The Community Oncology and Academic Medical Center Alliance in the Age of Precision Medicine: Cancer Genetics and Genomics Considerations
Abstract
1. Introduction
2. The Emergence and Evolution of the Community Health Care and Academic Medical Center Alliance
3. Community Oncology and Academic Cancer Center Alliance
4. The Age of Precision Oncology
5. The Community Oncology/Academic Cancer Center Alliance in Germline Cancer Genetics
6. The Community Oncology/Academic Cancer Center Alliance in Somatic Tumor Genomics
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wartman, S.A. The Transformation of Academic Health Centers: Meeting The Challenges Of Healthcare’s Changing Landscape; Academic Press: Fribourg, Switzerland, 2015. [Google Scholar]
- Dzau, V.J.; Ackerly, D.C.; Sutton-Wallace, P.; Merson, M.H.; Williams, R.S.; Krishnan, K.R.; Taber, R.C.; Califf, R.M. The role of academic health science systems in the transformation of medicine. Lancet 2010, 375, 949–953. [Google Scholar] [CrossRef]
- Desch, C.E.; Blayney, D.W. Making the choice between academic oncology and community practice: the big picture and details about each career. J. Oncol. Pract. 2006, 2, 132–136. [Google Scholar] [CrossRef]
- Todd, R.F., III. A guide to planning careers in hematology and oncology. Hematol. Am. Soc. Hemat. 2001, 2001, 499–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levit, L.A.; Kim, E.S.; McAneny, B.L.; Nadauld, L.D.; Levit, K.; Schenkel, C.; Schilsky, R.L. Implementing precision medicine in community-based oncology programs: Three models. J. Oncol. Pract. 2019, 15, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.A.; Godden, J.J.; Weissman, S.M.; Wham, D.; Wilson, A.; Ruggeri, A.; Mullane, M.P.; Weese, J.L. Implementing an oncology precision medicine clinic in a large community health system. Am. J. Manag. Care 2017, 23, SP425–SP427. [Google Scholar]
- Levine, D.M.; Becker, D.M.; Bone, L.R.; Hill, M.N.; Tuggle, M.B., II; Zeger, S.L. Community-academic health center partnerships for underserved minority populations. One solution to a national crisis. J. Am. Med. Assoc. 1994, 272, 309–311. [Google Scholar] [CrossRef]
- Shin, P.; Sharac, J.; Rosenbaum, S. Community health centers and medicaid at 50: An. Enduring relationship essential for health system transformation. Health Aff. (Millwood) 2015, 34, 1096–1104. [Google Scholar] [CrossRef]
- Sharma, A.E.; Huang, B.; Knox, M.; Willard-Grace, R.; Potter, M.B. Patient engagement in community health center leadership: How does it happen? J. Community Health 2018, 43, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.; Meyer, G.S. Academic health centers in a changing environment. Health Aff. (Millwood) 1996, 15, 200–215. [Google Scholar] [CrossRef]
- Nash, D.B.; Veloski, J.J. Emerging opportunities for educational partnerships between managed care organizations and academic health centers. West. J. Med. 1998, 168, 319–327. [Google Scholar] [PubMed]
- Roper, W.L.; Newton, W.P. The role of academic health centers in improving health. Ann. Fam. Med. 2006, 4, S55–S57. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.; Meyer, G.S. The future of the academic medical center under health care reform. N. Engl. J. Med. 1993, 329, 1812–1814. [Google Scholar] [CrossRef]
- Fox, P.D.; Wasserman, J. Academic medical centers and managed care: Uneasy partners. Health Aff. (Millwood) 1993, 12, 85–93. [Google Scholar] [CrossRef]
- Epstein, A.M. US teaching hospitals in the evolving health care system. J. Am. Med. Assoc. 1995, 273, 1203–1207. [Google Scholar] [CrossRef]
- Iglehart, J.K. Academic medical centers enter the market: The case of Philadelphia. N. Engl. J. Med. 1995, 333, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, R.; Karpf, M.; Perman, J.; Higdon, C.M. The U.S. health care system is in crisis: Implications for academic medical centers and their missions. Acad. Med. 2006, 81, 713–720. [Google Scholar] [CrossRef]
- Park, B.; Frank, B.; Likumahuwa-Ackman, S.; Brodt, E.; Gibbs, B.K.; Hofkamp, H.; DeVoe, J. Health equity and the tripartite mission: Moving from academic health centers to academic-community health systems. Acad. Med. 2019, 94, 1276–1282. [Google Scholar] [CrossRef]
- Bartlett, S.J.; Barnes, T.; McIvor, R.A. Integrating patients into meaningful real-world research. Ann. Am. Thorac. Soc. 2014, 11, S112–S117. [Google Scholar] [CrossRef]
- Gourevitch, M.N. Population health and the academic medical center: The time is right. Acad. Med. 2014, 89, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.; Newton, G.L.; Abraido-Lanza, A.F.; Aguirre, A.N.; Ahmed, S.; Esmond, S.L.; Evans, J.; Gelmon, S.B.; Hart, C.; Hendricks, D.; et al. Community engagement in academic health centers: A model for capturing and advancing our successes. J. Commun. Engagem. Scholarsh. 2018, 10, 81–90. [Google Scholar]
- Zerhouni, E. Medicine: The NIH roadmap. Science 2003, 302, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, T.L.; Green, L.A. The Michigan Clinical Research Collaboratory: Following the NIH Roadmap to the community. Ann. Fam. Med. 2006, 4, S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Zerhouni, E.A. Translational and clinical science—Time for a new vision. N. Engl. J. Med. 2005, 353, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Zerhouni, E.A. US biomedical research: Basic, translational, and clinical sciences. J. Am. Med. Assoc. 2005, 294, 1352–1358. [Google Scholar] [CrossRef]
- Kassirer, J.P. Academic medical centers under siege. N. Engl. J. Med. 1994, 331, 1370–1371. [Google Scholar] [CrossRef]
- Iglehart, J.K. Rapid changes for academic medical centers. 2. N. Engl. J. Med. 1995, 332, 407–411. [Google Scholar] [CrossRef]
- Iglehart, J.K. Rapid changes for academic medical centers. 1. N. Engl. J. Med. 1994, 331, 1391–1395. [Google Scholar] [CrossRef]
- Moses, H., III; Matheson, D.H.M.; Poste, G. Serving individuals and populations within integrated health systems: A bridge too far? J. Am. Med. Assoc. 2019, 321, 1975–1976. [Google Scholar] [CrossRef]
- Cutler, D.M.; Morton, F.S. Hospitals, market share, and consolidation. J. Am. Med. Assoc. 2013, 310, 1964–1970. [Google Scholar] [CrossRef]
- Moses, H., III; Matheson, D.H.; Dorsey, E.R.; George, B.P.; Sadoff, D.; Yoshimura, S. The anatomy of health care in the United States. J. Am. Med. Assoc. 2013, 310, 1947–1963. [Google Scholar] [CrossRef]
- Cohen, M.D.; Jennings, G. Mergers involving academic medical institutions: Impact on academic radiology departments. J. Am. Coll. Radiol. 2005, 2, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Denham, A.C.; Hay, S.S.; Steiner, B.D.; Newton, W.P. Academic health centers and community health centers partnering to build a system of care for vulnerable patients: Lessons from Carolina Health Net. Acad. Med. 2013, 88, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Rieselbach, R.E.; Rieselbach, R.E.; Epperly, T.; Friedman, A.; Keahey, D.; McConnell, E.; Nichols, K.; Nycz, G.; Roberts, J.; Schmader, K.; et al. A new community health center/academic medicine partnership for medicaid cost control, powered by the Mega Teaching Health Center. Acad. Med. 2018, 93, 406–413. [Google Scholar] [CrossRef]
- Blumenthal, D.; Campbell, E.G.; Weissman, J.S. The social missions of academic health centers. N. Engl. J. Med. 1997, 337, 1550–1553. [Google Scholar] [CrossRef]
- Fleishon, H.B.; Itri, J.N.; Boland, G.W.; Duszak, R., Jr. Academic medical centers and community hospitals integration: Trends and strategies. J. Am. Coll. Radiol. 2017, 14, 45–51. [Google Scholar] [CrossRef]
- Ellner, A.L.; Stout, S.; Sullivan, E.E.; Griffiths, E.P.; Mountjoy, A.; Phillips, R.S. Health systems innovation at academic health centers: Leading in a new era of health care delivery. Acad. Med. 2015, 90, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.T.; Inui, T.S.; Ludden, J.M.; Schoenbaum, S.C. The “teaching HMO”: A new academic partner. Acad. Med. 1994, 69, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, A.N.; Mazotti, L.A.; Blumberg, B.; Wamsley, M.A.; Grennan, T.; Shore, W.B. Creating a longitudinal integrated clerkship with mutual benefits for an academic medical center and a community health system. Perm. J. 2014, 18, 50–56. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Brown, P.; Brotman, D.J.; Deutschendorf, A.; Dunbar, L.; Everett, A.; Hickman, D.; Howell, E.; Purnell, L.; Sylvester, C.; et al. Case Study: Johns Hopkins Community Health Partnership: A model for transformation. Healthc (Amst.) 2016, 4, 264–270. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care; Smedley, B.D., Stith, A.Y., Nelson, A.R., Eds.; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Ahmed, S.M.; Maurana, C.; Nelson, D.; Meister, T.; Young, S.N.; Lucey, P. Opening the black box: Conceptualizing community engagement from 109 community-academic partnership programs. Prog. Community Health Partnersh. 2016, 10, 51–61. [Google Scholar] [CrossRef]
- Croft, C.R.; Dial, R.; Doyle, G.; Schaadt, J.; Merchant, L. Integrating a community hospital-based radiology department with an academic medical center. J. Am. Coll. Radiol. 2016, 13, 300–302. [Google Scholar] [CrossRef]
