Global Gene Expression Characterization of Circulating Tumor Cells in Metastasic Castration-Resistant Prostate Cancer Patients
Abstract
1. Introduction
2. Experimental Section
2.1. Patients
2.2. CTC Isolation
2.3. Global Gene Expression Analysis
2.4. CTCs Markers Validation by qRT-PCR
2.5. Statistical Analysis
3. Results
3.1. CTC Immunoisolation and Global Gene Expression Analysis
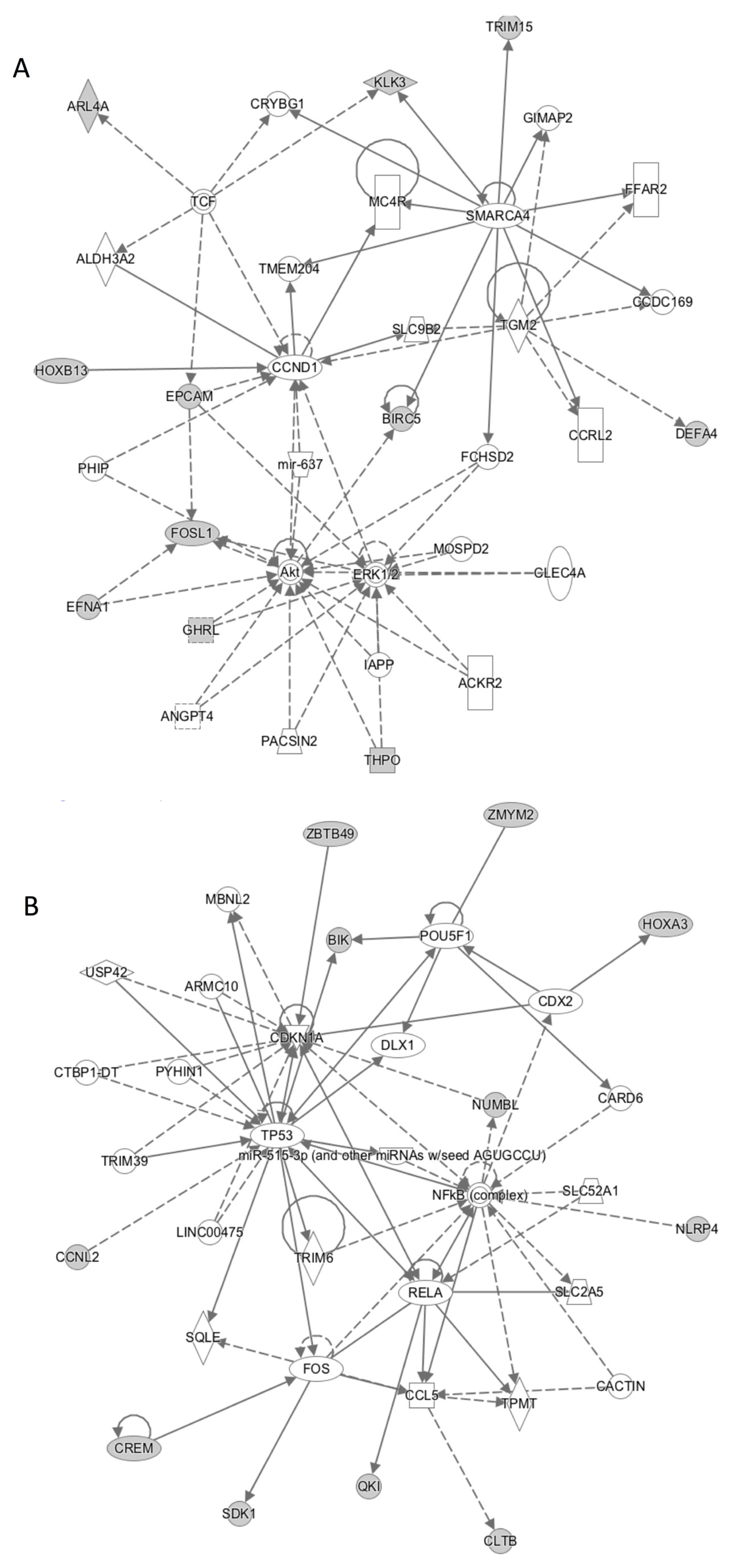
3.2. Biology of CTCs Isolated from mCRPC.
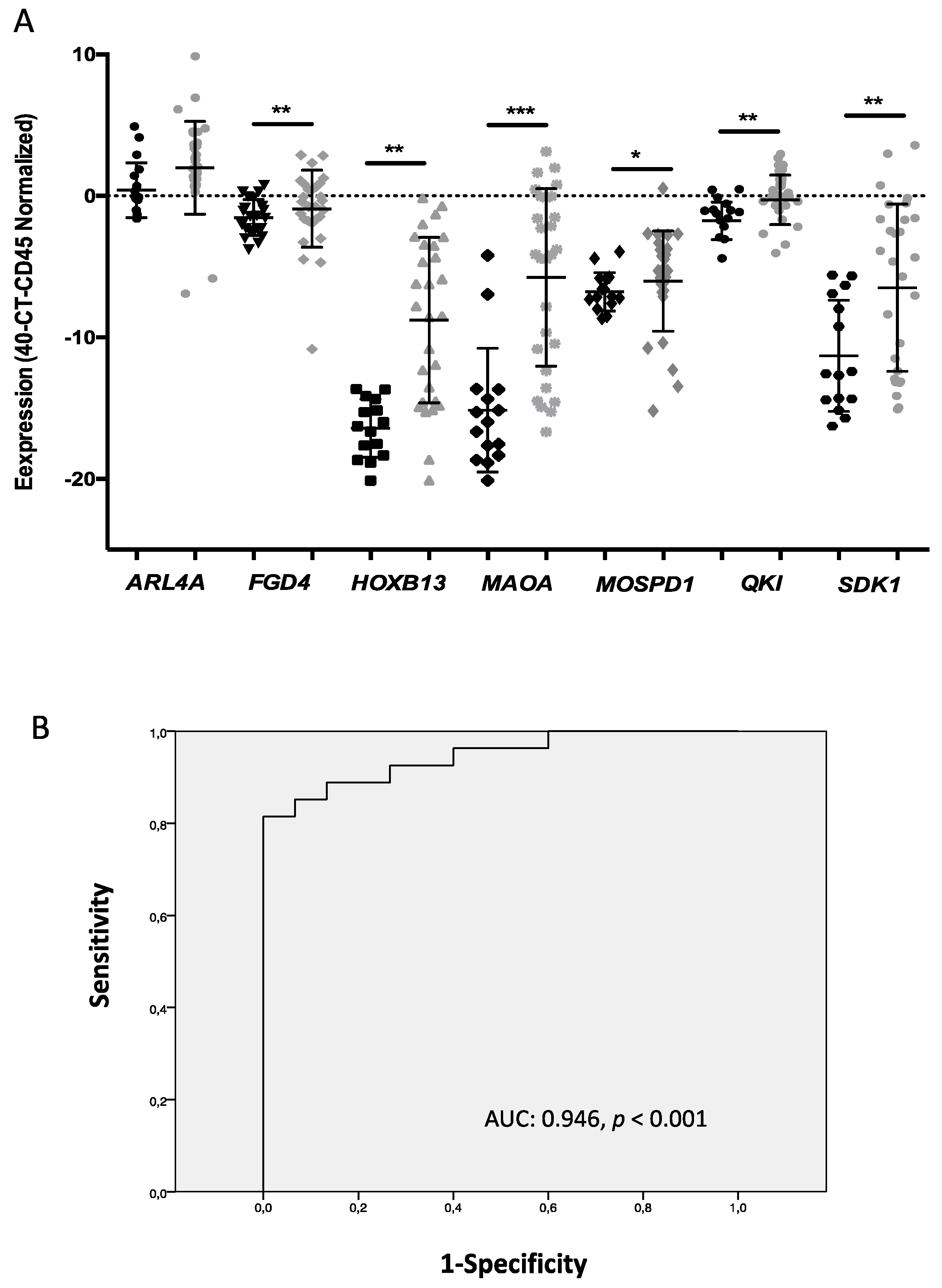
3.3. Validation of the CTC Gene Expression Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Climent, M.Á.; León-Mateos, L.; González del Alba, A.; Pérez-Valderrama, B.; Méndez-Vidal, M.J.; Mellado, B.; Arranz, J.Á.; Sánchez-Hernández, A.; Cassinello, J.; Olmos, D.; et al. Updated recommendations from the Spanish Oncology Genitourinary Group for the treatment of patients with metastatic castration-resistant prostate cancer. Crit. Rev. Oncol. Hematol. 2014, 96, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of advanced prostate cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Lu, C.; Chen, Y.; Paller, C.J.; Carducci, M.A.; Eisenberger, M.A.; Luo, J.; Antonarakis, E.S. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann. Oncol. 2015, 26, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Dianat-Moghadam, H.; Azizi, M.; Eslami-S, Z.; Cortés-Hernández, L.E.; Heidarifard, M.; Nouri, M.; Alix-Panabières, C. The role of circulating tumor cells in the metastatic cascade: Biology, technical challenges, and clinical relevance. Cancers 2020, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.W.M.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Heller, G.; Molina, A.; Attard, G.; Danila, D.C.; Jia, X.; Peng, W.; Sandhu, S.K.; Olmos, D.; Riisnaes, R.; et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2015, 33, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Goldkorn, A.; Ely, B.; Quinn, D.I.; Tangen, C.M.; Fink, L.M.; Xu, T.; Twardowski, P.; Van Veldhuizen, P.J.; Agarwal, N.; Carducci, M.A.; et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: A phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 1136–1142. [Google Scholar] [CrossRef]
