Relationships between Pulmonary Hypertension Risk, Clinical Profiles, and Outcomes in Dilated Cardiomyopathy
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population and Protocol
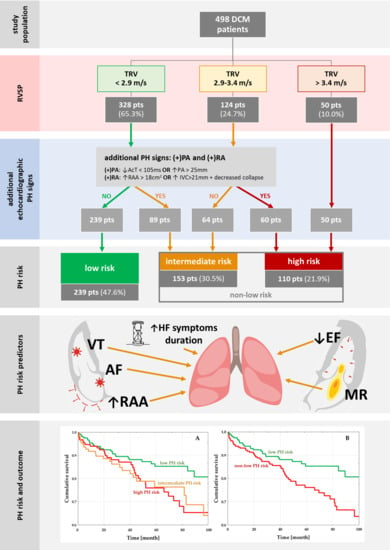
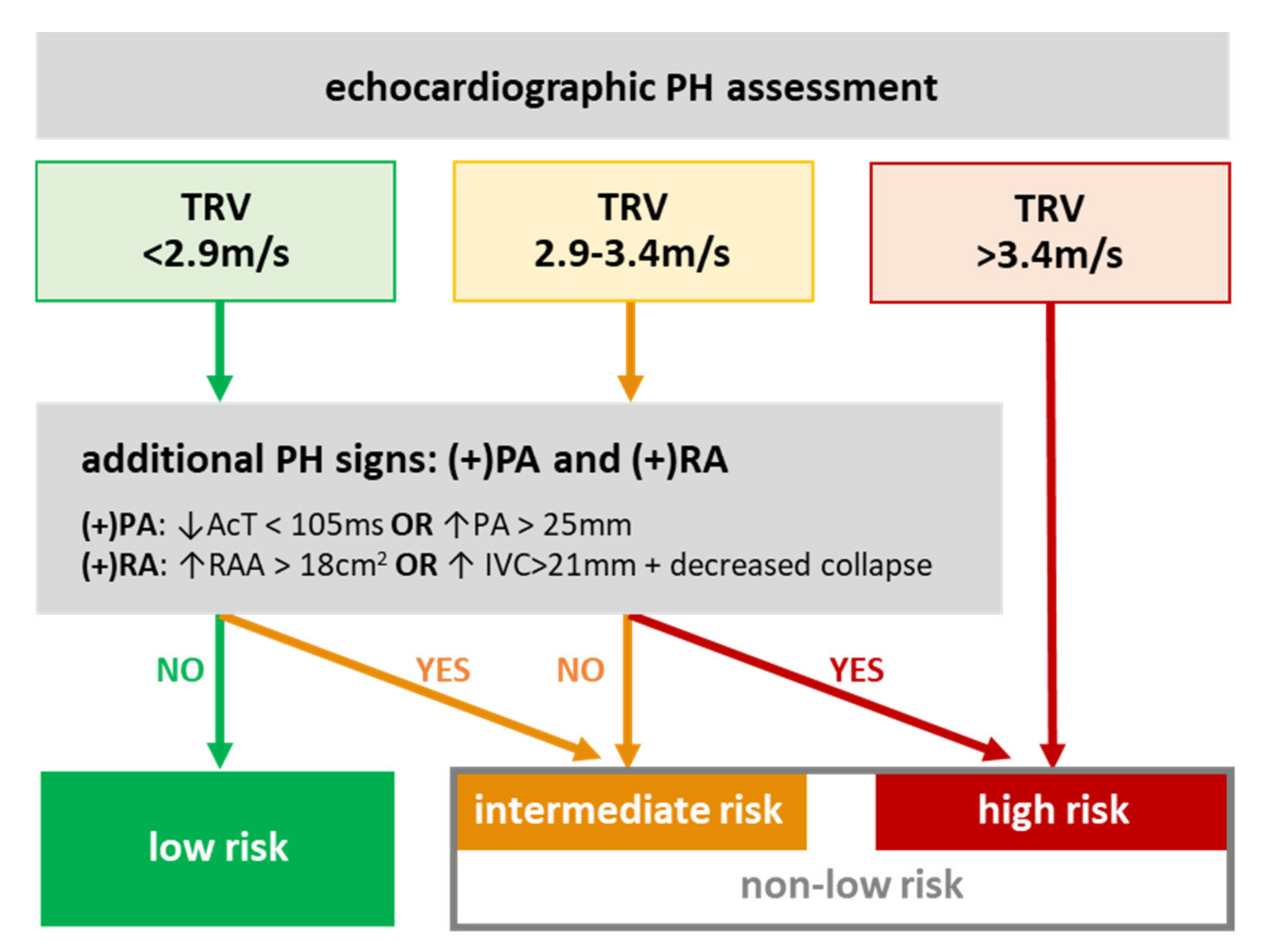
2.2. Pulmonary Hypertension Risks
2.3. Definitions of Endpoints
2.4. Statistical Analysis
3. Results
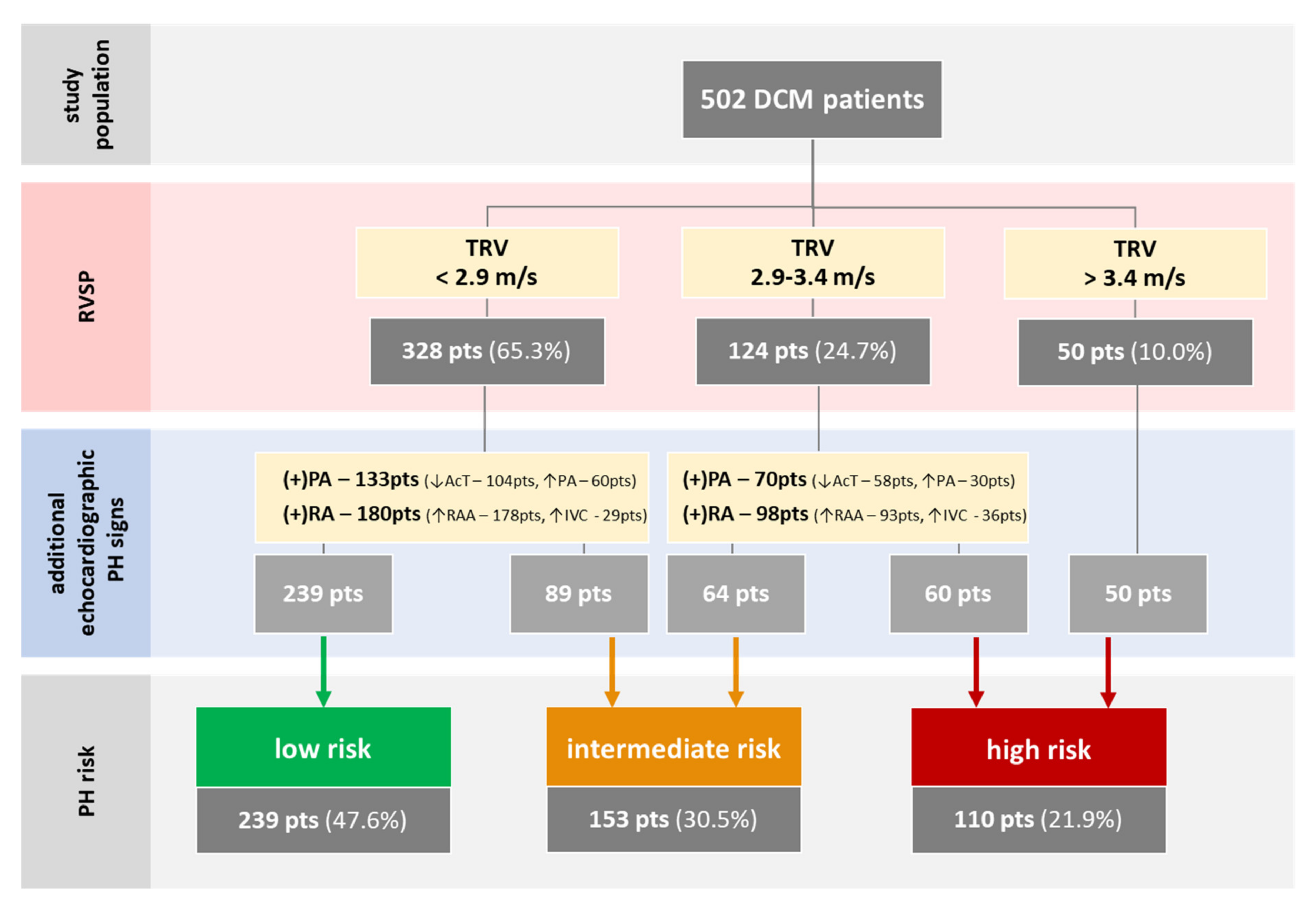
3.1. Baseline Characteristics
3.2. PH Risk and DCM Patients’ Profile
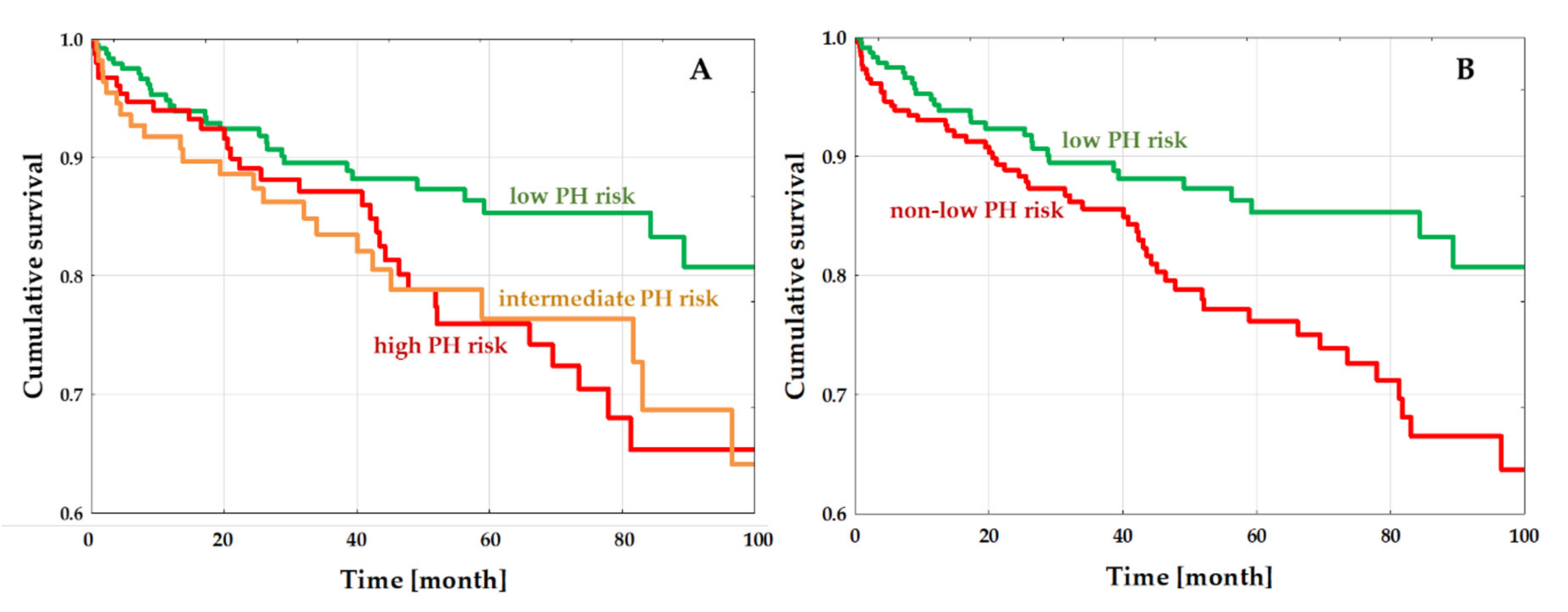
3.3. PH-Risk Impact on the Outcome
4. Discussion
4.1. Study Findings
4.2. Prevalence of Pulmonary Hypertension in LHD
4.2.1. Pulmonary Hypertension Assessment in HF
4.2.2. Pulmonary Hypertension in Dilated Cardiomyopathy
4.3. Pathology of Pulmonary Hypertension in Heart Failure and DCM
4.4. PH Risk and Outcome in DCM
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. Impact of RV and PH Risk on Outcomes in DCM
References
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Borlaug, B.A. Pulmonary Hypertension Due to Left Heart Disease. Circulation 2012, 126, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Vachiéry, J.L.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, J.S.R.; et al. Pulmonary Hypertension Due to Left Heart Diseases. J. Am. Coll. Cardiol. 2013, 62, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Gomez Sanchez, M.A.; Krishna Kumar, R.; Landzberg, M.; Machado, R.F.; et al. Updated Clinical Classification of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62. [Google Scholar] [CrossRef]
- Grymuza, M.; Malaczynska-Rajpold, K.; Jankiewicz, S.; Siniawski, A.; Grygier, M.; Mitkowski, P.; Kaluzna-Oleksy, M.; Lesiak, M.; Mularek-Kubzdela, T.; Araszkiewicz, A. Right Heart Catheterization Procedures in Patients with Suspicion of Pulmonary Hypertension. Experiences of a Tertiary Center. Postepy W Kardiol. Interwencyjnej 2017, 13, 295–301. [Google Scholar] [CrossRef]