- Sussman, A.J.; Otten, J.R.; Goldszer, R.C.; Hanson, M.; Trull, D.J.; Paulus, K.; Brown, M.; Dzau, V.; Brennan, T.A. Integration of an academic medical center and a community hospital: The Brigham and Women’s/Faulkner hospital experience. Acad. Med. 2005, 80, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Spalluto, L.B.; Thomas, D.; Beard, K.R.; Campbell, T.; Audet, C.M.; McBride Murry, V.; Shrubsole, M.J.; Barajas, C.P.; Joosten, Y.A.; Dittus, R.S.; et al. A community-academic partnership to reduce health care disparities in diagnostic imaging. J. Am. Coll. Radiol. 2019, 16, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Kelley-Quon, L.I.; Thomas, D.; Beard, K.R.; Campbell, T.; Audet, C.M.; McBride Murry, V.; Shrubsole, M.J.; Barajas, C.P.; Joosten, Y.A.; Dittus, R.S.; et al. Academic-community partnerships improve outcomes in pediatric trauma care. J. Pediatr. Surg. 2015, 50, 1032–1036. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phillip, C.R.; Mancera-Cuevas, K.; Leatherwood, C.; Chmiel, J.S.; Erickson, D.L.; Freeman, E.; Granville, G.; Dollear, M.; Walker, K.; McNeil, R.; et al. Implementation and dissemination of an African American popular opinion model to improve lupus awareness: An academic-community partnership. Lupus 2019, 28, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Rees, T. Academic medical center, community hospital partner to market center of excellence. Profiles Healthc. Mark. 1999, 15, 40–43. [Google Scholar]
- Yaggy, S.D.; Michener, J.L.; Yaggy, D.; Champagne, M.T.; Silberberg, M.; Lyn, M.; Johnson, F.; Yarnall, K.S. Just for Us: An academic medical center-community partnership to maintain the health of a frail low-income senior population. Gerontologist 2006, 46, 271–276. [Google Scholar] [CrossRef]
- Natesan, R.; Yang, W.T.; Tannir, H.; Parikh, J. Strategic expansion models in academic radiology. J. Am. Coll. Radiol. 2016, 13, 329–334. [Google Scholar] [CrossRef]
- Kirkwood, M.K.; Hanley, A.; Bruinooge, S.S.; Garrett-Mayer, E.; Levit, L.A.; Schenkel, C.; Seid, J.E.; Polite, B.N.; Schilsky, R.L. The State of oncology practice in America, 2018: results of the ASCO practice census survey. J. Oncol. Pract. 2018, 14, e412–e420. [Google Scholar] [CrossRef]
- OBR New Perspective Catalyst. Most Cancer Patients Will Be Treated in Integrated Delivery Networks (IDN) and Cancer Institutions by 2016, Predicts New Report; OBR New Perspective Catalyst: Sausalito, CA, USA, 2012; Volume 11. [Google Scholar]
- Genetech. The 2018 Genentech Oncology Trend Report; Genetech: San Francisco, CA, USA, 2018. [Google Scholar]
- Genetech. The 2019 Genentech Oncology Trend Report; Genetech: San Francisco, CA, USA, 2019. [Google Scholar]
- Academic Cancer Centers (NCCC). Trends Impacting Key Account. Management; Academic Cancer Centers: Pipersville, PA, USA, 2016. [Google Scholar]
- Nardi, E.A.; Wolfson, J.A.; Rosen, S.T.; Diasio, R.B.; Gerson, S.L.; Parker, B.A.; Alvarnas, J.C.; Levine, H.A.; Fong, Y.; Weisenburger, D.D.; et al. Value, access, and cost of cancer care delivery at academic cancer centers. J. Natl. Compr. Cancer Netw. 2016, 14, 837–847. [Google Scholar] [CrossRef]
- Speicher, P.J.; Englum, B.R.; Ganapathi, A.M.; Wang, X.; Hartwig, M.G.; D’Amico, T.A.; Berry, M.F. Traveling to a high-volume center is associated with improved survival for patients with esophageal cancer. Ann. Surg. 2017, 265, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Lidsky, M.E.; Sun, Z.; Nussbaum, D.P.; Adam, M.A.; Speicher, P.J.; Blazer, D.G., III. Going the extra mile: Improved survival for pancreatic cancer patients traveling to high-volume centers. Ann. Surg. 2017, 266, 333–338. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Ho, A.S.; Luu, M.; Yoshida, E.J.; Kim, S.; Mita, A.C.; Scher, K.S.; Shiao, S.L.; Tighiouart, M.; Zumsteg, Z.S. Treatment at high-volume facilities and academic centers is independently associated with improved survival in patients with locally advanced head and neck cancer. Cancer 2017, 123, 3933–3942. [Google Scholar] [CrossRef]
- Chen, A.Y.; Fedewa, S.; Pavluck, A.; Ward, E.M. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer 2010, 116, 4744–4752. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.G.; Rubin, D.M.; Elkin, E.B.; Neill, U.S.; Duck, E.; Radzyner, M.; Bach, P.B. Risk adjusting survival outcomes in hospitals that treat patients with cancer without information on cancer stage. JAMA Oncol. 2015, 1, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.; Adam, M.A.; Blazer, D.G., III. Overcoming a travel burden to high-volume centers for treatment of retroperitoneal sarcomas is associated with improved survival. World J. Surg. Oncol. 2019, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Dillman, R.O.; Chico, S.D. Cancer patient survival improvement is correlated with the opening of a community cancer center: Comparisons with intramural and extramural benchmarks. J. Oncol. Pract. 2005, 1, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Dinan, M.A.; Crawford, J. Survival comparison in patients with stage iv lung cancer in academic versus community centers in the United States. J. Thorac. Oncol. 2018, 13, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Carugo, A.; Draetta, G.F. Academic discovery of anticancer drugs: Historic and future perspectives. Ann. Rev. Cancer Biol. 2019, 3, 385–408. [Google Scholar] [CrossRef]
- Everett, J.R. Academic drug discovery: Current status and prospects. Expert Opin. Drug Discov. 2015, 10, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Matter, A. Bridging academic science and clinical research in the search for novel targeted anti-cancer agents. Cancer Biol. Med. 2015, 12, 316–327. [Google Scholar] [PubMed]
- Dorfman, G.S.; Lawrence, T.S.; Matrisian, L.M.; Translational Research Working Group. The Translational Research Working Group developmental pathway for interventive devices. Clin. Cancer Res. 2008, 14, 5700–5706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrios, C.H.; Reinert, T.; Werutsky, G. Global breast cancer research: Moving forward. Am. Soc. Clin. Oncol. Educ. Book. 2018, 38, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Kraut, N. Key drivers of biomedical innovation in cancer drug discovery. EMBO Mol. Med. 2015, 7, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Grodzinski, P.; Liu, C.H.; Hartshorn, C.M.; Morris, S.A.; Russell, L.M. NCI Alliance for Nanotechnology in Cancer—From academic research to clinical interventions. Biomed. Microdev. 2019, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Clauser, S.B.; Johnson, M.R.; O’Brien, D.M.; Beveridge, J.M.; Fennell, M.L.; Kaluzny, A.D. Improving clinical research and cancer care delivery in community settings: Evaluating the NCI community cancer centers program. Implement. Sci. 2009, 4, 63. [Google Scholar] [CrossRef]
- Hirsch, B.R.; Locke, S.C.; Abernethy, A.P. Experience of the national cancer institute community cancer centers program on community-based cancer clinical trials activity. J. Oncol. Pract. 2016, 12, e350-8. [Google Scholar] [CrossRef][Green Version]
- Copur, M.S.; Ramaekers, R.; Gonen, M.; Gulzow, M.; Hadenfeldt, R.; Fuller, C.; Scott, J.; Einspahr, S.; Benzel, H.; Mickey, M.; et al. Impact of the national cancer institute community cancer centers program on clinical trial and related activities at a community cancer center in rural Nebraska. J. Oncol. Pract. 2016, 12, 67–68. [Google Scholar] [CrossRef]
- DH, F. Clinical trials have the best medicine but do not enroll the patients who need it. Sci. Am. 2019, 320, 61–65. [Google Scholar]
- Copur, M.S. Inadequate awareness of and participation in cancer clinical trials in the community oncology setting. Oncology 2019, 33, 54–57. [Google Scholar]
- Green, M.A.; Michaels, M.; Blakeney, N.; Odulana, A.A.; Isler, M.R.; Richmond, A.; Long, D.G.; Robinson, W.S.; Taylor, Y.J.; Corbie-Smith, G. Evaluating a community-partnered cancer clinical trials pilot intervention with African American communities. J. Cancer Educ. 2015, 30, 158–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Best, A.; Hiatt, R.A.; Cameron, R.; Rimer, B.K.; Abrams, D.B. The evolution of cancer control research: An international perspective from Canada and the United States. Cancer Epidemiol Biomarkers Prev. 2003, 12, 705–712. [Google Scholar] [PubMed]
- Greenwald, P.; Cullen, J.W. The new emphasis in cancer control. J. Natl. Cancer. Inst. 1985, 74, 543–551. [Google Scholar] [CrossRef]
- Noel, L.; Phillips, F.; Tossas-Milligan, K.; Spear, K.; Vanderford, N.L.; Winn, R.A.; Vanderpool, R.C.; Eckhardt, S.G. Community-academic partnerships: Approaches to engagement. Am. Soc. Clin. Oncol. Educ. Book. 2019, 39, 88–95. [Google Scholar] [CrossRef]
- Harris, A.; Kumar, P.; Sutaria, S. Unlocking the Potential of Acdemic and Community Health System Partnerships; McKinsey and Company: New York, NY, USA, 2015. [Google Scholar]
- Johnson, T.M. Perspective on precision medicine in oncology. Pharmacotherapy 2017, 37, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Ersek, J.L.; Black, L.J.; Thompson, M.A.; Kim, E.S. Implementing precision medicine programs and clinical trials in the community-based oncology practice: Barriers and best practices. Am. Soc. Clin. Oncol. Educ. Book. 2018, 38, 188–196. [Google Scholar] [CrossRef]
- Nadauld, L.D.; Ford, J.M.; Pritchard, D.; Brown, T. Strategies for clinical implementation: Precision oncology at three distinct institutions. Health Aff. (Millwood) 2018, 37, 751–756. [Google Scholar] [CrossRef]
- Thompson, M.A.; Godden, J.J.; Wham, D.; Ruggeri, A.; Mullane, M.P.; Wilson, A.; Virani, S.; Weissman, S.M.; Ramczyk, B.; Vanderwall, P.; et al. Coordinating an oncology precision medicine clinic within an integrated health system: lessons learned in year one. J. Patient Cent. Res. Rev. 2019, 6, 36–45. [Google Scholar] [CrossRef]
- Carpten, J.C.; Mardis, E.R. The era of precision oncogenomics. Cold Spring Harb. Mol. Case Stud. 2018, 4. [Google Scholar] [CrossRef]
- International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature 2004, 431, 931–945. [Google Scholar] [CrossRef]
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.S.; Mills, G.B.; Mills Shaw, K.R. Precision oncology: Neither a silver bullet nor a dream. Pharmacogenomics 2017, 18, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.D.; Church, G.M. In situ localized amplification and contact replication of many individual DNA molecules. Nucleic Acids Res. 1999, 27, e34. [Google Scholar] [CrossRef]