- Heller, G.; McCormack, R.; Kheoh, T.; Molina, A.; Smith, M.R.; Dreicer, R.; Saad, F.; de Wit, R.; Aftab, D.T.; Hirmand, M.; et al. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: A comparison with prostate-specific antigen across five randomized phase III clinical trials. J. Clin. Oncol. 2018, 36, 572–580. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first & second-line abiraterone & enzalutamide. J. Clin. Oncol. 2017, 35, 2149–2156. [Google Scholar] [PubMed]
- Onstenk, W.; Sieuwerts, A.M.; Kraan, J.; Van, M.; Nieuweboer, A.J.M.; Mathijssen, R.H.J.; Hamberg, P.; Meulenbeld, H.J.; De Laere, B.; Dirix, L.Y.; et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur. Urol. 2015, 68, 939–945. [Google Scholar] [CrossRef]
- León-Mateos, L.; Casas, H.; Abalo, A.; Vieito, M.; Abreu, M.; Anido, U.; Gómez-Tato, A.; López, R.; Abal, M.; Muinelo-Romay, L. Improving circulating tumor cells enumeration and characterization to predict outcome in first line chemotherapy mCRPC patients. Oncotarget 2017, 8, 54708–54721. [Google Scholar] [CrossRef]
- Barbazán, J.; Vieito, M.; Abalo, A.; Alonso-Alconada, L.; Muinelo-Romay, L.; Alonso-Nocelo, M.; León, L.; Candamio, S.; Gallardo, E.; Anido, U.; et al. A logistic model for the detection of circulating tumour cells in human metastatic colorectal cancer. J. Cell. Mol. Med. 2012, 16, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, J.; Alonso-Nocelo, M.; Muinelo-Romay, L.; Barbazan, J.; Vieito, M.; Abalo, A.; Gomez-Tato, A.; de los Angeles, C.d.C.M.; Garcia-Caballero, T.; Rodriguez, C.; et al. Molecular profiling of circulating tumour cells identifies Notch1 as a principal regulator in advanced non-small cell lung cancer. Sci. Rep. 2016, 6, 37820. [Google Scholar] [CrossRef] [PubMed]
- Klebanov, L.; Yakovlev, A. How high is the level of technical noise in microarray data? Biol. Direct 2007, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Zahurak, M.; Parmigiani, G.; Yu, W.; Scharpf, R.B.; Berman, D.; Schaeffer, E.; Shabbeer, S.; Cope, L. Pre-processing Agilent microarray data. BMC Bioinform. 2007, 8, 142. [Google Scholar] [CrossRef]
- Went, P.; Vasei, M.; Bubendorf, L.; Terracciano, L.; Tornillo, L.; Riede, U.; Kononen, J.; Simon, R.; Sauter, G.; Baeuerle, P.A. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br. J. Cancer 2006, 94, 128–135. [Google Scholar] [CrossRef]
