Monomeric C-Reactive Protein Aggravates Secondary Degeneration after Intracerebral Haemorrhagic Stroke and May Function as a Sensor for Systemic Inflammation
Abstract
1. Introduction
2. Experimental Section
2.1. Human Brain Samples
2.2. Aquaporin 4 (AQP4), Glial Fibrillary Acidic Protein (GFAP) and mCRP Localization by Immunofluorescence
2.3. Counting of Colocalized mCRP/AQP4 and GFAP/AQP4 Positive Cells
2.4. Animal Procedures
2.5. Hippocampal Injection of mCRP
2.6. Histological Analysis of Mice Brain Tissue
2.7. Immunohistochemistry of Mice Brain Tissue
2.8. Statistical Analysis
3. Results
3.1. Neuropathological Macroscopic Examination
3.2. Immunohistochemical Analysis
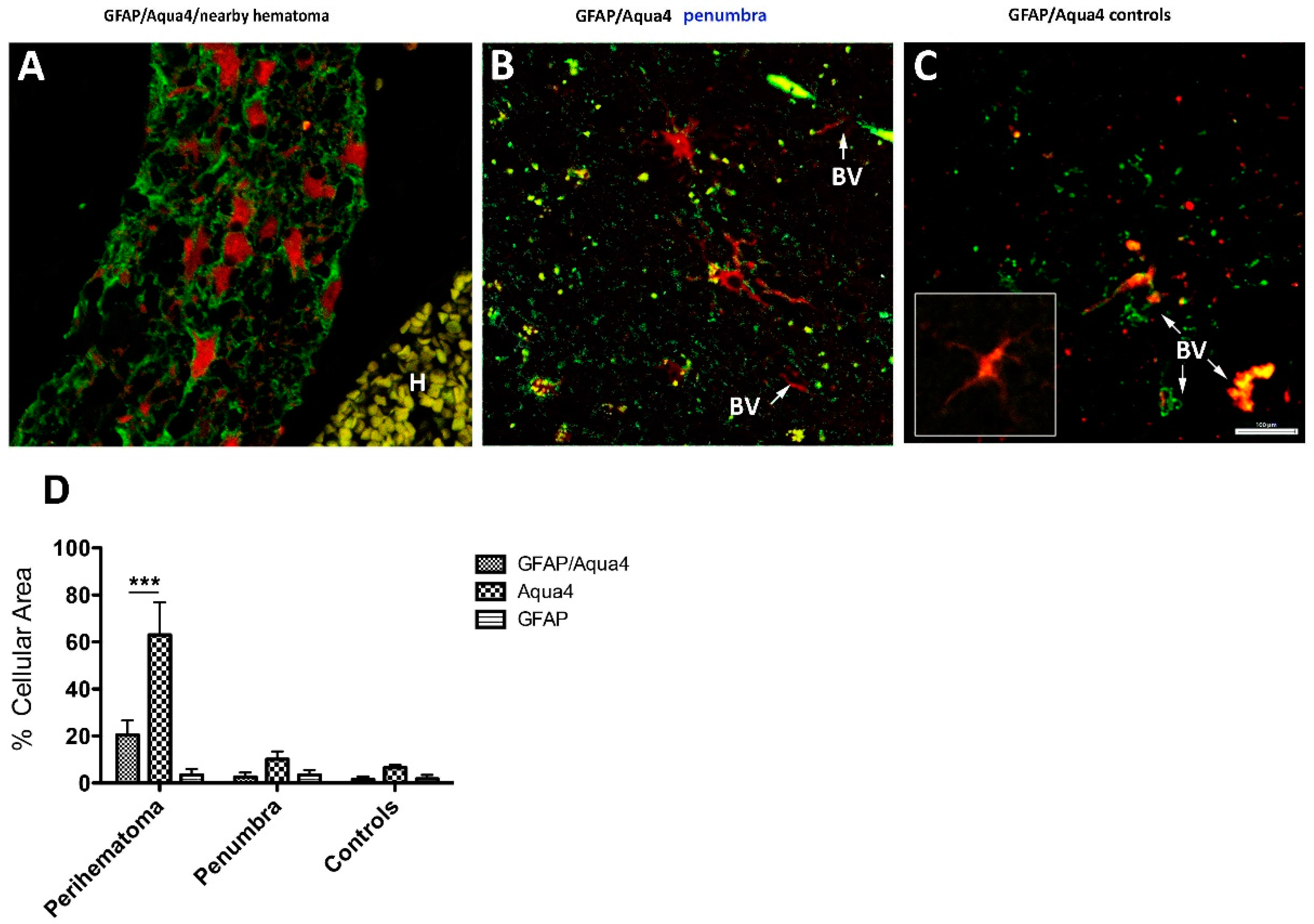
3.2.1. AQP4/mCRP Localization in the Perihaematomal Region
3.2.2. GFAP/AQP4 Localization in the Perihaematomal Region
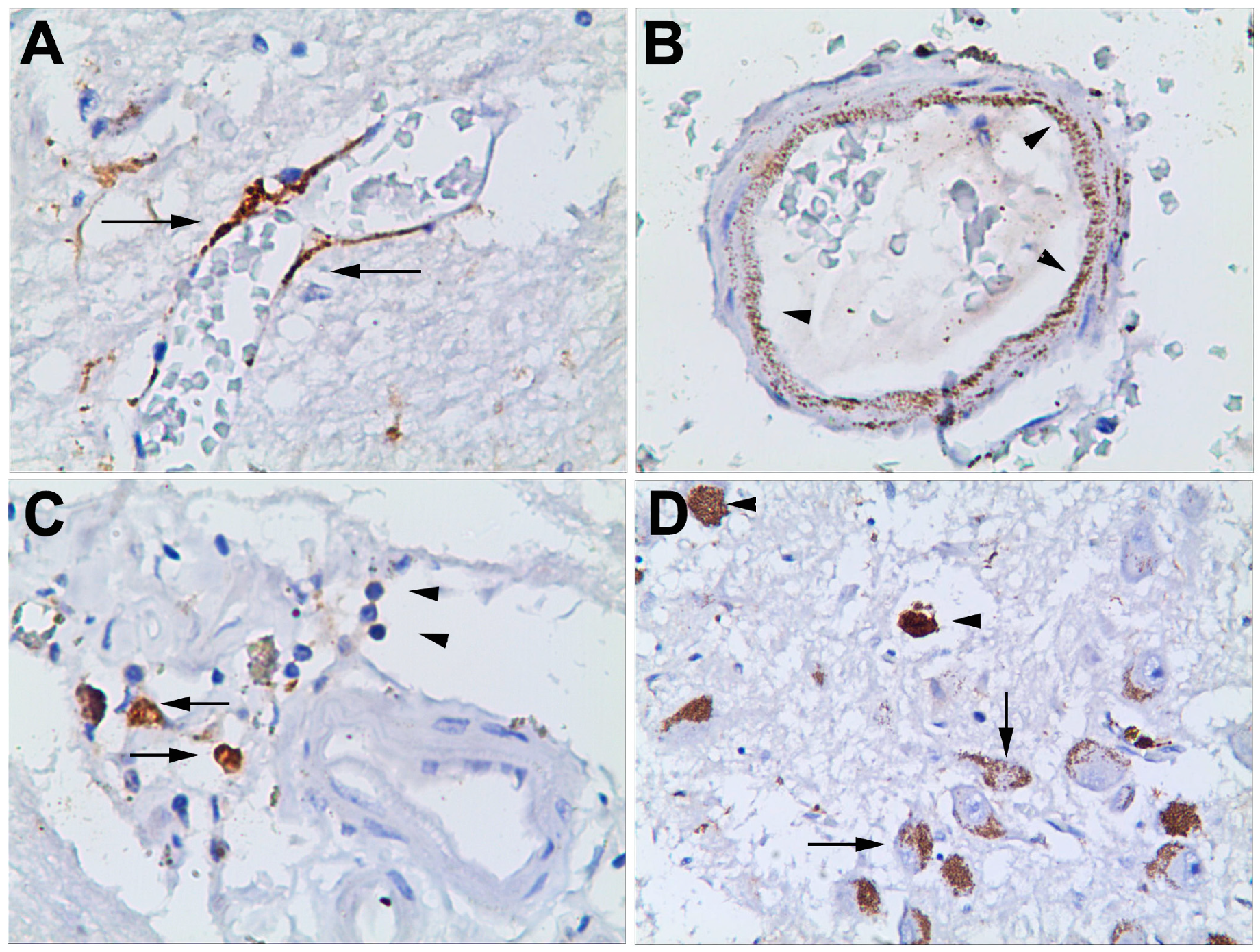
3.2.3. Haematoma, Peri-Haematoma and Hypothalamus mCRP Staining
3.2.4. mCRP Distribution after Local Delivery to Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460. [Google Scholar] [CrossRef]
- Garcia, P.Y.; Roussel, M.; Bugnicourt, J.M.; Lamy, C.; Canaple, S.; Peltier, J.; Loas, G.; Deramond, H.; Godefroy, O. Cognitive impairment and dementia after intracerebral hemorrhage: A cross-sectional study of a hospital-based series. J. Stroke Cerebrovasc. Dis. 2013, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Bailey, D.; Anderson, C.D.; Gurol, E.M.; Greenberg, S.M.; Rosand, J.; Viswanathan, A. Risk factors associated with early vs. delayed dementia after intracerebral hemorrhage. JAMA Neurol. 2016, 73, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N. Y.) 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. targets neuroinflammation after intracerebral hemorrhage and potential therapeutic. J. Stroke 2020, 22, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.R.; Habersberger, J.; Braig, D.; Schmidt, Y.; Goerendt, K.; Maurer, V.; Bannasch, H.; Scheichl, A.; Woollard, K.J.; von Dobschütz, E.; et al. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: In vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. 