Small Cell Lung Cancer from Traditional to Innovative Therapeutics: Building a Comprehensive Network to Optimize Clinical and Translational Research
Abstract
1. Introduction
2. Current Standard Therapies
2.1. Radiation Therapy
2.2. Chemotherapy
2.3. Surgery
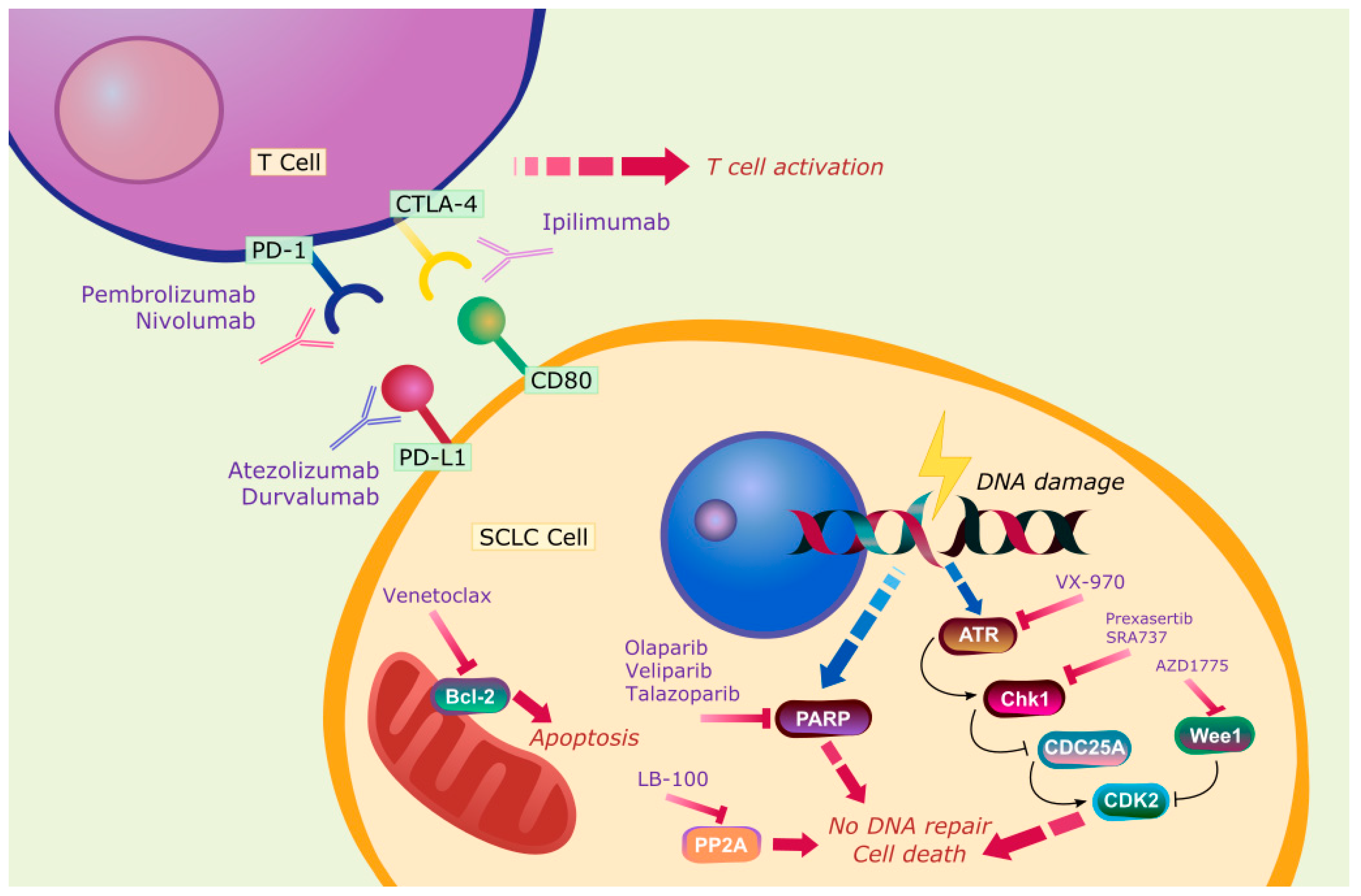
3. Novel Therapies
3.1. Atezolizumab
3.2. Durvalumab
3.3. Ipilimumab and Nivolumab
3.4. Pembrolizumab
4. Targeted Therapy
5. Protein Phosphatase 2A (PP2A)
6. Mitochondria
7. Stem Cell Therapy
8. Improving Outcomes
8.1. Biomarker-Based Therapy
8.2. Combination Therapy
8.3. Other Modalities
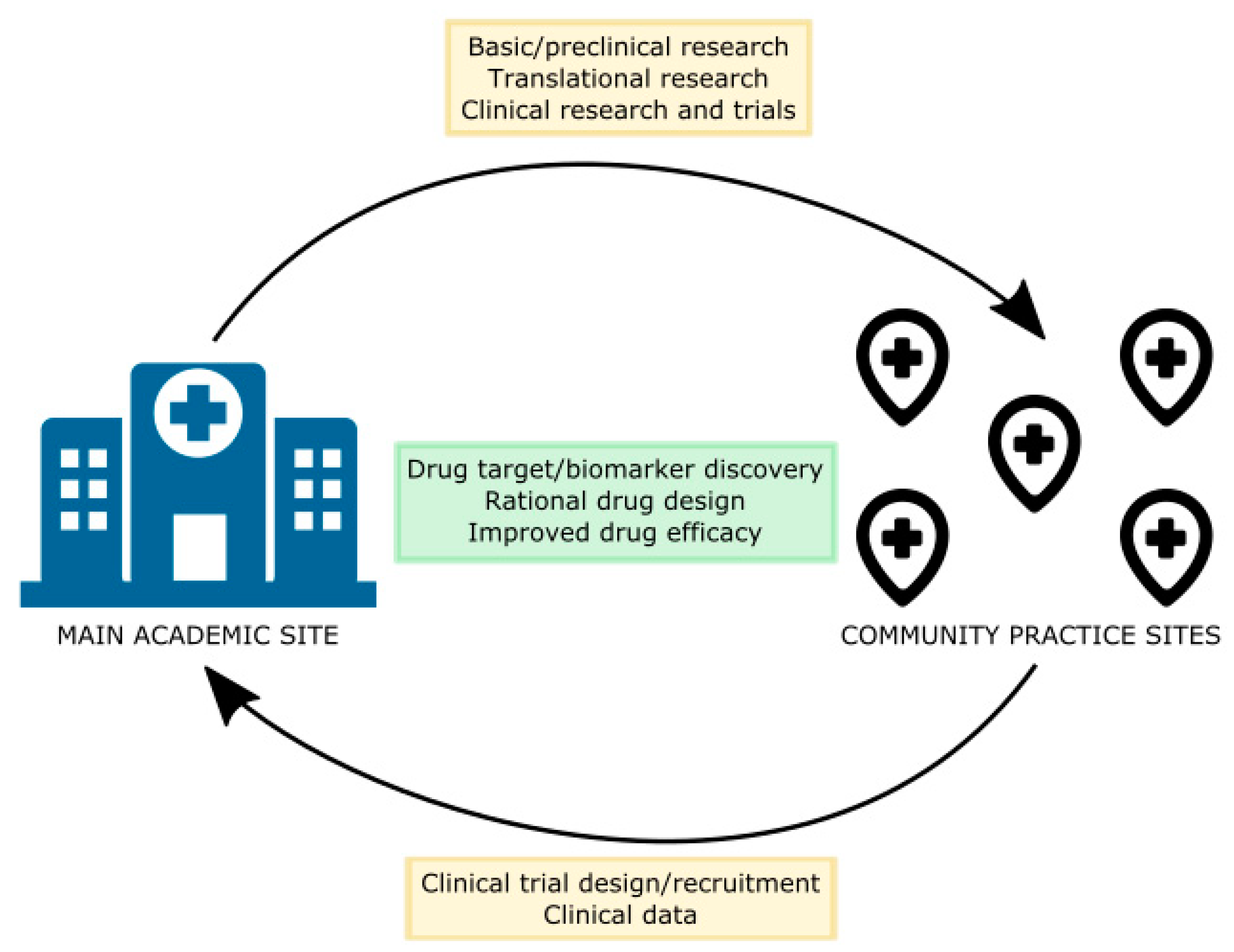
9. Community Network-City of Hope Experience
10. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Center for Health Statistics. CDC On-Line Database, Compiled from Compressed Mortality File 1999–2016 Series 20 No. 2V, 2017. Available online: https://www.cdc.gov/nchs/data_access/cmf.htm (accessed on 28 July 2020).
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 2017, 17, 765. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P. Staging and imaging of small cell Lung cancer. Cancer Imaging 2012, 11, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Felip, E. Pembrolizumab in advanced pretreated small cell lung cancer patients with PD-L1 expression: Data from the KEYNOTE-028 trial: A reason for hope? Transl. Lung Cancer Res. 2017, 6 (Suppl. S1), S78–S83. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Grants Nivolumab Accelerated Approval for Third-Line Treatment of Metastatic Small Cell Lung Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-third-line-treatment-metastatic-small-cell-lung-cancer (accessed on 28 July 2020).
- NCCN. NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer; The National Comprehensive Cancer Network (NCCN): Whitemarsh, PA, USA, 2019. [Google Scholar]
- Warde, P.; Payne, D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J. Clin. Oncol. 1992, 10, 890–895. [Google Scholar] [CrossRef]
- Pignon, J.P.; Arriagada, R.; Ihde, D.C.; Johnsosn, D.H.; Perry, M.C.; Souhami, R.L.; Brodin, O.; Joss, R.A.; Kies, M.S.; Lebeau, B.; et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N. Engl. J. Med. 1992, 327, 1618–1624. [Google Scholar] [CrossRef]
- Turrisi, A.T., III; Kim, K.; Blum, R.; Sause, W.T.; Livingston, R.B.; Komaki, R.; Wagner, H.; Aisner, S.; Johnson, D.H. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N. Engl. J. Med. 1999, 340, 265–271. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Snee, M.; Ashcroft, L.; Appel, W.; Barlesi, F.; Bhatnagar, A.; Bezjak, A.; Cardenal, F.; Fournel, P.; Harden, S.; et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): An open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017, 18, 1116–1125. [Google Scholar] [CrossRef]
- Fukuoka, M.; Furuse, K.; Saijo, N.; Nishiwaki, Y.; Ikegami, H.; Tamura, T.; Shimoyama, M.; Suemasu, K. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J. Natl. Cancer Inst. 1991, 83, 855–861. [Google Scholar] [CrossRef]
- Roth, B.J.; Johnson, D.H.; Einhorn, L.H.; Schacter, L.P.; Cherng, N.C.; Cohen, H.J.; Crawford, J.; Randolph, J.A.; Goodlow, J.L.; Broun, G.O.; et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: A phase III trial of the Southeastern cancer study group. J. Clin. Oncol. 1992, 10, 282–291. [Google Scholar] [CrossRef]
- Noda, K.; Nishiwaki, Y.; Kawahara, M.; Negoro, C.; Sugiura, T.; Yokoyama, A.; Fukuoka, M.; Mori, K.; Watanabe, K.; Tamura, T.; et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N. Engl. J. Med. 2002, 346, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jackman, D.M.; Johnson, B.E. Small-cell lung cancer. Lancet 2005, 366, 1385–1396. [Google Scholar] [CrossRef]
- O’Brien, M.E.R.; Ciuleanu, T.E.; Tsekov, H.; Shparyk, Y.; Cuceviá, B.; Juhasz, G.; Thatcher, N.; Ross, G.A.; Dane, G.C.; Crofts, T. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J. Clin. Oncol. 2006, 24, 5441–5447. [Google Scholar] [CrossRef] [PubMed]
- Von Pawel, J.; Schiller, J.H.; Shepherd, F.A.; Fields, S.Z.; Kleisbauer, J.P.; Chrysson, N.G.; Stewart, D.J.; Clark, P.I.; Palmer, M.C.; Depierre, A.; et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J. Clin. Oncol. 1999, 17, 658–667. [Google Scholar] [CrossRef]
- Horita, N.; Yamamoto, M.; Sato, T.; Tsukahara, T.; Nagakura, H.; Tashiro, K.; Shinata, Y.; Watanabe, H.; Nagai, K.; Inoue, M.; et al. Topotecan for relapsed small-cell lung cancer: Systematic review and meta-analysis of 1347 patients. Sci. Rep. 2015, 5, 15437. [Google Scholar] [CrossRef]