- Lee, C.H.; Kantoff, P. Treatment of metastatic prostate cancer in 2018. JAMA Oncol. 2019, 5, 263–264. [Google Scholar] [CrossRef]
- Friedlander, T.W.; Premasekharan, G.; Paris, P.L. Looking back, to the future of circulating tumor cells. Pharmacol. Ther. 2014, 142, 271–280. [Google Scholar] [CrossRef]
- Lack, J.; Gillard, M.; Cam, M.; Paner, G.P.; VanderWeele, D.J. Circulating tumor cells capture disease evolution in advanced prostate cancer. J. Transl. Med. 2017, 15, 44. [Google Scholar] [CrossRef]
- Miyamoto, D.T.; Zheng, Y.; Wittner, B.S.; Lee, R.J.; Zhu, H.; Broderick, K.T.; Desai, R.; Fox, D.B.; Brannigan, B.W.; Trautwein, J.; et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 2015, 349, 1351–1356. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Doggen, C.J.M.; Attard, G.; de Bono, J.S.; Terstappen, L.W.M.M. All circulating EpCAM+CK+CD45-objects predict overall survival in castration-resistant prostate cancer. Ann. Oncol. 2010, 21, 1851–1857. [Google Scholar] [CrossRef]
- Massoner, P.; Thomm, T.; Mack, B.; Untergasser, G.; Martowicz, A.; Bobowski, K.; Klocker, H.; Gires, O.; Puhr, M. EpCAM is overexpressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200c/205. Br. J. Cancer 2014, 111, 955–964. [Google Scholar] [CrossRef]
- He, Y.; Gu, J.; Strom, S.; Logothetis, C.J.; Kim, J.; Wu, X. The prostate cancer susceptibility variant rs2735839 near KLK3 gene is associated with aggressive prostate cancer and can stratify gleason score 7 patients. Clin. Cancer Res. 2014, 20, 5133–5139. [Google Scholar] [CrossRef]
- Dijkstra, S.; Leyten, G.H.J.M.; Jannink, S.A.; De Jong, H.; Mulders, P.F.A.; Van Oort, I.M.; Schalken, J.A. KLK3, PCA3, and TMPRSS2-ERG expression in the peripheral blood mononuclear cell fraction from castration-resistant prostate cancer patients and response to docetaxel treatment. Prostate 2014, 74, 1222–1230. [Google Scholar] [CrossRef]
- Chen, M.E.; Lin, S.H.; Chung, L.W.K.; Sikes, R.A. Isolation and characterization of PAGE-1 and GAGE-7. New genes expressed in the LNCaP prostate cancer progression model that share homology with melanoma-associated antigens. J. Biol. Chem. 1998, 273, 17618–17625. [Google Scholar] [CrossRef]