- Brenner, S.; Johnson, M.; Bridgham, J.; Golda, G.; Lloyd, D.H.; Johnson, D.; Luo, S.; McCurdy, S.; Foy, M.; Ewan, M.; et al. Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat. Biotechnol. 2000, 18, 630–634. [Google Scholar] [CrossRef]
- Mardis, E.R. Next-generation DNA sequencing methods. Annu. Rev. Genomics Hum. Genet. 2008, 9, 387–402. [Google Scholar] [CrossRef]
- Shendure, J.; Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 2008, 26, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.R.; Balasubramanian, S.; Swerdlow, H.P.; Smith, G.P.; Milton, J.; Brown, C.G.; Hall, K.P.; Evers, D.J.; Barnes, C.L.; Bignell, H.R.; et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 2008, 456, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008, 24, 133–141. [Google Scholar] [CrossRef]
- Tucker, T.; Marra, M.; Friedman, J.M. Massively parallel sequencing: The next big thing in genetic medicine. Am. J. Hum. Genet. 2009, 85, 142–154. [Google Scholar] [CrossRef]
- Ley, T.J.; Mardis, E.R.; Ding, L.; Fulton, B.; McLellan, M.D.; Chen, K.; Dooling, D.; Dunford-Shore, B.H.; McGrath, S.; Hickenbotham, M.; et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 2008, 456, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, J.; Bruyns, E.; Lin, J.Z.; Burcham, T.; Brenner, S.; Bowen, B.; Kramer, M.; Woychik, R. Massively parallel signature sequencing (MPSS) as a tool for in-depth quantitative gene expression profiling in all organisms. Brief. Funct. Genomic. Proteomic. 2002, 1, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.T.; Metta, M.; Ottenwalder, B.; Schlotterer, C. Gene expression profiling by massively parallel sequencing. Genome Res. 2008, 18, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research, N.; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar]
- Wang, H.; Nettleton, D.; Ying, K. Copy number variation detection using next generation sequencing read counts. BMC Bioinform. 2014, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, P.; Stanciu, M.; Brudno, M. Computational methods for discovering structural variation with next-generation sequencing. Nat. Methods 2009, 6, S13–S20. [Google Scholar] [CrossRef]
- Johnson, D.S.; Mortazavi, A.; Myers, R.M.; Wold, B. Genome-wide mapping of in vivo protein-DNA interactions. Science 2007, 316, 1497–1502. [Google Scholar] [CrossRef]
- Morel, D.; Jeffery, D.; Aspeslagh, S.; Almouzni, G.; Postel-Vinay, S. Combining epigenetic drugs with other therapies for solid tumours—Past lessons and future promise. Nat. Rev. Clin. Oncol. 2020, 17, 91–107. [Google Scholar] [CrossRef]
- Lazaris, C.; Aifantis, I.; Tsirigos, A. On epigenetic plasticity and genome topology. Trends Cancer 2020, 6, 177–180. [Google Scholar] [CrossRef]
- Segal, N.H.; Parsons, D.W.; Peggs, K.S.; Velculescu, V.; Kinzler, K.W.; Vogelstein, B.; Allison, J.P. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008, 68, 889–892. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; LChe. A paradigm shift in cancer immunotherapy: From enhancement to normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Zewde, M.; Kiyotani, K.; Park, J.H.; Fang, H.; Yap, K.L.; Yew, P.Y.; Alachkar, H.; Kato, T.; Mai, T.H.; Ikeda, Y.; et al. The era of immunogenomics/immunopharmacogenomics. J. Hum. Genet. 2018, 63, 865–875. [Google Scholar] [CrossRef]
- Dennard, R.H.; Gaensslen, F.H.; Yu, H.N.; Rideout, V.L.; Bassous, E.; Leblanc, A.R. Design of ion-implanted MOSFET’s with very small physical dimensions. IEEE J. Solid State Circuits 1974, 9, 256–268. [Google Scholar] [CrossRef]
- Gorder, P.F. Multicore processors for science and engineering. Comput. Sci. Eng. 2007, 9, 3–7. [Google Scholar] [CrossRef][Green Version]
- Denning, P.J.; Lewis, T.G. Exponential laws of computing growth. Commun. Acm 2017, 60, 54–65. [Google Scholar] [CrossRef]
- Thackray, A.; Brock, D.C. Moore’s Law: The Life of Gordon Moore, Silicon Valley’s Quiet Revolutionary; Basic Books: New York, NY, USA, 2015. [Google Scholar]
- Koomey, J.G.; Berard, S.; Sanchez, M.; Wong, H. Implications of historical trends in the electrical efficiency of computing. IEEE Comput. Soc. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Hill, M.D.; Marty, M.R. Amdahl’s law in the multicore era. Computer 2008, 41, 33. [Google Scholar] [CrossRef]
- Denning, P.J.; Tichy, W.F. Highly parallel computation. Science 1990, 250, 1217–1222. [Google Scholar] [CrossRef]
- Hinkson, I.V.; Davidsen, T.M.; Klemm, J.D.; Kerlavage, A.R.; Kibbe, W.A.; Chandramouliswaran, I. A comprehensive infrastructure for big data in cancer research: Accelerating cancer research and precision medicine. Front. Cell. Dev. Biol. 2017, 5, 83. [Google Scholar] [CrossRef]
- Wing, J.M. Computational thinking. Commun. Acm 2006, 49, 33–35. [Google Scholar] [CrossRef]
- Regev, A.; Shapiro, E. Cellular abstractions: Cells as computation. Nature 2002, 419, 343-343. [Google Scholar] [CrossRef] [PubMed]
- Searls, D.B. The roots of bioinformatics. PLoS Comput. Biol. 2010, 6. [Google Scholar] [CrossRef]
- Moorthie, S.; Hall, A.; Wright, C.F. Informatics and clinical genome sequencing: Opening the black box. Genet. Med. 2013, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Funari, V.; Canosa, S. The importance of bioinformatics in NGS: Breaking the bottleneck in data interpretation. Science 2014, 344, 653-653. [Google Scholar] [CrossRef]
- Oliver, G.R.; Hart, S.N.; Klee, E.W. Bioinformatics for clinical next generation sequencing. Clin. Chem. 2015, 61, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Gullapalli, R.R.; Lyons-Weiler, M.; Petrosko, P.; Dhir, R.; Becich, M.J.; LaFramboise, W.A. Clinical integration of next-generation sequencing technology. Clin. Lab. Med. 2012, 32, 585–599. [Google Scholar] [CrossRef]
- Hundal, J.; Miller, C.A.; Griffith, M.; Griffith, O.L.; Walker, J.; Kiwala, S.; Graubert, A.; McMichael, J.; Coffman, A.; Mardis, E.R. Cancer immunogenomics: computational neoantigen identification and vaccine design. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 105–111. [Google Scholar] [CrossRef]
- Tenenbaum, J.D. Translational bioinformatics: Past, present, and future. Genom. Proteomics Bioinform. 2016, 14, 31–41. [Google Scholar] [CrossRef]
- Weissenbach, J. The rise of genomics. C R Biol. 2016, 339, 231–239. [Google Scholar] [CrossRef]
- Morganti, S.; Tarantino, P.; Ferraro, E.; D’Amico, P.; Viale, G.; Trapani, D.; Duso, B.A.; Curigliano, G. Complexity of genome sequencing and reporting: Next generation sequencing (NGS) technologies and implementation of precision medicine in real life. Crit. Rev. Oncol. Hematol. 2019, 133, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Steinberg, K.M.; Larson, D.E.; Wilson, R.K.; Mardis, E.R. The next-generation sequencing revolution and its impact on genomics. Cell 2013, 155, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C.; Rowley, J.D.; Knudson, A.G., Jr. Cancer genetics, cytogenetics—Defining the enemy within. Nat. Med. 1998, 4, 1107–1111. [Google Scholar] [CrossRef]
- Druker, B.J.; Lydon, N.B. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J. Clin. Invest. 2000, 105, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Rossari, F.; Minutolo, F.; Orciuolo, E. Past, present, and future of Bcr-Abl inhibitors: From chemical development to clinical efficacy. J. Hematol. Oncol. 2018, 11, 84. [Google Scholar] [CrossRef]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef]
- Savage, D.G.; Antman, K.H. Imatinib mesylate—A new oral targeted therapy. N. Engl. J. Med. 2002, 346, 683–693. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Scholl, C.; Frohling, S. Exploiting rare driver mutations for precision cancer medicine. Curr. Opin. Genet. Dev. 2019, 54, 1–6. [Google Scholar] [CrossRef]
- Jackson, S.E.; Chester, J.D. Personalised cancer medicine. Int. J. Cancer 2015, 137, 262–266. [Google Scholar] [CrossRef]
- Neal, J.W.; Sledge, G.W. Decade in review-targeted therapy: Successes, toxicities and challenges in solid tumours. Nat. Rev. Clin. Oncol. 2014, 11, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Fojo, T.; MBrad. Precision oncology: Origins, optimism, and potential. Lancet Oncol. 2016, 17, e81–e86. [Google Scholar] [CrossRef]
- El-Deiry, W.S.; Goldberg, R.M.; Lenz, H.J.; Shields, A.F.; Gibney, G.T.; Tan, A.R.; Brown, J.; Eisenberg, B.; Heath, E.I.; Phuphanich, S.; et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. Cancer J. Clin. 2019, 69, 305–343. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Targeted Cancer Therapies. Available online: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet (accessed on 2 July 2020).
- Khotskaya, Y.B.; Mills, G.B.; Mills Shaw, K.R. Next-Generation Sequencing and Result Interpretation in Clinical Oncology: Challenges of Personalized Cancer Therapy. Annu. Rev. Med. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Buchanan, M. The law of accelerating returns. Nat. Phys. 2008, 4, 507-507. [Google Scholar] [CrossRef]
- Kurzweil, R. The Law of Accelerating Returns. 2001. Available online: https://www.kurzweilai.net/the-law-of-accelerating-returns (accessed on 12 April 2020).
- Blazer, K.R.; Nehoray, B.; Solomon, I.; Niell-Swiller, M.; Culver, J.O.; Uman, G.C.; Weitzel, J.N. Next-generation testing for cancer risk: Perceptions, experiences, and needs among early adopters in community healthcare settings. Genet. Test. Mol. Biomarkers 2015, 19, 657–665. [Google Scholar] [CrossRef]
- Mauer, C.B.; Pirzadeh-Miller, S.M.; Robinson, L.D.; Euhus, D.M. The integration of next-generation sequencing panels in the clinical cancer genetics practice: An. institutional experience. Genet. Med. 2014, 16, 407–412. [Google Scholar] [CrossRef]
- Sabour, L.; Sabour, M.; Ghorbian, S. Clinical applications of next-generation sequencing in cancer diagnosis. Pathol. Oncol. Res. 2017, 23, 225–234. [Google Scholar] [CrossRef]