- Masters, G.A.; Declerck, L.; Blanke, C.; Sandler, A.; DeVore, R.; Miller, K.; Johnson, D.; Eastern Cooperative Oncology Group. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J. Clin. Oncol. 2003, 21, 1550–1555. [Google Scholar] [CrossRef]
- Smyth, J.F.; Smith, I.E.; Sessa, C.; Schoffski, P.; Wanders, J.; Franklin, H.; Kaye, S.B. Activity of docetaxel (Taxotere) in small cell lung cancer: The Early Clinical Trials Group of the EORTC. Eur. J. Cancer 1994, 30A, 1058–1060. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tsurutani, J.; Yoshimura, N.; Asai, G.; Moriyama, A.; Nakagawa, K.; Kudoh, S.; Takada, M.; Minato, Y.; Fukuoka, M. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res. 2006, 26, 777–781. [Google Scholar]
- Pietanza, M.C.; Kadota, K.; Huberman, K.; Sima, C.S.; Fiore, J.J.; Sumner, D.K.; Travis, W.D.; Heguy, A.; Ginsberg, M.S.; Holodny, A.I.; et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin. Cancer Res. 2012, 18, 1138–1145. [Google Scholar] [CrossRef]
- Masuda, N.; Fukuoka, M.; Kusunoki, Y.; Matsui, K.; Takifuji, N.; Kudoh, S.; Negoro, S.; Nishioka, M.; Nakagawa, K.; Takada, M. CPT-11: A new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J. Clin. Oncol. 1992, 10, 1225–1229. [Google Scholar] [CrossRef]
- Jassem, J.; Karnicka-Mlodkowska, H.; van Pottelsberghe, C.; van Glabbeke, M.; Noseda, M.A.; Ardizzoni, A.; Gozzelino, F.; Planting, A.; van Zandwijk, N. Phase II study of vinorelbine (Navelbine) in previously treated small cell lung cancer patients: EORTC Lung Cancer Cooperative Group. Eur. J. Cancer 1993, 29A, 1720–1722. [Google Scholar] [CrossRef]
- Ding, Q.; Zhan, J. Amrubicin: Potential in combination with cisplatin or carboplatin to treat small-cell lung cancer. Drug Des. Dev. Ther. 2013, 7, 681–689. [Google Scholar]
- Miller, A.B.; Fox, W.; Tall, R. Five-year follow-up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small-celled or oat-celled carcinoma of the bronchus. Lancet 1969, 2, 501–505. [Google Scholar] [CrossRef]
- Fox, W.; Scadding, J.G. Treatment of oat-celled carcinoma of the bronchus. Lancet 1973, 2, 616–617. [Google Scholar] [CrossRef]
- Lad, T.; Piantadosi, S.; Thomas, P.; Payne, D.; Ruckdeschel, J.; Giaccone, G. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994, 106 (Suppl. S6), 320S–323S. [Google Scholar] [CrossRef]
- Rostad, H.; Naalsund, A.; Jacobsen, R.; Strand, T.E.; Scott, H.; Strøm, E.H.; Norstein, J. Small cell lung cancer in Norway. Should more patients have been offered surgical therapy? Eur. J. Cardiothorac. Surg. 2004, 26, 782–786. [Google Scholar] [CrossRef]
- Brock, M.V.; Hooker, C.M.; Syphard, J.E.; Westra, W.; Xu, L.; Alberg, A.J.; Mason, D.; Baylin, S.B.; Herman, J.G.; Yung, R.C.; et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J. Thorac. Cardiovasc. Surg. 2005, 129, 64–72. [Google Scholar] [CrossRef]
- Lim, E.; Belcher, E.; Yap, Y.K.; Nicholson, A.G.; Goldstraw, P. The role of surgery in the treatment of limited disease small cell lung cancer: Time to reevaluate. J. Thorac. Oncol. 2008, 3, 1267–1271. [Google Scholar] [CrossRef]
- Schreiber, D.; Rineer, J.; Weedon, J.; Vongtama, D.; Wortham, A.; Kim, A.; Han, P.; Choi, K.; Rotman, M. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: Should its role be re-evaluated? Cancer 2010, 116, 1350–1357. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Dang, J.; Li, G. The role of surgery in stage I to III small cell lung cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0210001. [Google Scholar] [CrossRef]
- Peifer, M.; Fernández-Cuesta, L.; Sos, M.L.; George, J.; Seidel, D.; Kasper, L.H.; Plenker, D.; Leenders, F.; Sun, R.; Zander, T.; et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat. Genet. 2012, 44, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børrensen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Reck, M.; Spigel, D.R. The future of immunotherapy in the treatment of small cell lung cancer. Oncologist 2016, 21, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Heigener, D.; Reinmuth, N. Immunotherapy for small-cell lung cancer: Emerging evidence. Future Oncol. 2016, 12, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; IMpower133 Study Group; et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.-W.; Akerley, W.; Pietanza, M.C.; Wu, Y.-L.; Zielinski, C.; Thomas, M.; et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 2016, 34, 3740–3748. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Kim, H.R.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodriguez-Cid, J.; Schenker, M.; et al. Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): Results from the double-blind, randomized phase III CheckMate 451 study. Ann. Oncol. 2019, 30 (Suppl. S2), ii77–ii80. [Google Scholar]