- Wu, J.B.; Shao, C.; Li, X.; Li, Q.; Hu, P.; Shi, C.; Li, Y.; Chen, Y.T.; Yin, F.; Liao, C.P.; et al. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J. Clin. Invest. 2014, 124, 2891–2908. [Google Scholar] [CrossRef]
- Zabalza, C.V.; Adam, M.; Burdelski, C.; Wilczak, W.; Wittmer, C.; Kraft, S.; Krech, T.; Steurer, S.; Koop, C.; Hube-Magg, C.; et al. HOXB13 overexpression is an independent predictor of early PSA recurrence in prostate cancer treated by radical prostatectomy. Oncotarget 2015, 6, 12822–12823. [Google Scholar] [CrossRef]
- Larkin, S.E.T.; Holmes, S.; Cree, I.A.; Walker, T.; Basketter, V.; Bickers, B.; Harris, S.; Garbis, S.D.; Townsend, P.A.; Aukim-Hastie, C. Identification of markers of prostate cancer progression using candidate gene expression. Br. J. Cancer 2012, 106, 157–165. [Google Scholar] [CrossRef]
- Kishi, H.; Igawa, M.; Kikuno, N.; Yoshino, T.; Urakami, S.; Shiina, H. Expression of the survivin gene in prostate cancer: Correlation with clinicopathological characteristics, proliferative activity and apoptosis. J. Urol. 2004, 171, 1855–1860. [Google Scholar] [CrossRef]
- Koike, H.; Sekine, Y.; Kamiya, M.; Nakazato, H.; Suzuki, K. Gene expression of survivin and its spliced isoforms associated with proliferation and aggressive phenotypes of prostate cancer. Urology 2008, 72, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Takeuchi, M.; Kinoyama, I.; Minematsu, T.; Shirasuna, K.; Matsuhisa, A.; Kita, A.; Tominaga, F.; Yamanaka, K.; Kudoh, M.; et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007, 67, 8014–8021. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Speicher, M.R. The biology of circulating tumor cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Latham, D.E.; Delaney, M.A.; Chakravarti, A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene 2005, 24, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 2003, 22, 8581–8589. [Google Scholar] [CrossRef] [PubMed]
- Carver, B.S.; Tran, J.; Gopalan, A.; Chen, Z.; Shaikh, S.; Carracedo, A.; Alimonti, A.; Nardella, C.; Varmeh, S.; Scardino, P.T.; et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat. Genet. 2009, 41, 619–624. [Google Scholar] [CrossRef]
- Gao, H.; Ouyang, X.; Banach-Petrosky, W.A.; Gerald, W.L.; Shen, M.M.; Abate-Shen, C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 14477–14482. [Google Scholar] [CrossRef]