- Berthelot, E.; Bailly, M.T.; El Hatimi, S.; Robard, I.; Rezgui, H.; Bouchachi, A.; Montani, D.; Sitbon, O.; Chemla, D.; Assayag, P. Pulmonary Hypertension Due to Left Heart Disease. Arch. Cardiovasc. Dis. 2017, 110, 420–431. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Ghio, S.; Gavazzi, A.; Campana, C.; Inserra, C.; Klersy, C.; Sebastiani, R.; Arbustini, E.; Recusani, F.; Tavazzi, L. Independent and Additive Prognostic Value of Right Ventricular Systolic Function and Pulmonary Artery Pressure in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2001, 37, 183–188. [Google Scholar] [CrossRef]
- Tatebe, S.; Fukumoto, Y.; Sugimura, K.; Miyamichi-Yamamoto, S.; Aoki, T.; Miura, Y.; Nochioka, K.; Satoh, K.; Shimokawa, H. Clinical Significance of Reactive Post-Capillary Pulmonary Hypertension in Patients with Left Heart Disease. Circ. J. 2012, 76, 1235–1244. [Google Scholar] [CrossRef]
- Abramson, S.V.; Burke, J.F.; Kelly, J.J.; Kitchen, J.G.; Dougherty, M.J.; Yih, D.F.; McGeehin, F.C.; Shuck, J.W.; Phiambolis, T.P. Pulmonary Hypertension Predicts Mortality and Morbidity in Patients with Dilated Cardiomyopathy. Ann. Intern. Med. 1992, 116, 888–895. [Google Scholar] [CrossRef]
- Guazzi, M.; Naeije, R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J. Am. Coll. Cardiol. 2017, 69, 1718–1734. [Google Scholar] [CrossRef]
- Tedford, R.J.; Beaty, C.A.; Mathai, S.C.; Kolb, T.M.; Damico, R.; Hassoun, P.M.; Leary, P.J.; Kass, D.A.; Shah, A.S. Prognostic Value of the Pre-Transplant Diastolic Pulmonary Artery Pressure-to-Pulmonary Capillary Wedge Pressure Gradient in Cardiac Transplant Recipients with Pulmonary Hypertension. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2014, 33, 289–297. [Google Scholar] [CrossRef]
- Kopeć, G.; Kurzyna, M.; Mroczek, E.; Chrzanowski, Ł.; Mularek-Kubzdela, T.; Skoczylas, I.; Kuśmierczyk, B.; Pruszczyk, P.; Błaszczak, P.; Lewicka, E.; et al. Data Base of PulmoNary HyPertension in the PoLish Population (BNP-PL)—Design of the Registry. Kardiol. Pol. 2019. [Google Scholar] [CrossRef]
- Kopeć, G.; Kurzyna, M.; Mroczek, E.; Chrzanowski, Ł.; Mularek-Kubzdela, T.; Skoczylas, I.; Kuśmierczyk, B.; Pruszczyk, P.; Błaszczak, P.; Lewicka, E.; et al. Characterization of Patients with Pulmonary Arterial Hypertension: Data from the Polish Registry of Pulmonary Hypertension (BNP-PL). J. Clin. Med. 2020, 9, 173. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, And. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the Cardiomyopathies: A Position Statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Codd, M.B.; Sugrue, D.D.; Gersh, B.J.; Melton, L.J. Epidemiology of Idiopathic Dilated and Hypertrophic Cardiomyopathy. A Population-Based Study in Olmsted County, Minnesota, 1975–1984. Circulation 1989, 80, 564–572. [Google Scholar] [CrossRef]
- Merlo, M.; Pyxaras, S.A.; Pinamonti, B.; Barbati, G.; Di Lenarda, A.; Sinagra, G. Prevalence and Prognostic Significance of Left Ventricular Reverse Remodeling in Dilated Cardiomyopathy Receiving Tailored Medical Treatment. J. Am. Coll. Cardiol. 2011, 57, 1468–1476. [Google Scholar] [CrossRef]
- Rubiś, P.; Wiśniowska-Śmiałek, S.; Biernacka-Fijałkowska, B.; Rudnicka-Sosin, L.; Wypasek, E.; Kozanecki, A.; Dziewięcka, E.; Faltyn, P.; Karabinowska, A.; Khachatryan, L.; et al. Left Ventricular Reverse Remodeling Is Not Related to Biopsy-Detected Extracellular Matrix Fibrosis and Serum Markers of Fibrosis in Dilated Cardiomyopathy, Regardless of the Definition Used for LVRR. Heart Vessel. 2017, 32, 714–725. [Google Scholar] [CrossRef]
- Rubiś, P. The Diagnostic Work-up of Genetic and Inflammatory Dilated Cardiomyopathy. E-J. Cardiol. Pract. 2015, 13, 19. [Google Scholar]
- Hatle, L.; Angelsen, B.A.J.; Tromsdal, A. Non-Invasive Estimation of Pulmonary Artery Systolic Pressure with Doppler Ultrasound. Br. Heart J. 1981, 45, 157–165. [Google Scholar] [CrossRef]
- Magnino, C.; Omedè, P.; Avenatti, E.; Presutti, D.; Iannaccone, A.; Chiarlo, M.; Moretti, C.; Gaita, F.; Veglio, F.; Milan, A. RIGHT1 Investigators. Inaccuracy of Right Atrial Pressure Estimates Through Inferior Vena Cava Indices. Am. J. Cardiol. 2017, 120, 1667–1673. [Google Scholar] [CrossRef]
- Ba, B.; Sv, P.; Cg, M. Accuracy of Echocardiographic Assessment of Pulmonary Hypertension Severity and Right Ventricular Dysfunction in Patients with Chronic Thromboembolic Pulmonary Hypertension. Minerva Cardioangiol. 2012, 60, 257–265. [Google Scholar]
