 1.23–3.43 * |
| Hypertensive sICH | Non-Hypertensive sICH | OR | CI95% | |
|---|---|---|---|---|
| End diastolic interventricular septum wall thickness, mm, median (IQR) | 13.0 (2.0) | 12.0 (2.0) | 1.35 | 1.17–1.58 * |
| Left ventricular hypertrophy | ||||
| any, n/N (%) | 113/159 (71.1) | 43/101 (42.6) | 3.31 | 1.97–5.62 * |
| mild, n/N (%) | 79/159 (49.7) | 28/101 (27.7) | 3.56 | 2.01–6.42 * |
| moderate, n/N (%) | 23/159 (14.5) | 12/101 (11.9) | 2.42 | 1.10–5.51 * |
| severe, n/N (%) | 11/159 (6.9) | 3/101 (3.0) | 4.62 | 1.35–21.33 * |
| Left atrial enlargement, n/N (%) | 82/157 (52.2) | 37/100 (37.0) | 1.86 | 1.12–3.13 * |
| Diastolic dysfunction, n/N (%) | 132/159 (83.0) | 82/101 (81.2) | 1.13 | 0.59–2.16 |
| Mitral valve regurgitation, n/N (%) | 81/159 (50.9) | 58/101 (57.4) | 0.77 | 0.46–1.27 |
| Tricuspid valve regurgitation, n/N (%) | 67/159 (42.1) | 50/100 (50.0) | 0.73 | 0.44–1.20 |
| Aortic valve regurgitation, n/N (%) | 36/159 (22.6) | 29/100 (29.0) | 0.72 | 0.41–1.27 |
| Aortic valve stenosis, n/N (%) | 8/159 (5.0) | 10/101 (9.9) | 0.48 | 0.18–1.27 |
| Diameter left atrium, mm, median (IQR) | 41.0 (8.0) | 38.0 (9.0) | 1.04 | 1.00–1.09 * |
| Left ventricular end-diastolic diameter, mm, median (IQR) | 46.0 (8.0) | 44.0 (7.0) | 1.02 | 0.98–1.06 |
| Left ventricular ejection fraction, per cent, median (IQR) | 60.0 (5.0) | 60.0 (0.0) | 0.99 | 0.95–1.04 |
| Right ventricular ejection fraction, per cent, median (IQR) | 60.0 (5.0) | 60.0 (0.0) | 1.00 | 0.95–1.07 |
| OR | CI95% | |
|---|---|---|
| LVH | 2.95 | 1.29–6.74 |
| NIHSS on admission | 0.96 | 0.92–1.00 |
| Systolic blood pressure on admission | 1.04 | 1.01–1.07 |
| Hyperlipidemia | 0.46 | 0.21–0.99 |
| Pre-existing ischemic stroke | 0.41 | 0.18–0.95 |
| Atrial fibrillation | 0.26 | 0.07–0.98 |
| Left atrial enlargement | 3.33 | 1.39–7.99 |
| Diastolic dysfunction | 0.28 | 0.09–0.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallesen, L.-P.; Wagner, J.; Lambrou, D.; Braun, S.; Weise, M.; Prakapenia, A.; Barlinn, J.; Siepmann, T.; Winzer, S.; Moustafa, H.; et al. Association of Hypertensive Intracerebral Hemorrhage with Left Ventricular Hypertrophy on Transthoracic Echocardiography. J. Clin. Med. 2020, 9, 2148. https://doi.org/10.3390/jcm9072148
Pallesen L-P, Wagner J, Lambrou D, Braun S, Weise M, Prakapenia A, Barlinn J, Siepmann T, Winzer S, Moustafa H, et al. Association of Hypertensive Intracerebral Hemorrhage with Left Ventricular Hypertrophy on Transthoracic Echocardiography. Journal of Clinical Medicine. 2020; 9(7):2148. https://doi.org/10.3390/jcm9072148
Chicago/Turabian StylePallesen, Lars-Peder, Jenny Wagner, Dimitris Lambrou, Silke Braun, Matthias Weise, Alexandra Prakapenia, Jessica Barlinn, Timo Siepmann, Simon Winzer, Haidar Moustafa, and et al. 2020. "Association of Hypertensive Intracerebral Hemorrhage with Left Ventricular Hypertrophy on Transthoracic Echocardiography" Journal of Clinical Medicine 9, no. 7: 2148. https://doi.org/10.3390/jcm9072148
APA StylePallesen, L.-P., Wagner, J., Lambrou, D., Braun, S., Weise, M., Prakapenia, A., Barlinn, J., Siepmann, T., Winzer, S., Moustafa, H., Kitzler, H. H., Barlinn, K., Reichmann, H., & Puetz, V. (2020). Association of Hypertensive Intracerebral Hemorrhage with Left Ventricular Hypertrophy on Transthoracic Echocardiography. Journal of Clinical Medicine, 9(7), 2148. https://doi.org/10.3390/jcm9072148