- Chappell, W.H.; Abrams, S.L.; Lertpiriyapong, K.; Fitzgerald, T.L.; Martelli, A.M.; Cocco, L.; Rakus, D.; Gizak, A.; Terrian, D.; Steelman, L.S.; et al. Novel roles of androgen receptor, epidermal growth factor receptor, TP53, regulatory RNAs, NF-kappa-B, chromosomal translocations, neutrophil associated gelatinase, and matrix metalloproteinase-9 in prostate cancer and prostate cancer stem cells. Adv. Biol. Regul. 2016, 60, 64–87. [Google Scholar] [CrossRef]
- Javed, S.; Langley, S.E.M. Importance of HOX genes in normal prostate gland formation, prostate cancer development and its early detection. BJU Int. 2014, 113, 535–540. [Google Scholar] [CrossRef]
- Ewing, C.M.; Ray, A.M.; Lange, E.M.; Zuhlke, K.A.; Robbins, C.M.; Tembe, W.D.; Wiley, K.E.; Isaacs, S.D.; Johng, D.; Wang, Y.; et al. Germline mutations in HOXB13 and prostate-cancer risk. N. Engl. J. Med. 2012, 366, 141–149. [Google Scholar] [CrossRef]
- Park, C.K.; Shin, S.J.; Cho, Y.A.; Joo, J.W.; Cho, N.H. HoxB13 expression in ductal type adenocarcinoma of prostate: Clinicopathologic characteristics and its utility as potential diagnostic marker. Sci. Rep. 2019, 9, 20205. [Google Scholar] [CrossRef]
- Miyamoto, D.T.; Lee, R.J.; Kalinich, M.; LiCausi, J.A.; Zheng, Y.; Chen, T.; Milner, J.D.; Emmons, E.; Ho, U.; Broderick, K.; et al. An RNA-based digital circulating tumor cell signature is predictive of drug response and early dissemination in prostate cancer. Cancer Discov. 2018, 8, 288–303. [Google Scholar] [CrossRef]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Liao, C.P.; Lin, T.P.; Li, P.C.; Geary, L.A.; Chen, K.; Vaikari, V.P.; Wu, J.B.; Lin, C.H.; Gross, M.E.; Shih, J.C. Loss of MAOA in epithelia inhibits adenocarcinoma development, cell proliferation and cancer stem cells in prostate. Oncogene 2018, 37, 5175–5190. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, F.; Lei, X.; Wei, D.; Lu, H.; Zhu, Z.; Xiang, A.; Ye, Z.; Wang, L.; Zheng, W.; et al. Androgen receptor-mediated upregulation of quaking affects androgen receptor-related prostate cancer development and anti-androgen receptor therapy. Mol. Med. Rep. 2018, 17, 8203–8211. [Google Scholar] [CrossRef]
- Bossan, A.; Ottman, R.; Andl, T.; Hasan, M.F.; Mahajan, N.; Coppola, D.; Chakrabarti, R. Expression of FGD4 positively correlates with the aggressive phenotype of prostate cancer. BMC Cancer 2018, 18, 1257. [Google Scholar] [CrossRef]