- Carballo, S.; Musso, P.; Garin, N.; Müller, H.; Serratrice, J.; Mach, F.; Carballo, D.; Stirnemann, J. Prognostic Value of the Echocardiographic Probability of Pulmonary Hypertension in Patients with Acute Decompensated Heart Failure. J. Clin. Med. 2019, 8, 1684. [Google Scholar] [CrossRef]
- Chen, C.; Liu, J.; Liu, Z.; He, X.; Yuan, X.; Ouyang, X.; Wang, L.; Li, X. Electrocardiogram Signs of Right Ventricular Hypertrophy May Help Identify Pulmonary Hypertension in Patients with Dilated Cardiomyopathy. Int. J. Cardiol. Heart Vasc. 2019, 22, 61–66. [Google Scholar] [CrossRef]
- Grzybowski, J.; Bilińska, Z.T.; RuzyŁŁo, W.; Kupść, W.; Michalak, E.; Szcześniewska, D.; Poplawska, W.; Rydlewska-Sadowska, W. Determinants of Prognosis in Nonischemic Dilated Cardiomyopathy. J. Card. Fail. 1996, 2, 77–85. [Google Scholar] [CrossRef]
- Hirashiki, A.; Kondo, T.; Okumura, T.; Kamimura, Y.; Nakano, Y.; Fukaya, K.; Sawamura, A.; Morimoto, R.; Adachi, S.; Takeshita, K.; et al. Cardiopulmonary Exercise Testing as a Tool for Diagnosing Pulmonary Hypertension in Patients with Dilated Cardiomyopathy. Ann. Noninvasive Electrocardiol. 2016, 21, 263–271. [Google Scholar] [CrossRef]
- Li, X.; Luo, R.; Fang, W.; Xu, X.; Niu, G.; Xu, Y.; Fu, M.; Hua, W.; Wu, X. Effects of Ventricular Conduction Block Patterns on Mortality in Hospitalized Patients with Dilated Cardiomyopathy: A Single-Center Cohort Study. BMC Cardiovasc. Disord. 2016, 16, 136. [Google Scholar] [CrossRef]
- Romeo, F.; Pelliccia, F.; Cianfrocca, C.; Barilla, F.; Reale, A.; Gallo, P.; Cristofani, R. Determinants of End-stage Idiopathic Dilated Cardiomyopathy: A Multivariate Analysis of 104 Patients. Clin. Cardiol. 1989, 12, 387–392. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Zheng, Y.; Zhao, X.; Mai, Q.; Liu, Q. Comparison on Clinical Features between Dilated Cardiomyopathy Patients with or without Pulmonary Hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2012, 40, 762–765. [Google Scholar] [PubMed]