- Ready, N.E.; Ott, P.A.; Hellmann, M.D.; Zugazagoitia, J.; Hann, C.L.; de Braud, F.; Antonia, S.J.; Ascierto, P.A.; Moreno, V.; Atmaca, A.; et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results from the CheckMate 032 Randomized Cohort. J. Thorac. Oncol. 2020, 15, 426–435. [Google Scholar] [CrossRef]
- Ott, P.A.; Elez, E.; Hiret, S.; Kim, D.-W.; Morosky, A.; Saraf, S.; Piperdi, B.; Mehnert, J.M. Pembrolizumab in patients with extensive-stage small-cell lung cancer: Results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol. 2017, 35, 3823–3829. [Google Scholar] [CrossRef]
- Chung, H.C.; Lopez-Martin, J.A.; Kao, S.C.-H.; Miller, W.H.; Ros, W.; Gao, B.; Marabelle, A.; Gottfried, M.; Zer, A.; Delord, J.-P.; et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J. Clin. Oncol. 2018, 15, 8506. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T. Small-cell lung cancer in 2016: Shining light on novel targets and therapies. Nat. Rev. Clin. Oncol. 2017, 14, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.W.; Simon, K.; Aslo, A.; Kok, Y.; Yokota, J.; Buys, C.H.; Terada, M.; Koeffler, H.P. p53 mutations in human lung tumors. Cancer Res. 1992, 52, 1695–1698. [Google Scholar] [PubMed]
- Takahashi, T.; Takahashi, T.; Suzuki, H.; Hida, T.; Sekido, Y.; Ariyoshi, Y.; Ueda, R. The p53 gene is very frequently mutated in small-cell lung cancer with a distinct nucleotide substitution pattern. Oncogene 1991, 6, 1775–1778. [Google Scholar] [PubMed]
- Helin, K.; Holm, K.; Niebuhr, A.; Eiberg, H.; Tommerup, N.; Hougaard, S.; Poulsen, H.S.; Spang-Thomsen, M.; Norgaard, P. Loss of the retinoblastoma protein-related p130 protein in small cell lung carcinoma. Proc. Natl. Acad. Sci. USA 1997, 94, 6933–6938. [Google Scholar] [CrossRef] [PubMed]
- Kaye, F.J. RB and cyclin dependent kinase pathways: Defining a distinction between RB and p16 loss in lung cancer. Oncogene 2002, 21, 6908–6914. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Behrens, C.; Virmani, A.K.; Mele, G.; Milchgrub, S.; Girard, L.; Fondon, J.W., 3rd; Garner, H.R.; McKay, B.; Latif, F.; et al. High resolution chromosome 3p allelotyping of human lung cancer and preneoplastic/preinvasive bronchial epithelium reveals multiple, discontinuous sites of 3p allele loss and 3 regions of frequent breakpoints. Cancer Res. 2000, 60, 1949–1960. [Google Scholar]
- Wistuba, I.I.; Berry, J.; Behrens, C.; Maitra, A.; Shivapurkar, N.; Milchgrub, S.; Mackay, B.; Minna, J.D.; Gazdar, A.F. Molecular changes in the bronchial epithelium of patients with small cell lung cancer. Clin. Cancer Res. 2000, 6, 2604–2610. [Google Scholar]
- Bordi, P.; Tiseo, M.; Barbieri, F.; Bavieri, M.; Sartori, G.; Marchetti, A.; Buttitta, F.; Bortesi, B.; Ambrosini-Spaltro, A.; Gnetti, L.; et al. Gene mutations in small-cell lung cancer (SCLC): Results of a panel of 6 genes in a cohort of Italian patients. Lung Cancer 2014, 86, 324–328. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Elkadi, O.R.; Tarasen, A.; Foulke, L.; Sheehan, C.E.; Otto, G.A.; Palmer, G.; Yelensky, R.; Lipson, D.; et al. Next-generation sequencing reveals frequent consistent genomic alterations in small cell undifferentiated lung cancer. J. Clin. Pathol. 2014, 67, 772–776. [Google Scholar] [CrossRef]
- Wakuda, K.; Kenmotsu, H.; Serizawa, M.; Koh, Y.; Isaka, M.; Takahashi, S.; Ono, A.; Taira, T.; Naito, T.; Murakami, H.; et al. Molecular profiling of small cell lung cancer in a Japanese cohort. Lung Cancer 2014, 84, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Metro, G.; Duranti, S.; Fischer, M.J.; Cappuzzo, F.; Crinò, L. Emerging drugs for small cell lung cancer—An update. Expert Opin. Emerg. Drugs 2012, 17, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Durinck, S.; Stawiski, E.W.; Poirier, J.T.; Modrusan, Z.; Shames, D.S.; Bergbower, E.A.; Guan, Y.; Shin, J.; Guillory, J.; et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012, 44, 1111–1116. [Google Scholar] [CrossRef]
- Umemura, S.; Mimaki, S.; Makinoshima, H.; Tada, S.; Ishii, G.; Ohmatsu, H.; Niho, S.; Yoh, K.; Matsumoto, S.; Takahashi, A.; et al. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J. Thorac. Oncol. 2014, 9, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Tsuchihara, K.; Goto, K. Genomic profiling of small-cell lung cancer: The era of targeted therapies. Jpn. J. Clin. Oncol. 2015, 45, 513–519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Badzio, A.; Wynes, M.W.; Dziadziuszko, R.; Merrick, D.T.; Pardo, M.; Rzyman, W.; Kowalczyk, A.; Singh, S.; Ranger-Moore, J.; Manriquez, G.; et al. Increased insulin-like growth factor 1 receptor protein expression and gene copy number in small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Potti, A.; Moazzam, N.; Ramar, K.; Hanekom, D.S.; Kargas, S.; Koch, M. CD117 (c-KIT) overexpression in patients with extensive-stage small-cell lung carcinoma. Ann. Oncol. 2003, 14, 894–897. [Google Scholar] [CrossRef]