- Sylvester, D.E.; Chen, Y.; Jamieson, R.V.; Dalla-Pozza, L.; Byrne, J.A. Investigation of clinically relevant germline variants detected by next-generation sequencing in patients with childhood cancer: A review of the literature. J. Med. Genet. 2018, 55, 785–793. [Google Scholar] [CrossRef]
- Obrochta, E.; Godley, L.A. Identifying patients with genetic predisposition to acute myeloid leukemia. Best Pract. Res. Clin. Haematol. 2018, 31, 373–378. [Google Scholar] [CrossRef]
- Gomy, I.; Mdel, P.D. Hereditary cancer risk assessment: Insights and perspectives for the next-generation sequencing era. Genet. Mol. Biol. 2016, 39, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Pensabene, M.; Spagnoletti, I.; Capuano, I.; Condello, C.; Pepe, S.; Contegiacomo, A.; Lombardi, G.; Bevilacqua, G.; Caligo, M.A. Two mutations of BRCA2 gene at exon and splicing site in a woman who underwent oncogenetic counseling. Ann. Oncol. 2009, 20, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Kamps, R.; Brandao, R.D.; Bosch, B.J.; Paulussen, A.D.; Xanthoulea, S.; Blok, M.J.; Romano, A. Next-generation sequencing in oncology: Genetic diagnosis, risk prediction and cancer classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Bradbury, A.; Garber, J.E.; Offit, K.; Robson, M.E. Multiplex genetic testing for cancer susceptibility: Out on the high wire without a net? J. Clin. Oncol. 2013, 31, 1267–1270. [Google Scholar] [CrossRef]
- Weitzel, J.N.; Blazer, K.R.; MacDonald, D.J.; Culver, J.O.; Offit, K. Genetics, genomics, and cancer risk assessment: State of the Art and future directions in the era of personalized medicine. Cancer J. Clin. 2011, 61, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Lynce, F.; Isaacs, C. How far do we go with genetic evaluation. Am. Soc. Clin. Oncol. Educ. Book. 2016, 35, e72–e78. [Google Scholar] [CrossRef]
- Lui, S.T.; Shuch, B. Genetic testing in kidney cancer patients: Who, when, and how? Eur. Urol. Focus 2019, 5, 973–976. [Google Scholar] [CrossRef]
- Piccinin, C.; Panchal, S.; Watkins, N.; Kim, R.H. An update on genetic risk assessment and prevention: The role of genetic testing panels in breast cancer. Expert Rev. Anticancer Ther. 2019, 19, 787–801. [Google Scholar] [CrossRef]
- Plichta, J.K.; Sebastian, M.L.; Smith, L.A.; Menendez, C.S.; Johnson, A.T.; Bays, S.M.; Euhus, D.M.; Clifford, E.J.; Jalali, M.; Kurtzman, S.H.; et al. Germline genetic testing: What the breast surgeon needs to know. Ann. Surg. Oncol. 2019, 26, 2184–2190. [Google Scholar] [CrossRef]
- Valle, L.; Vilar, E.; Tavtigian, S.V.; Stoffel, E.M. Genetic predisposition to colorectal cancer: Syndromes, genes, classification of genetic variants and implications for precision medicine. J. Pathol. 2019, 247, 574–588. [Google Scholar] [CrossRef]
- Suszynska, M.; Klonowska, K.; Jasinska, A.J.; Kozlowski, P. Large-scale meta-analysis of mutations identified in panels of breast/ovarian cancer-related genes—Providing evidence of cancer predisposition genes. Gynecol. Oncol. 2019, 153, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Muskens, I.S.; de Smith, A.J.; Zhang, C.; Hansen, H.M.; Morimoto, L.; Metayer, C.; Ma, X.; Walsh, K.M.; Wiemels, J.L. Germline cancer predisposition variants and pediatric glioma: A population-based study in California. Neuro. Oncol. 2020, 22, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.H.; Sample, K.M.; Pilarski, R.; Walsh, T.; Grosel, T.; Kinnamon, D.; Boru, G.; Massengill, J.B.; Schoenfield, L.; Kelly, B.; et al. Whole exome sequencing identifies candidate genes associated with hereditary predisposition to uveal melanoma. Ophthalmology 2020, 127, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.A.; Nathan, V.; Bourke, L.M.; Palmer, J.M.; Zhang, T.; Symmons, J.; Howlie, M.; Patch, A.M.; Read, J.; Holland, E.A.; et al. Evaluation of the contribution of germline variants in BRCA1 and BRCA2 to uveal and cutaneous melanoma. Melanoma Res. 2019, 29, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, M.; Miller, J.E.; Dasari, V.R.; Gogoi, R.; Kim, D. Exome-wide rare variant analysis from the discovEHR study identifies novel candidate predisposition genes for endometrial cancer. Front. Oncol. 2019, 9, 574. [Google Scholar] [CrossRef]
- Bertelsen, B.; Tuxen, I.V.; Yde, C.W.; Gabrielaite, M.; Torp, M.H.; Kinalis, S.; Oestrup, O.; Rohrberg, K.; Spangaard, I.; Santoni-Rugiu, E.; et al. High. frequency of pathogenic germline variants within homologous recombination repair in patients with advanced cancer. NPJ Genom. Med. 2019, 4, 13. [Google Scholar] [CrossRef]
- Akhavanfard, S.; Padmanabhan, R.; Yehia, L.; Cheng, F.; Eng, C. Comprehensive germline genomic profiles of children, adolescents and young adults with solid tumors. Nat. Commun. 2020, 11, 2206. [Google Scholar] [CrossRef]
- Jin, Z.B.; Li, Z.; Liu, Z.; Jiang, Y.; Cai, X.B.; Wu, J. Identification of de novo germline mutations and causal genes for sporadic diseases using trio-based whole-exome/genome sequencing. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1014–1031. [Google Scholar] [CrossRef]
- Johnson, L.M.; Hamilton, K.V.; Valdez, J.M.; Knapp, E.; Baker, J.N.; Nichols, K.E. Ethical considerations surrounding germline next-generation sequencing of children with cancer. Expert Rev. Mol. Diagn. 2017, 17, 523–534. [Google Scholar] [CrossRef]
- Stadler, Z.K.; Schrader, K.A.; Vijai, J.; Robson, M.E.; Offit, K. Cancer genomics and inherited risk. J. Clin. Oncol. 2014, 32, 687–698. [Google Scholar] [CrossRef]
- Backman, S.; Bajic, D.; Crona, J.; Hellman, P.; Skogseid, B.; Stalberg, P. Whole genome sequencing of apparently mutation-negative MEN1 patients. Eur. J. Endocrinol. 2020, 182, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Nissim, S.; Leshchiner, I.; Mancias, J.D.; Greenblatt, M.B.; Maertens, O.; Cassa, C.A.; Rosenfeld, J.A.; Cox, A.G.; Hedgepeth, J.; Wucherpfennig, J.I.; et al. Mutations in RABL3 alter KRAS prenylation and are associated with hereditary pancreatic cancer. Nat. Genet. 2019, 51, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Okur, V.; Chung, W.K. The impact of hereditary cancer gene panels on clinical care and lessons learned. Cold Spring Harb. Mol. Case Stud. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Lumish, H.S.; Steinfeld, H.; Koval, C.; Russo, D.; Levinson, E.; Wynn, J.; Duong, J.; Chung, W.K. Impact of panel gene testing for hereditary breast and ovarian cancer on patients. J. Genet. Couns. 2017, 26, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.T.; Bernhisel, R.; Brown, K.; Kidd, J.; Manley, S. Clinical testing with a panel of 25 genes associated with increased cancer risk results in a significant increase in clinically significant findings across a broad range of cancer histories. Cancer Genet. 2017, 218–219, 58–68. [Google Scholar] [CrossRef]
- Foley, S.B.; Rios, J.J.; Mgbemena, V.E.; Robinson, L.S.; Hampel, H.L.; Toland, A.E.; Durham, L.; Ross, T.S. Use of whole genome sequencing for diagnosis and discovery in the cancer genetics clinic. Ebiomedicine 2015, 2, 74–81. [Google Scholar] [CrossRef]
- Fewings, E.; Larionov, A.; Redman, J.; Goldgraben, M.A.; Scarth, J.; Richardson, S.; Brewer, C.; Davidson, R.; Ellis, I.; Evans, D.G.; et al. Germline pathogenic variants in PALB2 and other cancer-predisposing genes in families with hereditary diffuse gastric cancer without CDH1 mutation: A whole-exome sequencing study. Lancet Gastroenterol. Hepatol. 2018, 3, 489–498. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Eccles, D.M.; Mitchell, G.; Monteiro, A.N.; Schmutzler, R.; Couch, F.J.; Spurdle, A.B.; Gomez-Garcia, E.B.; ENIGMA Clinical Working Group. BRCA1 and BRCA2 genetic testing-pitfalls and recommendations for managing variants of uncertain clinical significance. Ann. Oncol. 2015, 26, 2057–2065. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Hoffman-Andrews, L. The known unknown: The challenges of genetic variants of uncertain significance in clinical practice. J. Law Biosci. 2017, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.Y.; Mozersky, J.; Cook-Deegan, R. Variants of uncertain significance in BRCA: A harbinger of ethical and policy issues to come? Genome. Med. 2014, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.L.; Hoskin, T.L.; Day, C.N.; Thomas, A.S.; Cogswell, J.A.; Couch, F.J.; Boughey, J.C. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 Genes. Ann. Surg. Oncol. 2017, 24, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Voelker, R. Quick uptakes: Taking the uncertainty out of interpreting BRCA variants. J. Am. Med. Assoc. 2019, 321, 1340–1341. [Google Scholar] [CrossRef]
- Domchek, S.; Weber, B.L. Genetic variants of uncertain significance: Flies in the ointment. J. Clin. Oncol. 2008, 26, 16–17. [Google Scholar] [PubMed]
- Medendorp, N.M.; Hillen, M.A.; Murugesu, L.; Aalfs, C.M.; Stiggelbout, A.M.; Smets, E.M.A. Uncertainty related to multigene panel testing for cancer: A qualitative study on counsellors’ and counselees’ views. J. Community Genet. 2019, 10, 303–312. [Google Scholar] [CrossRef]
- Medendorp, N.M.; Hillen, M.A.; van Maarschalkerweerd, P.E.A.; Aalfs, C.M.; Ausems, M.; Verhoef, S.; van der Kolk, L.E.; Berger, L.P.V.; Wevers, M.R.; Wagner, A.; et al. “We don’t know for sure”: Discussion of uncertainty concerning multigene panel testing during initial cancer genetic consultations. Fam. Cancer 2020, 19, 65–76. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Robson, M.E. Psychosocial effects of multigene panel testing in the context of cancer genomics. Hastings Cent. Rep. 2019, 49, S44–S52. [Google Scholar] [CrossRef]
- Afghahi, A.; AWKuria. The changing landscape of genetic testing for inherited breast cancer predisposition. Curr. Treat Option Oncol. 2017, 18, 27. [Google Scholar] [CrossRef]
- Richter, S.; Haroun, I.; Graham, T.C.; Eisen, A.; Kiss, A.; Warner, E. Variants of unknown significance in BRCA testing: Impact on risk perception, worry, prevention and counseling. Ann. Oncol. 2013, 24, viii69–viii74. [Google Scholar] [CrossRef]