- Bianco, F.; Bucciarelli, V.; Ammirati, E.; Occhi, L.; Musca, F.; Tonti, G.; Frigerio, M.; Gallina, S. Assessment of Right Ventricular Function in Advanced Heart Failure with Nonischemic Dilated Cardiomyopathy. J. Cardiovasc. Med. 2020, 21, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Mene-Afejuku, T.O.; Akinlonu, A.; Dumancas, C.; Lopez, P.D.; Cardenas, R.; Sueldo, C.; Veranyan, S.; Salazar, P.; Visco, F.; Pekler, G.; et al. Relationship between Pulmonary Hypertension and Outcomes among Patients with Heart Failure with Reduced Ejection Fraction. Hosp. Pract. (1995) 2019, 47, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Rihal, C.S.; Nishimura, R.A.; Hatle, L.K.; Bailey, K.R.; Tajik, A.J. Systolic and Diastolic Dysfunction in Patients with Clinical Diagnosis of Dilated Cardiomyopathy: Relation to Symptoms and Prognosis. Circulation 1994, 90, 2772–2779. [Google Scholar] [CrossRef]
- Hirashiki, A.; Kondo, T.; Adachi, S.; Nakano, Y.; Shimazu, S.; Shimizu, S.; Morimoto, R.; Okumura, T.; Murohara, T. Prognostic Value of Pulmonary Hypertension in Ambulatory Patients with Non-Ischemic Dilated Cardiomyopathy. Circ. J. 2014, 78, 1245–1253. [Google Scholar] [CrossRef]
- Delgado, J.F.; Conde, E.; Sánchez, V.; López-Ríos, F.; Gómez-Sánchez, M.A.; Escribano, P.; Sotelo, T.; de la Cámara, A.G.; Cortina, J.; de la Calzada, C.S. Pulmonary Vascular Remodeling in Pulmonary Hypertension Due to Chronic Heart Failure. Eur. J. Heart Fail. 2005, 7, 1011–1016. [Google Scholar] [CrossRef]
- Dziewięcka, E.; Gliniak, M.; Winiarczyk, M.; Karapetyan, A.; Wiśniowska-Śmiałek, S.; Karabinowska, A.; Holcman, K.; Kostkiewicz, M.; Hlawaty, M.; Leśniak-Sobelga, A.; et al. The Burden of Atrial Fibrillation and Its Prognostic Value in Patients with Dilated Cardiomyopathy. Kardiol. Pol. 2020, 78, 37–44. [Google Scholar] [CrossRef]
- Cappola, T.P.; Felker, G.M.; Kao, W.H.L.; Hare, J.M.; Baughman, K.L.; Kasper, E.K. Pulmonary Hypertension and Risk of Death in Cardiomyopathy: Patients with Myocarditis Are at Higher Risk. Circulation 2002, 105, 1663–1668. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Akkan, D.; Iversen, K.K.; Kjoller, E.; Køber, L.; Torp-Pedersen, C.; Hassager, C. Prognostic Importance of Pulmonary Hypertension in Patients with Heart Failure. Am. J. Cardiol. 2007, 99, 1146–1150. [Google Scholar] [CrossRef]