- Park, K.S.; Martelotto, L.G.; Peifer, M.; Sos, M.L.; Karnezis, A.N.; Mahjoub, M.R.; Bernard, K.; Conklin, J.F.; Szczepny, A.; Yuan, J.; et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat. Med. 2011, 17, 1504–1508. [Google Scholar] [CrossRef]
- Belani, C.P.; Dahlberg, S.E.; Rudin, C.M.; Fleisher, M.; Chen, H.X.; Takebe, N.; Ramalingam, S.S.; Schiller, J.H. Three-arm randomized phase II study of cisplatin and etoposide (CE) versus CE with either vismodegib (V) or cixutumumab (Cx) for patients with extensive stage-small cell lung cancer (ES-SCLC) (ECOG 1508). J. Clin. Oncol. 2013, 31, 7508. [Google Scholar] [CrossRef]
- Johnson, B.E.; Fischer, T.; Fischer, B.; Dunlop, D.; Rischin, D.; Silberman, S.; Kowalski, M.O.; Sayles, D.; Dimitrijevic, S.; Fletcher, C.; et al. Phase II study of imatinib in patients with small cell lung cancer. Clin. Cancer Res. 2003, 9, 5880–5887. [Google Scholar]
- Pandya, K.J.; Dahlberg, S.; Hidalgo, M.; Cohen, R.B.; Lee, M.W.; Schiller, J.H.; Johnson, D.H.; Eastern Cooperative Oncology Group. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small cell lung cancer who have responding or stable disease after induction chemotherapy: A trial of the Eastern Cooperative Oncology Group (E1500). J. Thorac. Oncol. 2007, 2, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Kalemkerian, G.P.; Ramnath, N.; Kraut, M.J.; Wozniak, A.J.; Worden, F.P.; Ruckdeschel, J.C.; Zhang, X.; Chen, W.; Gadgeel, S.M. Phase II trial of imatinib maintenance therapy after irinotecan and cisplatin in patients with c-Kit-positive, extensive-stage small-cell lung cancer. Clin. Lung Cancer 2010, 11, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Hainsworth, J.D.; Simons, L.; Meng, C.; Burris, H.A., 3rd; Yardley, D.A.; Grapski, R.; Schreeder, M.; Mallidi, P.V.; Greco, F.A.; et al. Irinotecan, carboplatin, and imatinib in untreated extensive-stage small-cell lung cancer: A phase II trial of the Minnie Pearl Cancer Research Network. J. Thorac. Oncol. 2007, 2, 854–861. [Google Scholar] [CrossRef]
- Krug, L.M.; Crapanzano, J.P.; Azzoli, C.G.; Miller, V.A.; Rizvi, N.; Gomez, J.; Kris, M.G.; Pizzo, B.; Tyson, L.; Dunne, M.; et al. Imatinib mesylate lacks activity in small cell lung carcinoma expressing c-kit protein: A phase II clinical trial. Cancer 2005, 103, 2128–2131. [Google Scholar] [CrossRef]
- Moore, A.M.; Einhorn, L.H.; Estes, D.; Govindan, R.; Axelson, J.; Vinson, J.; Breen, T.E.; Yu, M.; Hanna, N.H. Gefitinib in patients with chemo-sensitive and chemo-refractory relapsed small cell cancers: A Hoosier Oncology Group phase II trial. Lung Cancer 2006, 52, 93–97. [Google Scholar] [CrossRef]
- Tarhini, A.; Kotsakis, A.; Gooding, W.; Shuai, Y.; Petro, D.; Friedland, D.; Belani, C.P.; Dacic, S.; Argiris, A. Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin. Cancer Res. 2010, 16, 5900–5907. [Google Scholar] [CrossRef]
- Sattler, M.; Salgia, R. Molecular and cellular biology of small cell lung cancer. Semin. Oncol. 2003, 30, 57–71. [Google Scholar] [CrossRef]
- Bikfalvi, A.; Klein, S.; Pintucci, G.; Rifkin, D.B. Biological roles of fibroblast growth factor-2. Endocr. Rev. 1997, 18, 26–45. [Google Scholar]
- Ruotsalainen, T.; Joensuu, H.; Mattson, K.; Salven, P. High Pretreatment Serum Concentration of Basic Fibroblast Growth Factor Is a Predictor of Poor Prognosis in Small Cell Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1492–1495. [Google Scholar]
- Dai, S.; Zhou, Z.; Chen, Z.; Xu, G.; Chen, Y. Fibroblast Growth Factor Receptors (FGFRs): Structures and Small Molecule Inhibitors. Cells 2019, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.P.; Aebersold, D.M.; Zimmer, Y.; Medová, M. MET targeting: Time for a rematch. Oncogene 2020, 39, 2845–2862. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Adjei, A.A. FGFR signaling as a target for lung cancer therapy. J. Thorac. Oncol. 2016, 11, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Salgia, R.; Wang, X.; Hodgson, L.D.; Masters, G.A.; Green, M.; Vokes, E.E. Randomized phase II study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J. Clin. Oncol. 2008, 26, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; de Oliveira, M.R.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S.; et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Lochmann, T.L.; Floros, K.V.; Naseri, M.; Powell, K.M.; Cook, W.; March, R.J.; Stein, G.T.; Greninger, P.; Maves, Y.K.; Saunders, L.R.; et al. Venetoclax is effective in small-cell lung cancers with high BCL-2 expression. Clin. Cancer Res. 2018, 24, 360–369. [Google Scholar] [CrossRef]