- Idos, G.E.; Kurian, A.W.; Ricker, C.; Sturgeon, D.; Culver, J.O.; Kingham, K.E.; Koff, R.; Chun, N.M.; Rowe-Teeter, C.; Lebensohn, A.P.; et al. Multicenter prospective cohort study of the diagnostic yield and patient experience of multiplex gene panel testing for hereditary cancer risk. JCO Precis. Oncol. 2019. [Google Scholar] [CrossRef]
- Federici, G.; Soddu, S. Variants of uncertain significance in the era of high-throughput genome sequencing: A lesson from breast and ovary cancers. J. Exp. Clin. Cancer Res. 2020, 39, 46. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.A.; Husami, A.; Holle, J.; Johnson, J.A.; Qian, Y.; Mathur, A.; Wei, C.; Indugula, S.R.; Zou, F.; Meng, H.; et al. Clinical impact and cost-effectiveness of whole exome sequencing as a diagnostic tool: A pediatric center’s experience. Front. Pediatr. 2015, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Gieldon, L.; Mackenroth, L.; Kahlert, A.K.; Lemke, J.R.; Porrmann, J.; Schallner, J.; von der Hagen, M.; Markus, S.; Weidensee, S.; Novotna, B.; et al. Diagnostic value of partial exome sequencing in developmental disorders. PLoS ONE 2018, 13, e0201041. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.N.; Hart, S.N.; Vijai, J.; Schrader, K.A.; Slavin, T.P.; Thomas, T.; Wubbenhorst, B.; Ravichandran, V.; Moore, R.M.; Hu, C.; et al. Evaluation of ACMG-guideline-based variant classification of cancer susceptibility and non-cancer-associated genes in families affected by breast cancer. Am. J. Hum. Genet. 2016, 98, 801–817. [Google Scholar] [CrossRef]
- Eggington, J.M.; Bowles, K.R.; Moyes, K.; Manley, S.; Esterling, L.; Sizemore, S.; Rosenthal, E.; Theisen, A.; Saam, J.; Arnell, C.; et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin. Genet. 2014, 86, 229–237. [Google Scholar] [CrossRef]
- Sud, A.; Kinnersley, B.; Houlston, R.S. Genome-wide association studies of cancer: Current insights and future perspectives. Nat. Rev. Cancer 2017, 17, 692–704. [Google Scholar] [CrossRef]
- Turnbull, C.; Sud, A.; Houlston, R.S. Cancer genetics, precision prevention and a call to action. Nat. Genet. 2018, 50, 1212–1218. [Google Scholar] [CrossRef]
- Kurian, A.W.; Hare, E.E.; Mills, M.A.; Kingham, K.E.; McPherson, L.; Whittemore, A.S.; McGuire, V.; Ladabaum, U.; Kobayashi, Y.; Lincoln, S.E.; et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J. Clin. Oncol. 2014, 32, 2001–2009. [Google Scholar] [CrossRef]
- Grissom, A.A.; Friend, P.J. Multigene panel testing for hereditary cancer risk. J. Adv. Pract. Oncol. 2016, 7, 394–407. [Google Scholar]
- Kurian, A.W.; Ford, J.M. Multigene panel testing in oncology practice: How should we respond? JAMA Oncol. 2015, 1, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Secretary’s Advisory Committee on Genetics, Health and Society. Genetics Education and Training; The Honorable Kathleen Sebelius Secretary of Health and Human Services: Washington, DC, USA, 2011. [Google Scholar]
- Campion, M.; Goldgar, C.; Hopkin, R.J.; Prows, C.A.; Dasgupta, S. Genomic education for the next generation of health-care providers. Genet. Med. 2019, 21, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Guttmacher, A.E.; Porteous, M.E.; McInerney, J.D. Educating health-care professionals about genetics and genomics. Nat. Rev. Genet. 2007, 8, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Douma, K.F.; Smets, E.M.; Allain, D.C. Non-genetic health professionals’ attitude towards, knowledge of and skills in discussing and ordering genetic testing for hereditary cancer. Fam. Cancer 2016, 15, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Maiese, D.R.; Keehn, A.; Lyon, M.; Flannery, D.; Watson, M.; Working Groups of the National Coordinating Center for Seven Regional Genetics Service Collaboratives. Current conditions in medical genetics practice. Genet. Med. 2019, 21, 1874–1877. [Google Scholar] [CrossRef] [PubMed]
- Salari, K. The dawning era of personalized medicine exposes a gap in medical education. PLoS Med. 2009, 6, e1000138. [Google Scholar] [CrossRef]
- Stoll, K.; Kubendran, S.; Cohen, S.A. The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. Am. J. Med. Genet. C Semin. Med. Genet. 2018, 178, 24–37. [Google Scholar] [CrossRef]
- Daly, M.B.; Stearman, B.; Masny, A.; Sein, E.; Mazzoni, S. How to establish a high-risk cancer genetics clinic: Limitations and successes. Curr. Oncol. Rep. 2005, 7, 469–474. [Google Scholar] [CrossRef]
- Cohen, S.A.; Bradbury, A.; Henderson, V.; Hoskins, K.; Bednar, E.; Arun, B.K. Genetic counseling and testing in a community setting: Quality, access, and efficiency. Am. Soc. Clin. Oncol. Educ. Book. 2019, 39, e34–e44. [Google Scholar] [CrossRef]
- Stopfer, J.E. Genetic counseling and clinical cancer genetics services. Semin. Surg. Oncol. 2000, 18, 347–357. [Google Scholar] [CrossRef]
- Anderson, B.; McLosky, J.; Wasilevich, E.; Lyon-Callo, S.; Duquette, D.; Copeland, G. Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J. Cancer Epidemiol. 2012, 2012, 298745. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Marvin, M.L.; Riley, B.D.; Vig, H.S.; Rousseau, J.A.; Gustafson, S.L. Identification of genetic counseling service delivery models in practice: A report from the NSGC service delivery model task force. J. Genet. Couns. 2013, 22, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Ricker, C.; Lagos, V.; Feldman, N.; Hiyama, S.; Fuentes, S.; Kumar, V.; Farwell Hagman, K.; Palomares, M.; Blazer, K.; Lowstuter, K.; et al. If we build it…will they come?—Establishing a cancer genetics services clinic for an underserved predominantly Latina cohort. J. Genet. Couns. 2007, 15, 505–514. [Google Scholar] [CrossRef]
- Epstein, C.J.; Erickson, R.P.; Hall, B.D.; Golbus, M.S. The center-satellite system for the wide-scale distribution of genetic counseling services. Am. J. Hum. Genet. 1975, 27, 322–332. [Google Scholar] [PubMed]
- Reid, K.J.; Sakati, N.; Prichard, L.L.; Schneiderman, L.J.; Jones, O.W.; Dixson, B.K. Genetic counseling: An evaluation of public health genetic clinics. West. J. Med. 1976, 124, 6–12. [Google Scholar] [PubMed]
- Riccardi, V.M. Health care and disease prevention through genetic counseling: A regional approach. Am. J. Public Health 1976, 66, 268–272. [Google Scholar] [CrossRef][Green Version]
- Weissman, S.M.; Zellmer, K.; Gill, N.; Wham, D. Implementing a virtual health telemedicine program. in a community setting. J. Genet. Couns. 2018, 27, 323–325. [Google Scholar] [CrossRef]
- Brown, J.; Athens, A.; Tait, D.L.; Crane, E.K.; Higgins, R.V.; Naumann, R.W.; Gusic, L.H.; Amacker-North, L. A comprehensive program: Enabling effective delivery of regional genetic counseling. Int. J. Gynecol. Cancer 2018, 28, 996–1002. [Google Scholar] [CrossRef]
- Fournier, D.M.; Bazzell, A.F.; Dains, J.E. Comparing outcomes of genetic counseling options in breast and ovarian cancer: An integrative review. Oncol. Nurs. Forum 2018, 45, 96–105. [Google Scholar] [CrossRef]
- Buchanan, A.H.; Rahm, A.K.; Williams, J.L. Alternate service delivery models in cancer genetic counseling: A mini-review. Front. Oncol. 2016, 6, 120. [Google Scholar] [CrossRef]
- McDonald, E.; Lamb, A.; Grillo, B.; Lucas, L.; Miesfeldt, S. Acceptability of telemedicine and other cancer genetic counseling models of service delivery in geographically remote settings. J. Genet. Couns. 2014, 23, 221–228. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.J.; KRBlazer; JNWeitze. Extending comprehensive cancer center expertise in clinical cancer genetics and genomics to diverse communities: The power of partnership. J. Natl. Compr. Canc. Netw. 2010, 8, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.B.; McGraw, S.; Shen, J.; Albayrak, A.; Alterovitz, G.; Davies, M.; Fitz, C.D.V.; Freedman, R.A.; Lopez, L.N.; Sholl, L.M. Engaging patients in precision oncology: development and usability of a web-based patient-facing genomic sequencing report. JCO Precis. Oncol. 2020, 4, 307–318. [Google Scholar] [CrossRef]
- Blazer, K.R.; Macdonald, D.J.; Culver, J.O.; Huizenga, C.R.; Morgan, R.J.; Uman, G.C.; Weitzel, J.N. Personalized cancer genetics training for personalized medicine: Improving community-based healthcare through a genetically literate workforce. Genet. Med. 2011, 13, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, P.; Beitsch, P.; Arnell, C.; Cox, H.C.; Brown, K.; Kidd, J.; Lancaster, J.M. Impact of payer constraints on access to genetic testing. J. Oncol. Pract. 2017, 13, e47–e56. [Google Scholar] [CrossRef] [PubMed]
- Karlovich, C.A.; Williams, P.M. Clinical applications of next-generation sequencing in precision oncology. Cancer J. 2019, 25, 264–271. [Google Scholar] [CrossRef]
- Wadapurkar, R.; Vyas, R. Computational analysis of next generation sequencing data and its applications in clinical oncology. Inform. Med. Unlocked 2018, 11, 75–82. [Google Scholar] [CrossRef]
- Hyman, D.M.; Taylor, B.S.; Baselga, J. Implementing genome-driven oncology. Cell 2017, 168, 584–599. [Google Scholar] [CrossRef]
- Nangalia, J.; Campbell, P.J. Genome sequencing during a patient’s journey through cancer. N. Engl. J. Med. 2019, 381, 2145–2156. [Google Scholar] [CrossRef]
- Pleasance, E.D.; Cheetham, R.K.; Stephens, P.J.; McBride, D.J.; Humphray, S.J.; Greenman, C.D.; Varela, I.; Lin, M.L.; Ordonez, G.R.; Bignell, G.R.; et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010, 463, 191–196. [Google Scholar] [CrossRef]
- Campbell, P.J.; Getz, G.; Korbel, J.O.; Stuart, J.M.; Jennings, J.L.; Stein, L.D.; Perry, M.D.; Nahal-Bose, H.K.; Ouellette, B.F.F.; Li, C.H.; et al. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar]