- Bursi, F.; McNallan, S.M.; Redfield, M.M.; Nkomo, V.T.; Lam, C.S.P.; Weston, S.A.; Jiang, R.; Roger, V.L. Pulmonary Pressures and Death in Heart Failure: A Community Study. J. Am. Coll. Cardiol. 2012, 59, 222–231. [Google Scholar] [CrossRef]
| Parameters | All (n = 502) | Low PH Risk (n = 239, 47.6%) | Intermediate PH Risk (n= 153, 30.5%) | High PH Risk (n = 110, 21.9%) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 53.81 ± 13.84 | 52.08 ± 13.92 | 55.95 ± 12.86 | 54.62 ± 14.58 | 0.99 |
| BMI (kg/m2) | 27.69 ± 5.21 | 27.06 ± 5.06 | 28.43 ± 5.04 | 28.07 ± 5.60 | 0.10 |
| Male (n, %) | 403 (80.3%) | 182 (76.2%) | 131 (85.6%) | 90 (81.8%) | 0.06 |
| Urgent admission (n, %) | 76 (15.1%) | 25 (10.5%) | 27 (17.7%) | 24 (21.8%) | 0.0004 |
| Symptom duration (months) | 38.54 ± 56.63 | 30.43 ± 50.29 | 40.01 ± 50.53 | 54.12 ± 72.56 | 0.03 † |
| Mean NYHA class | 2.47 ± 0.90 | 2.2 ± 0.9 | 2.6 ± 0.9 | 2.8 ± 0.8 | 0.11 |
| NYHA III/IV (n, %) | 246 (49.0%) | 83 (34.7%) | 90 (58.8%) | 73 (66.4%) | <0.0001 |
| Killip 3–4 (n, %) | 12 (2.4%) | 5 (2.1%) | 4 (2.6%) | 3 (2.7%) | 0.92 |
| Ankle edema (n, %) | 153 (30.5%) | 52 (21.8%) | 55 (36.0%) | 46 (41.8%) | 0.0002 |
| DM (n, %) | 112 (22.3%) | 44 (18.4%) | 40 (26.1%) | 28 (25.5%) | 0.13 |
| AF (n, %) | 160 (31.9%) | 60 (25.1%) | 59 (38.6%) | 41 (37.3%) | 0.008 |
| COPD (n, %) | 34 (6.8%) | 13 (5.4%) | 14 (9.2%) | 7 (6.4%) | 0.35 |
| Prior stroke (n, %) | 30 (6.0%) | 11 (4.6%) | 7 (4.6%) | 12 (10.9%) | 0.047 |
| Dyslipidemia (n, %) | 345 (68.7%) | 66 (27.6%) | 48 (31.3%) | 43 (39.1%) | 0.10 |
| Smoker (n, %) | 0.11 | ||||
| current | 150 (31.1%) | 81 (33.9%) | 50 (32.9%) | 25 (22.9%) | |
| previously | 90 (15.9%) | 33 (13.8%) | 30 (19.7%) | 17 (15.6%) | |
| SBP/DBP (mmHg) | 119.62 ± 19.09/ 75.74 ± 11.98 | 122.46 ± 19.48/ 75.95 ± 11.96 | 118.32 ± 18.64/ 76.18 ± 12.53 | 115.22 ± 17.96/ 74.73 ± 11.33 | 0.09/0.26 |
| Heart rate (bpm) | 79.87 ± 19.17 | 77.20 ± 18.35 | 83.21 ± 20.34 | 81.04 ± 18.55 | 0.32 |
| LBBB (n, %) | 117 (23.3%) | 57 (23.9%) | 31 (20.3%) | 29 (26.4%) | 0.49 |
| VT (n, %) | 115 (22.9%) | 38 (15.9%) | 42 (27.5%) | 35 (31.8%) | 0.002 |
| LVEDd (mm) | 65.93 ± 10.44 | 63.07 ± 9.73 | 67.26 ± 10.28 | 70.28 ± 10.35 | 0.35 |
| LVEF (%) | 26.43 ± 10.34 | 29.39 ± 10.20 | 25.09 ± 10.24 | 21.84 ± 8.69 | 0.02 ‡ |