- Rudin, C.M.; Poirier, J.T.; Byers, L.A.; Dive, C.; Dowlati, A.; George, J.; Heymach, J.V.; Johnson, J.E.; Lehman, J.M.; MacPherson, D.; et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer 2019, 19, 289–297. [Google Scholar] [CrossRef]
- Simos, D.; Sajjady, G.; Sergi, M.; Liew, M.S.; Califano, R.; Ho, C.; Leighl, N.; White, S.; Summers, Y.; Petrcich, W.; et al. Third-line chemotherapy in small-cell lung cancer: An international analysis. Clin. Lung Cancer 2014, 15, 110–118. [Google Scholar] [CrossRef]
- Sos, M.L.; Dietlein, F.; Peifer, M.; Schöttle, J.; Balke-Want, H.; Müller, C.; Koker, M.; Richters, A.; Heynck, S.; Malchers, F.; et al. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 17034–17039. [Google Scholar] [CrossRef]
- Mertz, J.A.; Conery, A.R.; Bryant, B.M.; Sandy, P.; Balasubramanian, S.; Mele, D.A.; Bergeron, L.; Sims, R.J., 3rd. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. USA 2011, 108, 16669–16674. [Google Scholar] [CrossRef]
- Owonikoko, T.; Nackaerts, K.; Csoszi, T.; Ostoros, G.; Baik, C.; Ullmann, C.D.; Zagadailov, E.; Sheldon-Waniga, E.; Huebner, D. OA05.05 randomized phase 2 study: Alisertib (MLN8237) or placebo + paclitaxel as second-line therapy for small-cell lung cancer (SCLC). J. Thorac. Oncol. 2017, 12, S261–S262. [Google Scholar] [CrossRef]
- Mir, S.E.; De Witt Hamer, P.C.; Krawczyk, P.M.; Balaj, L.; Claes, A.; Niers, J.M.; Van Tilborg, A.A.G.; Zwinderman, A.H.; Geerts, D.; Kaspers, G.J.L.; et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 2010, 18, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Tong, P.; Diao, L.; Li, L.; Fan, Y.; Hoff, J.; Heymach, J.V.; Wang, J.; Byers, L.A. Targeting AXL and mTOR pathway overcomes primary and acquired resistance to WEE1 inhibition in small-cell lung cancer. Clin. Cancer Res. 2017, 23, 6239–6253. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; McGrail, D.J.; Sun, C.; Labrie, M.; Chen, X.; Zhang, D.; Ju, Z.; Vellano, C.P.; Lu, Y.; Li, Y.; et al. Sequential therapy with PARP and WEE1 inhibitors minimizes toxicity while maintaining efficacy. Cancer Cell 2019, 35, 851.e7–867.e7. [Google Scholar] [CrossRef] [PubMed]
- Cardnell, R.J.; Feng, Y.; Mukherjee, S.; Diao, L.; Tong, P.; Stewart, C.A.; Masrorpour, F.; Fan, Y.; Nilsson, M.; Shen, Y.; et al. Activation of the PI3K/mTOR pathway following PARP inhibition in small cell Lung cancer. PLoS ONE 2016, 11, e0152584. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Dahlberg, S.E.; Sica, G.L.; Wagner, L.I.; Wade, J.L.; Srkalovic, G.; Lash, B.W.; Leach, J.W.; Leal, T.B.; Aggarwal, C.; et al. Randomized phase II trial of cisplatin and etoposide in combination with veliparib or placebo for extensive-stage small-cell lung cancer: ECOG-ACRIN 2511 study. J. Clin. Oncol. 2018, 3, 222–229. [Google Scholar] [CrossRef]
- Inno, A.; Stagno, A.; Gori, S. Schlafen-11 (SLFN11): A step forward towards personalized medicine in small-cell lung cancer? Transl. Lung Cancer Res. 2018, 7 (Suppl. S4), S341–S345. [Google Scholar] [CrossRef]
- Laird, J.H.; Lok, B.H.; Ma, J.; Bell, A.; de Stanchina, E.; Poirier, J.T.; Rudin, C.M. Talazoparib is a potent radiosensitizer in small cell lung cancer cell lines and xenografts. Clin. Cancer Res. 2018, 24, 5143–5152. [Google Scholar] [CrossRef]
- Kunnimalaiyaan, M.; Chen, H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist 2007, 12, 535–542. [Google Scholar] [CrossRef]
- Chapman, G.; Sparrow, D.B.; Kremmer, E.; Dunwoodie, S.L. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum. Mol. Genet. 2011, 20, 905–916. [Google Scholar] [CrossRef]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C.; Aujay, M.A.; Bheddah, S.; Black, K.; Desai, R.; Escarpe, P.A.; Hampl, J.; Laysang, A.; et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor initiating cells in vivo. Sci. Transl. Med. 2015, 7, 302ra136. [Google Scholar] [CrossRef]
- Morgensztern, D.; Besse, B.; Greillier, L.; Santana-Davila, R.; Ready, N.; Hann, C.L.; Glisson, B.S.; Farago, A.F.; Dowlati, A.; Rudin, C.M.; et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-Expressing, relapsed/refractory small-cell lung cancer: Results from the phase II trinity study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Cancer stem cell candidate Rova-T discontinued. Nat. Rev. Drug Discov. 2019, 18, 814. [Google Scholar] [CrossRef] [PubMed]