2014, 130, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.R.; Zeller, J.; Kiefer, J.; Braig, D.; Kreuzaler, S.; Lenz, Y.; Potempa, L.A.; Grahammer, F.; Huber, T.B.; Huber-Lang, M.; et al. A conformational change in C-reactive protein enhances leukocyte recruitment and reactive oxygen species generation in ischemia/reperfusion injury. Front. Immunol. 2018, 9, 675. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H. Mechanisms of neuroinflammation and inflammatory mediators involved in brain injury following subarachnoid hemorrhage. Histol. Histopathol. 2020, 6, 18208. [Google Scholar] [CrossRef]
- Di Napoli, M.; Behrouz, R.; Topel, C.H.; Misra, V.; Pomero, F.; Giraudo, A.; Pennati, P.; Masotti, L.; Schreuder, F.H.B.M.; Staals, J.; et al. Investigators. Hypoalbuminemia, systemic inflammatory response syndrome, and functional outcome in intracerebral hemorrhage. J. Crit. Care. 2017, 41, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Peña, E.; Arderiu, G.; Padró, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G.; Boehme, A.K.; Comeau, M.E.; Langefeld, C.D.; et al. C-reactive protein in atherothrombosis and angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Soto-Tinoco, E.; Guerrero-Vargas, N.N.; Buijs, R.M. Interaction between the hypothalamus and the immune system. Exp. Physiol. 2016, 101, 1463–1471. [Google Scholar] [CrossRef]
- Aoyama, S.; Shibata, S. Time-of-day-dependent physiological responses to meal and exercise. Front. Nutr. 2020, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A. The HPA axis and the immune system: A perspective. NeuroImmun. Biol. 2008, 7, 3–15. [Google Scholar] [CrossRef]
- Jiang, Y.; Peng, T.; Gaur, U.; Silva, M.; Little, P.; Chen, Z.; Qiu, W.; Zhang, Y.; Zheng, W. Role of corticotropin releasing factor in the neuroimmune mechanisms of depression: Examination of current pharmaceutical and herbal therapies. Front. Cell. Neurosci. 2019, 13, 290. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.; Matou, S.; Zeinolabediny, Y.; Corpas, R.; Weston, R.; Liu, D.; Boras, E.; Di Napoli, M.; Petcu, E.; Sarroca, S.; et al. Monomeric C-reactive protein—A key molecule driving development of Alzheimer’s disease associated with brain ischaemia? Sci. Rep. 2015, 5, 13281. [Google Scholar] [CrossRef]