| LVEF < 35% (%) | 384 (76.5%) | 161 (67.4%) | 121 (79.1%) | 102 (92.7%) | <0.0001 |
| RVOT (mm) | 32.66 ± 7.22 | 30.13 ± 5.87 | 34.37 ± 7.27 | 35.62 ± 7.97 | 0.27 |
| TAPSE (mm) | 18.3 ± 5.05 | 19.82 ± 4.96 | 17.50 ± 4.99 | 16.29 ± 4.36 | 0.10 |
| LAA (cm2) | 29.11 ± 8.79 | 25.06 ± 6.88 | 31.28 ± 8.13 | 34.91 ± 9.11 | 0.92 |
| RAA (cm2) | 22.97 ± 8.2 | 19.07 ± 6.22 | 25.30 ± 7.97 | 27.53 ± 8.46 | 0.008‡ |
| Moderate/Severe PR (n, %) | 23 (4.6%) | 5 (2.1%) | 9 (5.9%) | 9 (8.5%) | 0.03 |
| Moderate/Severe MR (n, %) | 253 (50.4%) | 76 (31.8%) | 97 (63.4%) | 80 (72.7%) | <0.0001 |
| Moderate/Severe TR (n, %) | 138 (27.5%) | 17 (7.1%) | 52 (34.0%) | 69 (62.7%) | <0.0001 |
| TRV (m/s) | 2.58 ± 1.92 | 2.05 ± 1.42 | 2.64 ± 1.64 | 3.40 ± 1.55 | 0.03‡ |
| Hb (g/dl) | 14.26 ± 1.58 | 14.38 ± 1.51 | 14.2 ± 1.66 | 14.09 ± 1.63 | 0.71 |
| Creatinine (μmol/l) | 94.44 ± 40.89 | 88.89 ± 39.99 | 95.76 ± 36.41 | 104.65 ± 46.59 | 0.46 |
| LDL cholesterol (mmol/l) | 2.92 ± 0.97 | 3.04 ± 0.96 | 3.01 ± 1.00 | 2.54 ± 0.85 | 0.64 |
| Fasting glucose (mg/dl) | 6.23 ± 1.92 | 6.00 ± 1.50 | 6.48 ± 2.44 | 6.38 ± 1.89 | 0.41 |
| CRP (mg/l] | 9.02 ± 23.89 | 5.19 ± 9.91 | 9.55 ± 18.87 | 16.60 ± 42.66 | 0.12 |
| NT-proBNP (ng/mL) | 3724 ± 7648 | 2278 ± 5207 | 3854 ± 5257 | 6655 ± 12464 | 0.56 |
| ACEI/ARB/ARNI (n, %) | 454 (90.4%) | 220 (92.1%) | 138 (90.2%) | 96 (87.3%) | 0.37 |
| BB (n, %) | 482 (96%) | 233 (97.9%) | 143 (93.5%) | 106 (97.3%) | 0.06 |
| MRA (n, %) | 437 (87.1%) | 205 (86.1%) | 133 (86.9%) | 99 (90.8%) | 0.46 |
| Digoxin (n, %) | 117 (23.3%) | 33 (13.9%) | 50 (32.7%) | 34 (31.2%) | <0.0001 |
| Loop diuretic daily dosage (mg/day) | 65.22 ± 101.92 | 49.73 ± 91.03 | 71.36 ± 112.93 | 90.32 ± 103.23 | 0.57 |
| ICD (n, %) | 55 (11%) | 10 (4.2%) | 20 (13.1%) | 25 (22.7%) | <0.0001 |
| CRT (n, %) | 16 (3.2%) | 5 (2.1%) | 3 (2.0%) | 8 (7.3%) | 0.02 |
| Parameters | Univariate Regression (OR (95%CI)) | p-Value | Multivariate Regression (OR (95%CI)) | p-Value |
|---|---|---|---|---|
| Urgent admission | 0.486 (0.292–0.808) | 0.005 | 1.257 (0.585–2.698) | 0.56 |
| Symptom duration | 0.995 (0.991–0.998) | 0.003 | 0.995 (0.990–1.001) | 0.03 |