- Puiffe, M.L.; Le Page, C.; Filali-Mouhim, A.; Zietarska, M.; Ouellet, V.; Tonin, P.N.; Chevrette, M.; Provencher, D.M.; Mes-Masson, A.M. Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 2007, 9, 820–829. [Google Scholar] [CrossRef]
- Verone, A.R.; Duncan, K.; Godoy, A.; Yadav, N.; Bakin, A.; Koochekpour, S.; Jin, J.P.; Heemers, H.V. Androgen-responsive serum response factor target genes regulate prostate cancer cell migration. Carcinogenesis 2013, 34, 1737–1746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Mao, X.Y.; Liu, X.; Song, R.R.; Berney, D.; Lu, Y.J.; Ren, G. High frequency of the SDK1:AMACR fusion transcript in Chinese prostate cancer. Int. J. Clin. Exp. Med. 2015, 8, 15127–15136. [Google Scholar]
- Hawksworth, D.; Ravindranath, L.; Chen, Y.; Furusato, B.; Sesterhenn, I.A.; Mcleod, D.G.; Srivastava, S.; Petrovics, G. Overexpression of C-MYC oncogene in prostate cancer predicts biochemical recurrence. Prostate Cancer Prostatic Dis. 2010, 13, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Monga, J.; Subramani, D.; Bharathan, A.; Ghosh, J. Pharmacological and genetic targeting of 5-lipoxygenase interrupts c-Myc oncogenic signaling and kills enzalutamide-resistant prostate cancer cells via apoptosis. Sci. Rep. 2020, 10, 6649. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | Mean (Range) |
|---|---|
| 69.6 (52–80) | |
| Previous PT * surgery | n (%) |
| yes | 6 (21.4) |
| no | 22 (78.6) |
| ECOG, PS ** | |
| 0 | 7 (25) |
| 1 | 18 (64.3) |
| 2 | 3 (10.7) |
| Gleason score | |
| ≤7 | 15 (53.6) |
| >7 | 10 (35.7) |
| Unknown | 3 (10.7) |
| Metastasis location | |
| Bone only | 15 (53.6) |
| Lymph node + Bone | 10 (35.7) |
| Any + Lung | 3 (10.7) |
| PSA*** (ng/dL) | |
| Mean, range | 445 (2–3238) |
| Median, range | 136 (2–3238) |
| AP ****, (UI/L) | |
| Mean, range | 617 (77–3115) |
| Median, range | 461 (77–3115) |
| LDH ***** (UI/L) | |
| Mean, range | 503 (121–1136) |
| Median, range | 454 (121–1136) |
| Overall Survival (OS) | Progression Free Survival (PFS) | |||
|---|---|---|---|---|
| Marker | Mean (95% CI) | p-Value | Mean (95% CI) | p-Value |
| HOXB13 | ||||
| low | 31.77 (22.76-40.79) | 0.014 * | 8.20 (6.20–10.20) | 0.344 |