- Demircioglu, D.; Cukuroglu, E.; Kindermans, M.; Nandi, T.; Calabrese, C.; Fonseca, N.A.; Kahles, A.; Lehmann, K.V.; Stegle, O.; Brazma, A.; et al. A pan-cancer transcriptome analysis reveals pervasive regulation through alternative promoters. Cell 2019, 178, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Shimada, Y.; Ichikawa, H.; Kameyama, H.; Takabe, K.; Okuda, S.; Wakai, T. Next generation sequencing-based gene panel tests for the management of solid tumors. Cancer Sci. 2019, 110, 6–15. [Google Scholar] [CrossRef] [PubMed]
- El Achi, H.; Khoury, J.D.; Loghavi, S. Liquid biopsy by next-generation sequencing: A multimodality test for management of cancer. Curr. Hematol. Malig. Rep. 2019, 14, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Kancherla, J.; Rao, S.; Bhuvaneshwar, K.; Riggins, R.B.; Beckman, R.A.; Madhavan, S.; Bravo, H.C.; Boca, S.M. Evidence-based network approach to recommending targeted cancer therapies. JCO Clin. Cancer Inform. 2020, 4, 71–88. [Google Scholar] [CrossRef]
- Zhang, W.; Chien, J.; Yong, J.; Kuang, R. Network-based machine learning and graph theory algorithms for precision oncology. NPJ Precis. Oncol. 2017, 1, 25. [Google Scholar] [CrossRef]
- Jiang, X.L.; Martinez-Ledesma, E.; Morcos, F. Revealing protein networks and gene-drug connectivity in cancer from direct information. Sci. Rep. 2017, 7, 3739. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, C. Network analysis of cancer-focused association network reveals distinct network association patterns. Cancer Inform. 2014, 13, 45–51. [Google Scholar]
- Cheng, F.; Liu, C.; Jiang, J.; Lu, W.; Li, W.; Liu, G.; Zhou, W.; Huang, J.; Tang, Y. Prediction of drug-target interactions and drug repositioning via network-based inference. PLoS Comput. Biol. 2012, 8, e1002503. [Google Scholar] [CrossRef]
- Garber, K. In a major shift, cancer drugs go “tissue-agnostic”. Science 2017, 356, 1111–1112. [Google Scholar] [CrossRef]
- Luoh, S.W.; Flaherty, K.T. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann. Intern. Med. 2018, 169, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves landmark tissue-agnostic cancer drug. Nat. Rev. Drug Discov. 2018, 1, 87. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ayuso, P.; Sahoo, S.; Ashton, G.; An, E.; Simms, N.; Galvin, M.; Leong, H.S.; Frese, K.K.; Simpson, K.; Cook, N.; et al. Signaling pathway screening platforms are an efficient approach to identify therapeutic targets in cancers that lack known driver mutations: A case report for a cancer of unknown primary origin. NPJ Genom. Med. 2018, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.L.; Conley, B.A.; Boerner, S.; LoRusso, P.M. Next-generation sequencing to guide clinical trials. Clin. Cancer Res. 2015, 21, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Beaubier, N.; Bontrager, M.; Huether, R.; Igartua, C.; Lau, D.; Tell, R.; Bobe, A.M.; Bush, S.; Chang, A.L.; Hoskinson, D.C.; et al. Integrated genomic profiling expands clinical options for patients with cancer. Nat. Biotechnol. 2019, 37, 1351–1360. [Google Scholar] [CrossRef]
- Lih, C.J.; Harrington, R.D.; Sims, D.J.; Harper, K.N.; Bouk, C.H.; Datta, V.; Yau, J.; Singh, R.R.; Routbort, M.J.; Luthra, R.; et al. Analytical validation of the next-generation sequencing assay for a nationwide signal—Finding clinical trial: Molecular analysis for therapy choice clinical trial. J. Mol. Diagn. 2017, 19, 313–327. [Google Scholar] [CrossRef]
- Bitzer, M.; Ostermann, L.; Horger, M.; Biskup, S.; Schulze, M.; Ruhm, K.; Hilke, F.; Öner, Ö.; Nikolaou, K.; Schroeder, C.; et al. Next-generation sequencing of advanced gi tumors reveals individual treatment options. JCO Precis. Oncol. 2020, 4, 258–271. [Google Scholar] [CrossRef]
- Sicklick, J.K.; Kato, S.; Okamura, R.; Schwaederle, M.; Hahn, M.E.; Williams, C.B.; De, P.; Krie, A.; Piccioni, D.E.; Miller, V.A.; et al. Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat. Med. 2019, 25, 744–750. [Google Scholar] [CrossRef]
- Rodon, J.; Soria, J.C.; Berger, R.; Miller, W.H.; Rubin, E.; Kugel, A.; Tsimberidou, A.; Saintigny, P.; Ackerstein, A.; Brana, I.; et al. Genomic and transcriptomic profiling expands precision cancer medicine: The WINTHER trial. Nat. Med. 2019, 25, 751–758. [Google Scholar] [CrossRef]
- Rothwell, D.G.; Ayub, M.; Cook, N.; Thistlethwaite, F.; Carter, L.; Dean, E.; Smith, N.; Villa, S.; Dransfield, J.; Clipson, A.; et al. Utility of ctDNA to support patient selection for early phase clinical trials: The TARGET study. Nat. Med. 2019, 25, 738–743. [Google Scholar] [CrossRef]
- Reitsma, M.; Fox, J.; Vanden Borre, P.; Cavanaugh, M.; Chudnovsky, Y.; Erlich, R.L.; Gribbin, T.E.; Anhorn, R. Effect of a collaboration between a health plan, oncology practice, and comprehensive genomic profiling company from the payer perspective. J. Manag. Care Spec. Pharm. 2019, 25, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Luh, F.; Yen, Y. Benefits and harms of the centers for medicare & medicaid services ruling on next-generation sequencing. JAMA Oncol. 2018, 4, 1171–1172. [Google Scholar] [PubMed]
- Phillips, K.A.; Trosman, J.R.; Deverka, P.A.; Quinn, B.; Tunis, S.; Neumann, P.J.; Chambers, J.D.; Garrison, L.P., Jr.; Douglas, M.P.; Weldon, C.B. Insurance coverage for genomic tests. Science 2018, 360, 278–279. [Google Scholar]
- Trosman, J.R.; Weldon, C.B.; Gradishar, W.J.; Benson, A.; Cristofanilli, M.; Kurian, A.W.; Ford, J.M.; Balch, A.; Watkins, J.; Phillips, K.A. From the past to the present: Insurer coverage frameworks for next-generation tumor sequencing. Value Health 2018, 21, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Freedman, A.N.; Klabunde, C.N.; Wiant, K.; Enewold, L.; Gray, S.W.; Filipski, K.K.; Keating, N.L.; Leonard, D.G.B.; Lively, T.; McNeel, T.S.; et al. Use of next-generation sequencing tests to guide cancer treatment: Results from a nationally representative survey of oncologists in the United States. JCO Precision Oncol. 2018, 13, 1–13. [Google Scholar] [CrossRef]
- Miller, F.A.; Krueger, P.; Christensen, R.J.; Ahern, C.; Carter, R.F.; Kamel-Reid, S. Postal survey of physicians and laboratories: Practices and perceptions of molecular oncology testing. BMC Health Serv. Res. 2009, 9, 131. [Google Scholar] [CrossRef]
- Gray, S.W.; Hicks-Courant, K.; Cronin, A.; Rollins, B.J.; Weeks, J.C. Physicians’ attitudes about multiplex tumor genomic testing. J. Clin. Oncol. 2014, 32, 1317–1323. [Google Scholar] [CrossRef]
- Gray, S.W.; Park, E.R.; Najita, J.; Martins, Y.; Traeger, L.; Bair, E.; Gagne, J.; Garber, J.; Janne, P.A.; Lindeman, N.; et al. Oncologists’ and cancer patients’ views on whole-exome sequencing and incidental findings: Results from the CanSeq study. Genet. Med. 2016, 18, 1011–1019. [Google Scholar] [CrossRef]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef]
- Bieg-Bourne, C.C.; Millis, S.Z.; Piccioni, D.E.; Fanta, P.T.; Goldberg, M.E.; Chmielecki, J.; Parker, B.A.; Kurzrock, R. Next-generation sequencing in the clinical setting clarifies patient characteristics and potential actionability. Cancer Res. 2017, 77, 6313–6320. [Google Scholar] [CrossRef]
- Clark, D.F.; Maxwell, K.N.; Powers, J.; Lieberman, D.B.; Ebrahimzadeh, J.; Long, J.M.; McKenna, D.; Shah, P.; Bradbury, A.; Morrissette, J.J.D.; et al. Identification and confirmation of potentially actionable germline mutations in tumor-only genomic sequencing. JCO Precis. Oncol. 2019, 3. [Google Scholar] [CrossRef]
- Madhavan, S.; Subramaniam, S.; Brown, T.D.; Chen, J.L. Art and challenges of precision medicine: Interpreting and integrating genomic data into clinical practice. Am. Soc. Clin. Oncol. Educ. Book. Educ. Book. 2018, 38, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.E.; Choi, K.; Lanman, R.B.; Licitra, E.J.; Skrzypczak, S.M.; Pe Benito, R.; Wu, T.; Arunajadai, S.; Kaur, S.; Harper, H.; et al. Genomic profiling of advanced non-small cell lung cancer in community settings: Gaps and opportunities. Clin. Lung. Cancer 2017, 18, 651–659. [Google Scholar] [CrossRef]
- Trosman, J.R.; Weldon, C.B.; Kelley, R.K.; Phillips, K.A. Challenges of coverage policy development for next-generation tumor sequencing panels: Experts and payers weigh in. J. Natl. Compr. Canc. Netw. 2015, 13, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.S.; Ambinder, E.P.; Hess, G.P.; Yu, P.P.; Bernstam, E.V.; Routbort, M.J.; Clemenceau, J.R.; Hamm, J.T.; Febbo, P.G.; Domchek, S.M.; et al. Identifying health information technology needs of oncologists to facilitate the adoption of genomic medicine: Recommendations from the 2016 American Society of Clinical Oncology Omics and Precision Oncology Workshop. J. Clin. Oncol. 2017, 35, 3153–3159. [Google Scholar] [CrossRef]
- Griffith, M.; Spies, N.C.; Krysiak, K.; McMichael, J.F.; Coffman, A.C.; Danos, A.M.; Ainscough, B.J.; Ramirez, C.A.; Rieke, D.T.; Kujan, L.; et al. CIViC is a community knowledgebase for expert crowdsourcing the clinical interpretation of variants in cancer. Nat. Genet. 2017, 49, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Phillips, S.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; Chang, M.T.; et al. OncoKB: A precision oncology knowledge base. JCO Precis. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Bailey, A.M.; Zeng, J.; Johnson, A.M.; Shufean, M.A.; Brusco, L.; Litzenburger, B.C.; Sanchez, N.S.; Khotskaya, Y.B.; Holla, V.; et al. “Personalized cancer therapy”: A publicly available precision oncology resource. Cancer Res. 2017, 77, e123–e126. [Google Scholar] [CrossRef]
- My Cancer Genome: Genetically Informed Cancer Medicine. Available online: https://www.mycancergenome.org/ (accessed on 25 April 2020).
- Warner, J.L.; Prasad, I.; Bennett, M.; Arniella, M.; Beeghly-Fadiel, A.; Mandl, K.D.; Alterovitz, G. SMART cancer navigator: A framework for implementing ASCO workshop recommendations to enable precision cancer medicine. JCO Precis. Oncol. 2018. [Google Scholar] [CrossRef]
- City of Hope. Molecular Oncology. Available online: https://www.cityofhope.org/clinical-molecular-diagnostic-laboratory/list-of-cmdl-tests/molecular-oncology (accessed on 3 June 2020).