| NYHA III/IV | 0.326 (0.226–0.47) | <0.0001 | 0.635 (0.377–1.069) | 0.09 |
| Ankle edema | 0.446 (0.3–0.663) | <0.0001 | 0.911 (0.502–1.653) | 0.76 |
| AF | 0.546 (0.372–0.803) | 0.002 | 0.534 (0.311–0.915) | 0.02 |
| Prior stroke | 0.62 (0.288–1.333) | 0.22 | - | - |
| VT | 0.457 (0.29–0.72) | 0.0007 | 0.507 (0.292–0.881) | 0.02 |
| LVEF | 1.059 (1.039–1.08) | <0.0001 | 1.033 (1.005–1.061) | 0.02 |
| RAA | 0.856 (0.827–0.887) | <0.0001 | 0.878 (0.842–0.915) | <0.0001 |
| PR | 0.291 (0.106–0.798] | 0.02 | 0.591 (0.159–2.196) | 0.43 |
| MR | 0.227 (0.156–0.33] | <0.0001 | 0.368 (0.225–0.604) | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziewięcka, E.; Wiśniowska-Śmiałek, S.; Karabinowska, A.; Holcman, K.; Gliniak, M.; Winiarczyk, M.; Karapetyan, A.; Kaciczak, M.; Podolec, P.; Kostkiewicz, M.; et al. Relationships between Pulmonary Hypertension Risk, Clinical Profiles, and Outcomes in Dilated Cardiomyopathy. J. Clin. Med. 2020, 9, 1660. https://doi.org/10.3390/jcm9061660
Dziewięcka E, Wiśniowska-Śmiałek S, Karabinowska A, Holcman K, Gliniak M, Winiarczyk M, Karapetyan A, Kaciczak M, Podolec P, Kostkiewicz M, et al. Relationships between Pulmonary Hypertension Risk, Clinical Profiles, and Outcomes in Dilated Cardiomyopathy. Journal of Clinical Medicine. 2020; 9(6):1660. https://doi.org/10.3390/jcm9061660
Chicago/Turabian StyleDziewięcka, Ewa, Sylwia Wiśniowska-Śmiałek, Aleksandra Karabinowska, Katarzyna Holcman, Matylda Gliniak, Mateusz Winiarczyk, Arman Karapetyan, Monika Kaciczak, Piotr Podolec, Magdalena Kostkiewicz, and et al. 2020. "Relationships between Pulmonary Hypertension Risk, Clinical Profiles, and Outcomes in Dilated Cardiomyopathy" Journal of Clinical Medicine 9, no. 6: 1660. https://doi.org/10.3390/jcm9061660
APA StyleDziewięcka, E., Wiśniowska-Śmiałek, S., Karabinowska, A., Holcman, K., Gliniak, M., Winiarczyk, M., Karapetyan, A., Kaciczak, M., Podolec, P., Kostkiewicz, M., Hlawaty, M., Leśniak-Sobelga, A., & Rubiś, P. (2020). Relationships between Pulmonary Hypertension Risk, Clinical Profiles, and Outcomes in Dilated Cardiomyopathy. Journal of Clinical Medicine, 9(6), 1660. https://doi.org/10.3390/jcm9061660