- Mumby, M. PP2A: Unveiling a reluctant tumor suppressor. Cell 2007, 130, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, D.; Neviani, P. Protein phosphatase 2A: A target for anticancer therapy. Lancet Oncol. 2013, 14, e229–e238. [Google Scholar] [CrossRef]
- Neviani, P.; Perrotti, D. SETting OP449 into the PP2A-activating drug family. Clin. Cancer Res. 2014, 20, 2026–2028. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Nagahara, Y.; Ikekita, M.; Shinomiya, T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br. J. Pharmacol. 2003, 138, 1303–1312. [Google Scholar] [CrossRef]
- Cristóbal, I.; Madoz-Gúrpide, J.; Manso, R.; González-Alonso, P.; Rojo, F.; García-Foncillas, J. Potential anti-tumor effects of FTY720 associated with PP2A activation: A brief review. Curr. Med. Res. Opin. 2016, 32, 1137–1141. [Google Scholar] [CrossRef]
- Gutierrez, A.; Pan, L.; Groen, R.W.J.; Baleydier, F.; Kentsis, A.; Marineau, J.; Grebliunaite, R.; Kozakewich, E.; Reed, C.; Pflumio, F.; et al. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J. Clin. Investig. 2014, 124, 644–655. [Google Scholar] [CrossRef]
- Kauko, O.; O’Connor, C.M.; Kulesskiy, E.; Sangodkar, J.; Aakula, A.; Izadmehr, S.; Yetukuri, L.; Yadav, B.; Padzik, A.; Laajala, T.D.; et al. PP2A inhibition is a druggable MEK inhibitor resistance mechanism in KRAS-mutant lung cancer cells. Sci. Transl. Med. 2018, 10, eaaq1093. [Google Scholar] [CrossRef]
- Shimizu, T.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Smith, L.S.; Gunn, S.; Smetzer, L.; Mays, T.A.; Kaiser, B.; et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin. Cancer Res. 2012, 18, 2316–2325. [Google Scholar] [CrossRef]
- Chung, V.; Mansfield, A.S.; Braiteh, F.; Richards, D.; Durivage, H.; Ungerleider, R.S.; Johnson, F.; Kovach, J.S. Safety, Tolerability, and Preliminary Activity of LB-100, an Inhibitor of Protein Phosphatase 2A, in Patients with Relapsed Solid Tumors: An Open-Label, Dose Escalation, First-in-Human, Phase I Trial. Clin. Cancer Res. 2017, 23, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; Wang, H.; Maggio, D.; Kovach, J.S.; Zhang, Q.; Song, Q.; Marincola, F.M.; Heiss, J.D.; Gilbert, M.R.; Lu, R.; et al. Pharmacologic inhibition of protein phosphatase-2A achieves durable immune-mediated antitumor activity when combined with PD-1 blockade. Nat. Commun. 2018, 9, 2126. [Google Scholar] [CrossRef] [PubMed]
- Taffs, R.E.; Redegeld, F.A.; Sitkovsky, M.V. Modulation of cytolytic T lymphocyte functions by an inhibitor of serine/threonine phosphatase, okadaic acid. Enhancement of cytolytic T lymphocyte-mediated cytotoxicity. J. Immunol. 1991, 147, 722–728. [Google Scholar] [PubMed]
- Cui, J.; Wang, H.; Medina, R.; Zhang, Q.; Xu, C.; Indig, I.H.; Zhou, J.; Song, Q.; Dmitriev, P.; Sun, M.Y.; et al. Inhibition of PP2A with LB-100 Enhances Efficacy of CAR-T Cell Therapy Against Glioblastoma. Cancers 2020, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Ho, W.; Zhang, C.; Yang, C.; Elder, J.B.; Zhuang, Z. LB100, a small molecule inhibitor of PP2A with potent chemo- and radio-sensitizing potential. Cancer Biol. Ther. 2015, 16, 821–833. [Google Scholar] [CrossRef]
- Cui, Q.; Wen, S.; Huang, P. Targeting cancer cell mitochondria as a therapeutic approach: Recent updates. Future Med. Chem. 2017, 9, 929–949. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M.; Quari, M.; Lopez, M.; Joseph, J.; Zielonka, J.; Dwinell, M.B. A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox Biol. 2018, 14, 316–327. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Birrer, M.J.; DiCarlo, B.A.; Gaillard, S.; Martin, L.P.; Nemunaitis, J.J.; Perez, R.P.; Schilder, R.J.; Annunziata, C.M.; Begley, C.G.; et al. A phase 1b, open-label, non-randomized multicenter study of birinapant in combination with conatumumab in subjects with relapsed epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer. J. Clin. Oncol. 2015, 33, 5571. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Smith, D.C.; Pitot, H.C.; Brill, J.M.; Chugh, R.; Rouits, E.; Rubin, J.; Strickler, J.; Vuagniaux, G.; Sorensen, J.M.; et al. Safety, pharmacokinetics and pharmacodynamics properties of oral DEBIO1143 (AT-406) in patients with advanced cancer: Results of a first-in-man study. Cancer Chemother. Pharmacol. 2015, 75, 851–859. [Google Scholar] [CrossRef]
- Bendell, J.C.; Patel, M.R.; Infante, J.R.; Kurkjian, C.D.; Jones, S.F.; Pant, S.; Burris, H.A., 3rd; Moreno, O.; Esquibel, V.; Levin, W.; et al. Phase 1, open-label, dose escalation, safety, and pharmacokinetics study of ME-344 as a single agent in patients with refractory solid tumors. Cancer 2015, 121, 1056–1063. [Google Scholar] [CrossRef]