- Ellis, P.G. Development and implementation of oncology care pathways in an integrated care network: The via oncology pathways experience. J. Oncol. Pract. 2013, 9, 171–173. [Google Scholar] [CrossRef]
- Luchini, C.; Lawlor, R.T.; Milella, M.; Scarpa, A. Molecular tumor boards in clinical practice. Trends Cancer 2020. [Google Scholar] [CrossRef]
- Rolfo, C.; Manca, P.; Salgado, R.; Van Dam, P.; Dendooven, A.; Machado Coelho, A.; Ferri Gandia, J.; Rutten, A.; Lybaert, W.; Vermeij, J.; et al. Multidisciplinary molecular tumour board: A tool to improve clinical practice and selection accrual for clinical trials in patients with cancer. ESMO Open. 2018, 3, e000398. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Dienstmann, R. Precision oncology: Separating the wheat from the chaff. ESMO Open 2018, 3, e000446. [Google Scholar] [CrossRef] [PubMed]
- Romero, D. Optimizing access to matched therapies. Nat. Rev. Clin. Oncol. 2019, 16, 401. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Mardis, E.; Salm, M.; Soria, J.C.; Siu, L.L.; Swanton, C. Prioritizing targets for precision cancer medicine. Ann. Oncol. 2014, 25, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Dalton, W.S.; Sullivan, D.; Ecsedy, J.; Caligiuri, M.A. Patient enrichment for precision-based cancer clinical trials: Using prospective cohort surveillance as an approach to improve clinical trials. Clin. Pharmacol. Ther. 2018, 104, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Chakradhar, S. Matching up. Nat. Med. 2018, 24, 882–884. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Iyer, M.K.; Robinson, D.R.; Lonigro, R.J.; Wu, Y.M.; Cao, X.; Kalyana-Sundaram, S.; Sam, L.; Balbin, O.A.; Quist, M.J.; et al. Personalized oncology through integrative high-throughput sequencing: A pilot study. Sci. Transl. Med. 2011, 3, 111ra121. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Dumbrava, E.E.I.; Litzenburger, B.; Khotskaya, Y.B.; Johnson, A.M.; Yap, T.A.; Rodon, J.; Zeng, J.; Shufean, M.A.; Bailey, A.M.; et al. Precision oncology decision support: Current approaches and strategies for the future. Clin. Cancer Res. 2018, 24, 2719–2731. [Google Scholar] [CrossRef]
- Dalton, W.B.; Forde, P.M.; Kang, H.; Connolly, R.M.; Stearns, V.; Gocke, C.D.; Eshleman, J.R.; Axilbund, J.; Petry, D.; Geoghegan, C.; et al. Personalized medicine in the oncology clinic: Implementation and outcomes of the Johns Hopkins Molecular Tumor Board. JCO Precis. Oncol. 2017. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.; Holla, V.; Bailey, A.M.; Brusco, L.; Chen, K.; Routbort, M.; Patel, K.P.; Zeng, J.; Kopetz, S.; et al. A decision support framework for genomically informed investigational cancer therapy. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Hyman, D.M.; Solit, D.B.; Arcila, M.E.; Cheng, D.T.; Sabbatini, P.; Baselga, J.; Berger, M.F.; Ladanyi, M. Precision medicine at Memorial Sloan Kettering Cancer Center: Clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov. Today 2015, 20, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.D.; Fernandes, H.; Zia, H.; Tavassoli, P.; Rennert, H.; Pisapia, D.; Imielinski, M.; Sboner, A.; Rubin, M.A.; Kluk, M.; et al. The cancer precision medicine knowledge base for structured clinical-grade mutations and interpretations. J. Am. Med. Inform. Assoc. 2017, 24, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Eng, K.; Mosquera, J.M.; Sigaras, A.; Romanel, A.; Rennert, H.; Kossai, M.; Pauli, C.; Faltas, B.; Fontugne, J.; et al. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol. 2015, 1, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Schwaederle, M.; Parker, B.A.; Schwab, R.B.; Daniels, G.A.; Piccioni, D.E.; Kesari, S.; Helsten, T.L.; Bazhenova, L.A.; Romero, J.; Fanta, P.T.; et al. Precision oncology: The UC San Diego Moores Cancer Center PREDICT Experience. Mol. Cancer Ther. 2016, 15, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Said, R.; Tsimberidou, A.M. Basket trials and the MD Anderson Precision Medicine Clinical trials platform. Cancer J. 2019, 25, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, H.; Acharya, D.; Chamarthy, U.; Meunier, J.; Ali-Ahmad, H.; Hamdan, M.; Herman, J.; Srkalovic, G. Implementation and outcomes of a molecular tumor board at Herbert-Herman Cancer Center, Sparrow Hospital. Acta Med. Acad. 2019, 48, 105–115. [Google Scholar]
- Grandori, C.; Kemp, C.J. Personalized cancer models for target. discovery and precision medicine. Trends Cancer 2018, 4, 634–642. [Google Scholar] [CrossRef]
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017, 7, 462–477. [Google Scholar] [CrossRef]
- Chen, H.Z.; Bonneville, R.; Roychowdhury, S. Implementing precision cancer medicine in the genomic era. Semin. Cancer Biol. 2019, 55, 16–27. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Sacks, L.V.; Shamsuddin, H.H.; Yasinskaya, Y.I.; Bouri, K.; Lanthier, M.L.; Sherman, R.E. Scientific and regulatory reasons for delay and denial of FDA approval of initial applications for new drugs, 2000–2012. J. Am. Med. Assoc. 2014, 311, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Khozin, S.; Liu, K.; Jarow, J.P.; Pazdur, R. Regulatory watch: Why do oncology drugs fail to gain US regulatory approval? Nat. Rev. Drug. Discov. 2015, 14, 450–451. [Google Scholar] [CrossRef]
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017, 18, 122. [Google Scholar] [CrossRef]
- Seruga, B.; Ocana, A.; Amir, E.; Tannock, I.F. Failures in phase III: Causes and consequences. Clin. Cancer Res. 2015, 21, 4552–4560. [Google Scholar] [CrossRef]
- Schmidt, C. The struggle to do no harm. Nature 2017, 552, S74–S75. [Google Scholar] [CrossRef]
- Mullard, A. How much do phase III trials cost? Nat. Rev. Drug Discov. 2018, 1, 7777-7777. [Google Scholar] [CrossRef]
- Vincent Rajkumar, S. The high cost of prescription drugs: Causes and solutions. Blood Cancer J. 2020, 10, 71. [Google Scholar] [CrossRef]
- Sanders, A.B.; Fulginiti, J.V.; Witzke, D.B.; Bangs, K.A. Characteristics influencing career decisions of academic and nonacademic emergency physicians. Ann. Emerg. Med. 1994, 23, 81–87. [Google Scholar] [CrossRef]
- Tsang, J.L.Y.; Ross, K. It’s time to increase community hospital-based health research. Acad. Med. 2017, 92, 727. [Google Scholar] [CrossRef]
- Dimond, E.P.; St Germain, D.; Nacpil, L.M.; Zaren, H.A.; Swanson, S.M.; Minnick, C.; Carrigan, A.; Denicoff, A.M.; Igo, K.E.; Acoba, J.D.; et al. Creating a “culture of research” in a community hospital: Strategies and tools from the National Cancer Institute Community Cancer Centers Program. Clin. Trials 2015, 12, 246–256. [Google Scholar] [CrossRef]
- Boaz, A.; Hanney, S.; Jones, T.; Soper, B. Does the engagement of clinicians and organisations in research improve healthcare performance: A three-stage review. BMJ Open 2015, 5, e009415. [Google Scholar] [CrossRef]
- Knepper, T.C.; Bell, G.C.; Hicks, J.K.; Padron, E.; Teer, J.K.; Vo, T.T.; Gillis, N.K.; Mason, N.T.; McLeod, H.L.; Walko, C.M. Key lessons learned from moffitt’s molecular tumor board: The clinical genomics action committee experience. Oncologist 2017, 22, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Morris, V.; Kee, B.; Fogelman, D.; Xiao, L.; Eng, C.; Dasari, A.; Shroff, R.; Mazard, T.; Shaw, K.; et al. Utility of a molecular prescreening program in advanced colorectal cancer for enrollment on biomarker-selected clinical trials. Ann. Oncol. 2016, 27, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Garralda, E.; Aguilar, S.; Sala, G.; Viaplana, C.; Ruiz-Pace, F.; González-Zorelle, J.; LoGiacco, D.G.; Ogbah, Z.; Masdeu, L.R.; et al. Evolving landscape of molecular prescreening strategies for oncology early clinical trials. JCO Precis. Oncol. 2020, 4, 505–513. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Bender, R.J.; Rao, S.; Boca, S.M.; Chung, V.; Hendifar, A.E.; Mikhail, S.; Sohal, D.P.S.; Pohlmann, P.R.; et al. A virtual molecular tumor board to improve efficiency and scalability of delivering precision oncology to physicians and their patients. JAMIA Open 2019, 2, 505–515. [Google Scholar] [CrossRef]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Lazar, V.; Leyland-Jones, B.; Schilsky, R.L.; Mendelsohn, J.; Kurzrock, R. Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: A meta-analysis. JAMA Oncol. 2016, 2, 1452–1459. [Google Scholar] [CrossRef]
- Schwaederle, M.; Zhao, M.; Lee, J.J.; Eggermont, A.M.; Schilsky, R.L.; Mendelsohn, J.; Lazar, V.; Kurzrock, R. Impact of precision medicine in diverse cancers: A meta-analysis of phase II clinical trials. J. Clin. Oncol. 2015, 33, 3817–3825. [Google Scholar] [CrossRef]
- Stockley, T.L.; Oza, A.M.; Berman, H.K.; Leighl, N.B.; Knox, J.J.; Shepherd, F.A.; Chen, E.X.; Krzyzanowska, M.K.; Dhani, N.; Joshua, A.M.; et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: The Princess Margaret IMPACT/COMPACT trial. Genome Med. 2016, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Haslem, D.S.; Van Norman, S.B.; Fulde, G.; Knighton, A.J.; Belnap, T.; Butler, A.M.; Rhagunath, S.; Newman, D.; Gilbert, H.; Tudor, B.P.; et al. A retrospective analysis of precision medicine outcomes in patients with advanced cancer reveals improved progression-free survival without increased health care costs. J. Oncol. Pract. 2017, 13, e108–e119. [Google Scholar] [CrossRef] [PubMed]
- Radovich, M.; Kiel, P.J.; Nance, S.M.; Niland, E.E.; Parsley, M.E.; Ferguson, M.E.; Jiang, G.; Ammakkanavar, N.R.; Einhorn, L.H.; Cheng, L.; et al. Clinical benefit of a precision medicine based approach for guiding treatment of refractory cancers. Oncotarget 2016, 7, 56491–56500. [Google Scholar] [CrossRef]
- Aust, S.; Schwameis, R.; Gagic, T.; Mullauer, L.; Langthaler, E.; Prager, G.; Grech, C.; Reinthaller, A.; Krainer, M.; Pils, D.; et al. Precision medicine tumor boards: Clinical applicability of personalized treatment concepts in ovarian cancer. Cancers 2020, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Tafe, L.J.; Gorlov, I.P.; de Abreu, F.B.; Lefferts, J.A.; Liu, X.; Pettus, J.R.; Marotti, J.D.; Bloch, K.J.; Memoli, V.A.; Suriawinata, A.A.; et al. Implementation of a molecular tumor board: The impact on treatment decisions for 35 patients evaluated at Dartmouth-Hitchcock Medical Center. Oncologist 2015, 20, 1011–1018. [Google Scholar] [CrossRef]
- Lamping, M.; Benary, M.; Leyvraz, S.; Messerschmidt, C.; Blanc, E.; Kessler, T.; Schutte, M.; Lenze, D.; Johrens, K.; Burock, S.; et al. Support of a molecular tumour board by an evidence-based decision management system for precision oncology. Eur. J. Cancer 2020, 127, 41–51. [Google Scholar] [CrossRef]
- Boddu, S.; Walko, C.M.; Bienasz, S.; Bui, M.M.; Henderson-Jackson, E.; Naghavi, A.O.; Mullinax, J.E.; Joyce, D.M.; Binitie, O.; Letson, G.D.; et al. Clinical utility of genomic profiling in the treatment of advanced sarcomas: A single-center experience. JCO Precis. Oncol. 2018, 1–8. [Google Scholar] [CrossRef]
- Lane, B.R.; Bissonnette, J.; Waldherr, T.; Ritz-Holland, D.; Chesla, D.; Cottingham, S.L.; Alberta, S.; Liu, C.; Thompson, A.B.; Graveel, C.; et al. Development of a center for personalized cancer care at a regional cancer center: Feasibility trial of an institutional tumor sequencing advisory board. J. Mol. Diagn. 2015, 17, 695–704. [Google Scholar] [CrossRef]
- Overton, L.C.; Corless, C.L.; Agrawal, M.; Assikis, V.J.; Beegle, N.L.; Blau, S.; Chernoff, M.; Divers, S.G.; Henry, D.H.; Nikolinakos, P.; et al. Impact of next-generation sequencing (NGS) on treatment decisions in the community oncology setting. J. Clin. Oncol. 2014, 32, 11028-11028. [Google Scholar] [CrossRef]
- ASCO eLearning. Multidisciplinary Molecular Tumor Boards (MMTBs). Available online: https://elearning.asco.org/product-details/multidisciplinary-molecular-tumor-boards-mmtbs (accessed on 1 May 2020).
- Healio Learn Genomics. Available online: https://www.healio.com/hematology-oncology/learn-genomics (accessed on 1 May 2020).