- Di Napoli, M.; Slevin, M.; Popa-Wagner, A.; Singh, P.; Lattanzi, S.; Divani, A.A. Monomeric C-reactive protein and cerebral hemorrhage: From bench to bedside. Front. Immunol. 2018, 9, 1921. [Google Scholar] [CrossRef]
- Di Napoli, M.; Godoy, D.A.; Campi, V.; Masotti, L.; Smith, C.J.; Parry Jones, A.R.; Hopkins, S.J.; Slevin, M.; Papa, F.; Mogoanta, L.; et al. C-reactive protein in intracerebral hemorrhage: Time course, tissue localization, and prognosis. Neurology 2012, 14, 690–699. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Stöcker, K.; Balseanu, A.T.; Rogalewski, A.; Diederich, K.; Minnerup, J.; Margaritescu, C.; Schäbitz, W.R. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke 2010, 41, 1027–1031. [Google Scholar] [CrossRef]
- Slevin, M.; Matou-Nasri, S.; Turu, M.; Luque, A.; Rovira, N.; Badimon, L.; Boluda, S.; Potempa, L.; Sanfeliu, C.; De Vera, N. Modified C-reactive protein is expressed by stroke neovessels and is a potent activator of angiogenesis in vitro. Brain Pathol. 2010, 20, 151–165. [Google Scholar] [CrossRef]
- Fang, L.; Yujie, J. Cortical infarction of the right parietal lobe and neurogenic heart disease. A report of three cases. Neural. Regen. Res. 2012, 7, 943–947. [Google Scholar]
- Martini, S.; Testai, F.D.; Woo, D.; Elkind, M.S.V. Systemic inflammatory response syndrome, infection, and outcome in intracerebral hemorrhage. Neurol. Neuroimmunol. Neuroinflamm. 2017, 5, e428. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Romero, J.R.; Beiser, A.; Himali, J.J.; Shoamanesh, A.; DeCarli, C.; Seshadri, S. Cerebral microbleeds and risk of incident dementia: The Framingham heart study. Neurobiol. Aging 2017, 54, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.; Liu, D.; Ferris, G.; Al-Hsinawi, M.; Al-Baradie, R.; Krupinski, J. Expression of monomeric C-reactive protein in infarcted brain tissue from patients with Alzheimer’s disease. Turk. J. Pathol. 2017, 33, 25–29. [Google Scholar]