- Diamond, J.R.; Goff, B.; Forster, M.D.; Bendell, J.C.; Britten, C.D.; Gordon, M.S.; Gabra, H.; Waterhouse, D.M.; Poole, M.; Camidge, D.R.; et al. Phase Ib study of the mitochondrial inhibitor ME-344 plus topotecan in patients with previously treated, locally advanced or metastatic small cell lung, ovarian and cervical cancers. Investig. New Drugs 2017, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Greeno, E.; Borazanci, E.H.; Gockerman, J.P.; Korn, R.L.; Saluja, A.; VonHoff, D.D. Phase I dose escalation and pharmokinetic study of a modified schedule of 14-ophosphonooxymethyltriptolide. J. Clin. Oncol. 2016, 34, TPS472. [Google Scholar] [CrossRef]
- Stein, M.N.; Bertino, J.R.; Kaufman, H.L.; Mayer, T.; Moss, R.; Silk, A.; Chan, N.; Malhotra, J.; Rodriguez, L.; Aisner, J.; et al. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin. Cancer Res. 2017, 23, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Roth, K.G.; Mambetsariev, I.; Kulkarni, P.; Salgia, R. The mitochondrion as an emerging therapeutic target in cancer. Trends Mol. Med. 2020, 26, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.A.; Shimono, Y.; Qian, D.; Clarke, M.F. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 2007, 23, 675–699. [Google Scholar] [CrossRef]
- Yang, Y.M.; Chang, J.W. Current status and issues in cancer stem cell study. Cancer Investig. 2008, 26, 741–755. [Google Scholar] [CrossRef]
- Wicha, M.S.; Liu, S.; Dontu, G. Cancer stem cells: An old idea—A paradigm shift. Cancer Res. 2006, 66, 1883–1896. [Google Scholar] [CrossRef]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef]
- Codony-Servat, J.; Verlicchi, A.; Rosell, R. Cancer stem cells in small cell lung cancer. Transl. Lung Cancer Res. 2016, 5, 16–25. [Google Scholar]
- Stewart, C.A.; Byers, L.A. Altering the course of small cell lung cancer: Targeting cancer stem cells via LSD1 inhibition. Cancer Cell 2015, 28, 4–6. [Google Scholar] [CrossRef]
- Bauer, T.M.; Besse, B.; Martinez-Marti, A.; Trigo, J.M.; Moreno, V.; Garrido, P.; Ferron-Brady, G.; Wu, Y.; Park, J.; Collingwood, T.; et al. Phase I, Open-Label, Dose-Escalation Study of the Safety, Pharmacokinetics, Pharmacodynamics, and Efficacy of GSK2879552 in Relapsed/Refractory SCLC. J. Thorac. Oncol. 2019, 14, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.P.; Kruger, R.G. Antitumor activity of LSD1 inhibitors in Lung cancer. Mol. Cell Oncol. 2016, 3, e1117700. [Google Scholar] [CrossRef] [PubMed]
- Ropacki, M.; Navarro, A.; Maes, T.; Gutierrez, S.; Bullock, R.; Buesa, C. P2.12-04 CLEPSIDRA: A Phase II Trial Combining Iadademstat with Platinum-Etoposide in Platinum-Sensitive Relapsed SCLC Patients. J. Thorac. Oncol. 2019, 14, S813. [Google Scholar] [CrossRef]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.-H.; et al. LSD1 ablation stimulates anti-tumor immunity and enables checkpoint blockade. Cell 2018, 174, 549–563. [Google Scholar] [CrossRef]
- Morgensztern, D.; Rose, M.; Waqar, S.N.; Morris, J.; Ma, P.C.; Reid, T.; Brzezniak, C.E.; Zeman, K.G.; Padmanabhan, A.; Hirth, J.; et al. RRx-001 followed by platinum plus etoposide in patients with previously treated small-cell lung cancer. Br. J. Cancer 2019, 121, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, K.; Jahchan, N.S.; Schnorr, P.J.; Cristea, S.; Ring, A.M.; Maute, R.L.; Volkmer, A.K.; Volkmer, J.-P.; Liu, J.; Lim, J.S.; et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J. Clin. Investig. 2016, 126, 2610–2620. [Google Scholar] [CrossRef]
- Bonanno, L.; Pavan, A.; Dieci, M.V.; Di Liso, E.; Schiavon, M.; Comacchio, G.; Attili, I.; Pasello, G.; Calabrese, F.; Rea, F.; et al. The role of immune microenvironment in small-cell lung cancer: Distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. Eur. J. Cancer 2018, 101, 191–200. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rizvi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.; et al. Tumor mutational burden and efficacy of Nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018, 33, 853.e4–861.e4. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P.; Dahlberg, S.E.; Rudin, C.M.; Fleisher, M.; Chen, H.X.; Takebe, N.; Velasco, M.R., Jr.; Tester, W.J.; Sturtz, K.; Hann, C.L.; et al. Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508). Cancer 2016, 122, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Pietanza, M.C.; Litvak, A.M.; Varghese, A.M.; Krug, L.M.; Fleisher, M.; Teitcher, J.B.; Holodny, A.I.; Sima, C.S.; Woo, K.M.; Ng, K.K.; et al. A phase I trial of the Hedgehog inhibitor, sonidegib (LDE225), in combination with etoposide and cisplatin for the initial treatment of extensive stage small cell lung cancer. Lung Cancer 2016, 99, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Almodovar, K.; Iams, W.T.; Meador, C.B.; Zhao, Z.; York, S.; Horn, L.; Yan, Y.; Hernandez, J.; Chen, H.; Shyr, Y.; et al. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J. Thorac. Oncol. 2018, 13, 112–123. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Trigo Perez, J.M.; Besse, B.; Moreno, V.; Lopez, R.; Sala, M.A.; Aix, S.P.; Fernandez, C.M.; Siguero, M.; Kahatt, C.M.; et al. Efficacy and safety profile of lurbinectedin in second-line SCLC patients: Results from a phase II single-agent trial. J. Clin. Oncol. 2019, 37, 8506. [Google Scholar] [CrossRef]
- Kontogianni, K.; Nicholson, A.G.; Butcher, D.; Sheppard, M.N. CD56: A useful tool for the diagnosis of small cell lung carcinomas on biopsies with extensive crush artefact. J. Clin. Pathol. 2005, 58, 978–980. [Google Scholar] [CrossRef]
- Yu, L.; Lu, Y.; Yao, Y.; Liu, Y.; Wang, Y.; Lai, Q.; Zhang, R.; Li, W.; Wang, R.; Fu, Y.; et al. Promiximab-duocarmycin, a new CD56 antibody-drug conjugates, is highly efficacious in small cell lung cancer xenograft models. Oncotarget 2017, 9, 5197–5207. [Google Scholar] [CrossRef]
- Zaman, S.; Jadid, H.; Denson, A.C.; Gray, J.E. Targeting Trop-2 in solid tumors: Future prospects. Onco Targets Ther. 2019, 12, 1781–1790. [Google Scholar] [CrossRef]
- Ocean, A.J.; Starodub, A.N.; Bardia, A.; Vahdat, L.T.; Isakoff, S.J.; Guarino, M.; Messersmith, W.A.; Picozzi, V.J.; Mayer, I.A.; Wegener, W.A.; et al. Sacituzumab govitecan (IMMU-132), an Anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer 2017, 123, 3843–3854. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd; Berman, A.T.; Jabbour, S.K. Harnessing the potential synergy of combining radiation therapy and immunotherapy for thoracic malignancies. Transl. Lung Cancer Res. 2017, 6, 109–112. [Google Scholar] [CrossRef]
- McColl, K.; Wildey, G.; Sakre, N.; Lipka, M.B.; Behtaj, M.; Kresak, A.; Chen, Y.; Yang, M.; Velcheti, V.; Fu, P.; et al. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer. Oncotarget 2017, 8, 73745–73756. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Klingbeil, O.; He, X.Y.; Wu, X.S.; Arun, G.; Lu, B.; Somerville, T.; Milazzo, J.P.; Wilkinson, J.E.; Demerdash, O.E.; et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev. 2018, 32, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Polley, E.; Kunkel, M.; Evans, D.; Silvers, T.; Delosh, R.; Laudeman, J.; Ogle, C.; Reinhart, R.; Selby, M.; Connelly, J.; et al. Small cell lung cancer screen of oncology drugs, investigational agents, and gene and microRNA expression. J. Natl. Cancer Inst. 2016, 108, djw122. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Mollaoglu, G.; Guthrie, M.R.; Böhm, S.; Brägelmann, J.; Can, I.; Ballieu, P.M.; Marx, A.; George, J.; Heinen, C.; Chalishazar, M.D.; et al. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell 2017, 31, 270–285. [Google Scholar] [CrossRef]
- Sonkin, D.; Vural, S.; Thomas, A.; Teicher, B.A. Neuroendocrine negative SCLC is mostly RB1 WT and may be sensitive to CDK4/6 inhibition: Supplemental Tables. bioRxiv 2019. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subbiah, S.; Nam, A.; Garg, N.; Behal, A.; Kulkarni, P.; Salgia, R. Small Cell Lung Cancer from Traditional to Innovative Therapeutics: Building a Comprehensive Network to Optimize Clinical and Translational Research. J. Clin. Med. 2020, 9, 2433. https://doi.org/10.3390/jcm9082433
Subbiah S, Nam A, Garg N, Behal A, Kulkarni P, Salgia R. Small Cell Lung Cancer from Traditional to Innovative Therapeutics: Building a Comprehensive Network to Optimize Clinical and Translational Research. Journal of Clinical Medicine. 2020; 9(8):2433. https://doi.org/10.3390/jcm9082433
Chicago/Turabian StyleSubbiah, Shanmuga, Arin Nam, Natasha Garg, Amita Behal, Prakash Kulkarni, and Ravi Salgia. 2020. "Small Cell Lung Cancer from Traditional to Innovative Therapeutics: Building a Comprehensive Network to Optimize Clinical and Translational Research" Journal of Clinical Medicine 9, no. 8: 2433. https://doi.org/10.3390/jcm9082433
APA StyleSubbiah, S., Nam, A., Garg, N., Behal, A., Kulkarni, P., & Salgia, R. (2020). Small Cell Lung Cancer from Traditional to Innovative Therapeutics: Building a Comprehensive Network to Optimize Clinical and Translational Research. Journal of Clinical Medicine, 9(8), 2433. https://doi.org/10.3390/jcm9082433