| high | 16.39 (8.86–23.92) | 6.68 (4.18–9.18) | ||
| MAOA | ||||
| low | 30.66 (18.97–42.35) | 0.338 | 8.73 (5.42–12.05) | 0.028 * |
| high | 23.51 (15.55–31.47) | 6.47 (4.42–8.51) | ||
| FGD4 | ||||
| low | 28.65 (18.49–38.81) | 0.725 | 8.74 (6.63–10.85) | 0.068 |
| high | 24.77 (15.55–34.00) | 6.06 (4.00–8.12) | ||
| MOSPD1 | ||||
| low | 30.01 (20.95–39.07) | 0.264 | 8.74 (6.74–10.74) | 0.006 * |
| high | 20.57 (10.15–30.98) | 5.39 (3.75–7.04) | ||
| QKI | ||||
| low | 29.27 (20.17–38.37) | 0.507 | 8.78 (6.87–10.70) | 0.022 * |
| high | 22.32 (11.38–33.27) | 5.34 (3.11–7.57) | ||
| SDK1 | ||||
| low | 28.59 (19.64–37.53) | 0.546 | 8.89 (7.07–10.56) | 0.014 * |
| high | 23.28 (12.21–34.35) | 4.70 (2.15–7.23) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Mateos, L.; Abalo, A.; Casas, H.; Anido, U.; Rapado-González, Ó.; Vieito, M.; Suárez-Cunqueiro, M.; Gómez-Tato, A.; Abal, M.; López-López, R.; et al. Global Gene Expression Characterization of Circulating Tumor Cells in Metastasic Castration-Resistant Prostate Cancer Patients. J. Clin. Med. 2020, 9, 2066. https://doi.org/10.3390/jcm9072066
León-Mateos L, Abalo A, Casas H, Anido U, Rapado-González Ó, Vieito M, Suárez-Cunqueiro M, Gómez-Tato A, Abal M, López-López R, et al. Global Gene Expression Characterization of Circulating Tumor Cells in Metastasic Castration-Resistant Prostate Cancer Patients. Journal of Clinical Medicine. 2020; 9(7):2066. https://doi.org/10.3390/jcm9072066
Chicago/Turabian StyleLeón-Mateos, Luis, Alicia Abalo, Helena Casas, Urbano Anido, Óscar Rapado-González, María Vieito, Mercedes Suárez-Cunqueiro, Antonio Gómez-Tato, Miguel Abal, Rafael López-López, and et al. 2020. "Global Gene Expression Characterization of Circulating Tumor Cells in Metastasic Castration-Resistant Prostate Cancer Patients" Journal of Clinical Medicine 9, no. 7: 2066. https://doi.org/10.3390/jcm9072066
APA StyleLeón-Mateos, L., Abalo, A., Casas, H., Anido, U., Rapado-González, Ó., Vieito, M., Suárez-Cunqueiro, M., Gómez-Tato, A., Abal, M., López-López, R., & Muinelo-Romay, L. (2020). Global Gene Expression Characterization of Circulating Tumor Cells in Metastasic Castration-Resistant Prostate Cancer Patients. Journal of Clinical Medicine, 9(7), 2066. https://doi.org/10.3390/jcm9072066