- Pishvaian, M.J.; Bender, R.J.; Halverson, D.; Rahib, L.; Hendifar, A.E.; Mikhail, S.; Chung, V.; Picozzi, V.J.; Sohal, D.; Blais, E.M.; et al. Molecular profiling of patients with pancreatic cancer: Initial results from the know your tumor initiative. Clin. Cancer Res. 2018, 24, 5018–5027. [Google Scholar] [CrossRef]
- Burkard, M.E.; Deming, D.A.; Parsons, B.M.; Kenny, P.A.; Schuh, M.R.; Leal, T.; Uboha, N.; Lang, J.M.; Thompson, M.A.; Warren, R.; et al. Implementation and clinical utility of an integrated academic-community regional molecular tumor board. JCO Precis. Oncol. 2017, 1–10. [Google Scholar] [CrossRef]
- Heifetz, L.J.; Christensen, S.D.; Devere-White, R.W.; Meyers, F.J. A model for rural oncology. J. Oncol. Pract. 2011, 7, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Shea, C.M.; Teal, R.; Haynes-Maslow, L.; McIntyre, M.; Weiner, B.J.; Wheeler, S.B.; Jacobs, S.R.; Mayer, D.K.; Young, M.; Shea, T.C. Assessing the feasibility of a virtual tumor board program: A case study. J. Healthc. Manag. 2014, 59, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Farhangfar, C. Utilization of consultative molecular tumor board in community setting. J. Clin. Oncol. 2017, 35, 6508. [Google Scholar] [CrossRef]
- Marshall, C.L.; Petersen, N.J.; Naik, A.D.; Vander Velde, N.; Artinyan, A.; Albo, D.; Berger, D.H.; Anaya, D.A. Implementation of a regional virtual tumor board: A prospective study evaluating feasibility and provider acceptance. Telemed. J. E Health 2014, 20, 705–711. [Google Scholar] [CrossRef]
- Nasser, S.; Kurdolgu, A.A.; Izatt, T.; Aldrich, J.; Russell, M.L.; Christoforides, A.; Tembe, W.; Keifer, J.A.; Corneveaux, J.J.; Byron, S.A.; et al. An Integrated framework for reporting clinically relevant biomarkers from paired tumor/normal genomic and transcriptomic sequencing data in support of clinical trials in personalized medicine. Pac. Symp. Biocomput. 2015, 2015, 56–67. [Google Scholar]
- Von Hoff, D.; Han, H. Precision Medicine in Cancer Therapy; Springer International Publishing: New York, NY, USA, 2019. [Google Scholar]
- Weiss, G.J.; Byron, S.A.; Aldrich, J.; Sangal, A.; Barilla, H.; Kiefer, J.A.; Carpten, J.D.; Craig, D.W.; Whitsett, T.G. A prospective pilot study of genome-wide exome and transcriptome profiling in patients with small cell lung cancer progressing after first-line therapy. PLoS ONE 2017, 12, e0179170. [Google Scholar] [CrossRef]
- Byron, S.A.; Tran, N.L.; Halperin, R.F.; Phillips, J.J.; Kuhn, J.G.; de Groot, J.F.; Colman, H.; Ligon, K.L.; Wen, P.Y.; Cloughesy, T.F.; et al. Prospective feasibility trial for genomics-informed treatment in recurrent and progressive glioblastoma. Clin. Cancer Res. 2018, 24, 295–305. [Google Scholar] [CrossRef]
- Mueller, S.; Jain, P.; Liang, W.S.; Kilburn, L.; Kline, C.; Gupta, N.; Panditharatna, E.; Magge, S.N.; Zhang, B.; Zhu, Y.; et al. A pilot precision medicine trial for children with diffuse intrinsic pontine glioma-PNOC003: A report from the Pacific Pediatric Neuro-Oncology Consortium. Int. J. Cancer 2019, 145, 1889–1901. [Google Scholar] [CrossRef]
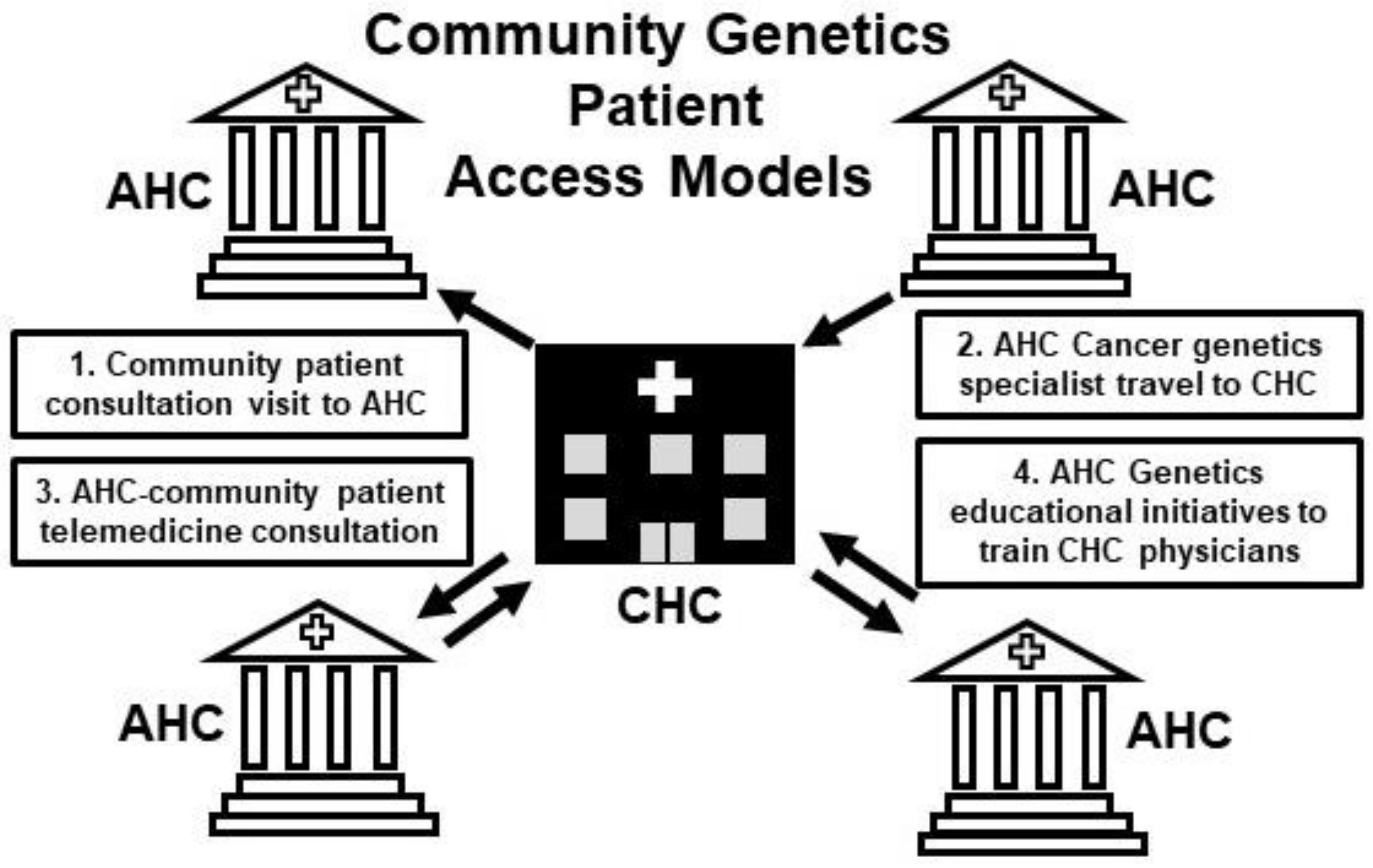
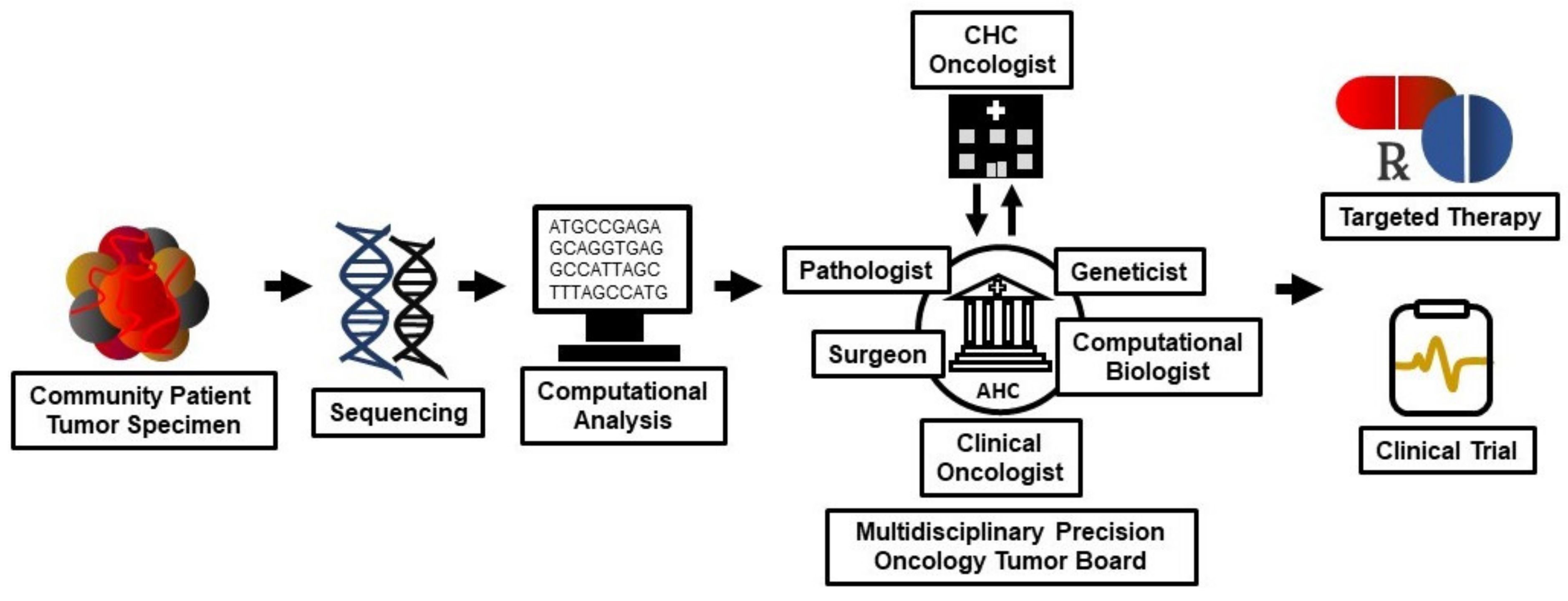
| WEB-BASED RESOURCE | URL |
|---|---|
| CIViC | civicdb.org |
| OncoKB | oncokb.org |
| Personalized Cancer Therapy | pct.mdanderson.org |
| My Cancer Genome | mycancergenome.org |
| SMART Cancer Navigator | smart-cancer-navigator.github.io/home |
| ASCO Multidisciplinary Molecular Tumor Boards | elearning.asco.org/product-details/multidisciplinary-molecular-tumor/boards-mmtbs |
| Helio Learn Genomics | healio.com |
| Know Your Tumor | pancan.org |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melas, M.; Subbiah, S.; Saadat, S.; Rajurkar, S.; McDonnell, K.J. The Community Oncology and Academic Medical Center Alliance in the Age of Precision Medicine: Cancer Genetics and Genomics Considerations. J. Clin. Med. 2020, 9, 2125. https://doi.org/10.3390/jcm9072125
Melas M, Subbiah S, Saadat S, Rajurkar S, McDonnell KJ. The Community Oncology and Academic Medical Center Alliance in the Age of Precision Medicine: Cancer Genetics and Genomics Considerations. Journal of Clinical Medicine. 2020; 9(7):2125. https://doi.org/10.3390/jcm9072125
Chicago/Turabian StyleMelas, Marilena, Shanmuga Subbiah, Siamak Saadat, Swapnil Rajurkar, and Kevin J. McDonnell. 2020. "The Community Oncology and Academic Medical Center Alliance in the Age of Precision Medicine: Cancer Genetics and Genomics Considerations" Journal of Clinical Medicine 9, no. 7: 2125. https://doi.org/10.3390/jcm9072125
APA StyleMelas, M., Subbiah, S., Saadat, S., Rajurkar, S., & McDonnell, K. J. (2020). The Community Oncology and Academic Medical Center Alliance in the Age of Precision Medicine: Cancer Genetics and Genomics Considerations. Journal of Clinical Medicine, 9(7), 2125. https://doi.org/10.3390/jcm9072125