- Strang, F.; Scheichl, A.; Chen, Y.C.; Wang, X.; Htun, N.M.; Bassler, N.; Eisenhardt, S.U.; Habersberger, J.; Peter, K. Amyloid plaques dissociate pentameric to monomeric C-reactive protein: A novel pathomechanism driving cortical inflammation in Alzheimer’s disease? Brain Pathol. 2012, 22, 337–346. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Zeller, J.; Potempa, L.A.; Pietersz, G.A.; Eisenhardt, S.U.; Peter, K. C-reactive protein and its structural isoforms: An evolutionary conserved marker and central player in inflammatory diseases and beyond. Subcell. Biochem. 2020, 94, 499–520. [Google Scholar] [CrossRef]
- Wang, J.; Tang, B.; Liu, X.; Wu, X.; Wang, H.; Xu, D.; Guo, Y. Increased monomeric CRP levels in acute myocardial infarction: A possible new and specific biomarker for diagnosis and severity assessment of disease. Atherosclerosis 2015, 239, 343–349. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slevin, M.; García-Lara, E.; Capitanescu, B.; Sanfeliu, C.; Zeinolabediny, Y.; AlBaradie, R.; Olah, P.; Guo, B.; Pirici, D.; Di Napoli, M.; et al. Monomeric C-Reactive Protein Aggravates Secondary Degeneration after Intracerebral Haemorrhagic Stroke and May Function as a Sensor for Systemic Inflammation. J. Clin. Med. 2020, 9, 3053. https://doi.org/10.3390/jcm9093053
Slevin M, García-Lara E, Capitanescu B, Sanfeliu C, Zeinolabediny Y, AlBaradie R, Olah P, Guo B, Pirici D, Di Napoli M, et al. Monomeric C-Reactive Protein Aggravates Secondary Degeneration after Intracerebral Haemorrhagic Stroke and May Function as a Sensor for Systemic Inflammation. Journal of Clinical Medicine. 2020; 9(9):3053. https://doi.org/10.3390/jcm9093053
Chicago/Turabian StyleSlevin, Mark, Elisa García-Lara, Bogdan Capitanescu, Coral Sanfeliu, Yasmin Zeinolabediny, Raid AlBaradie, Peter Olah, Baoqiang Guo, Daniel Pirici, Mario Di Napoli, and et al. 2020. "Monomeric C-Reactive Protein Aggravates Secondary Degeneration after Intracerebral Haemorrhagic Stroke and May Function as a Sensor for Systemic Inflammation" Journal of Clinical Medicine 9, no. 9: 3053. https://doi.org/10.3390/jcm9093053
APA StyleSlevin, M., García-Lara, E., Capitanescu, B., Sanfeliu, C., Zeinolabediny, Y., AlBaradie, R., Olah, P., Guo, B., Pirici, D., Di Napoli, M., & Popa-Wagner, A. (2020). Monomeric C-Reactive Protein Aggravates Secondary Degeneration after Intracerebral Haemorrhagic Stroke and May Function as a Sensor for Systemic Inflammation. Journal of Clinical Medicine, 9(9), 3053. https://doi.org/10.3390/jcm9093053






