Virtual Histology to Evaluate Mechanisms of Pulmonary Artery Lumen Enlargement in Response to Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Evaluation of Patients
2.3. Balloon Pulmonary Angioplasty
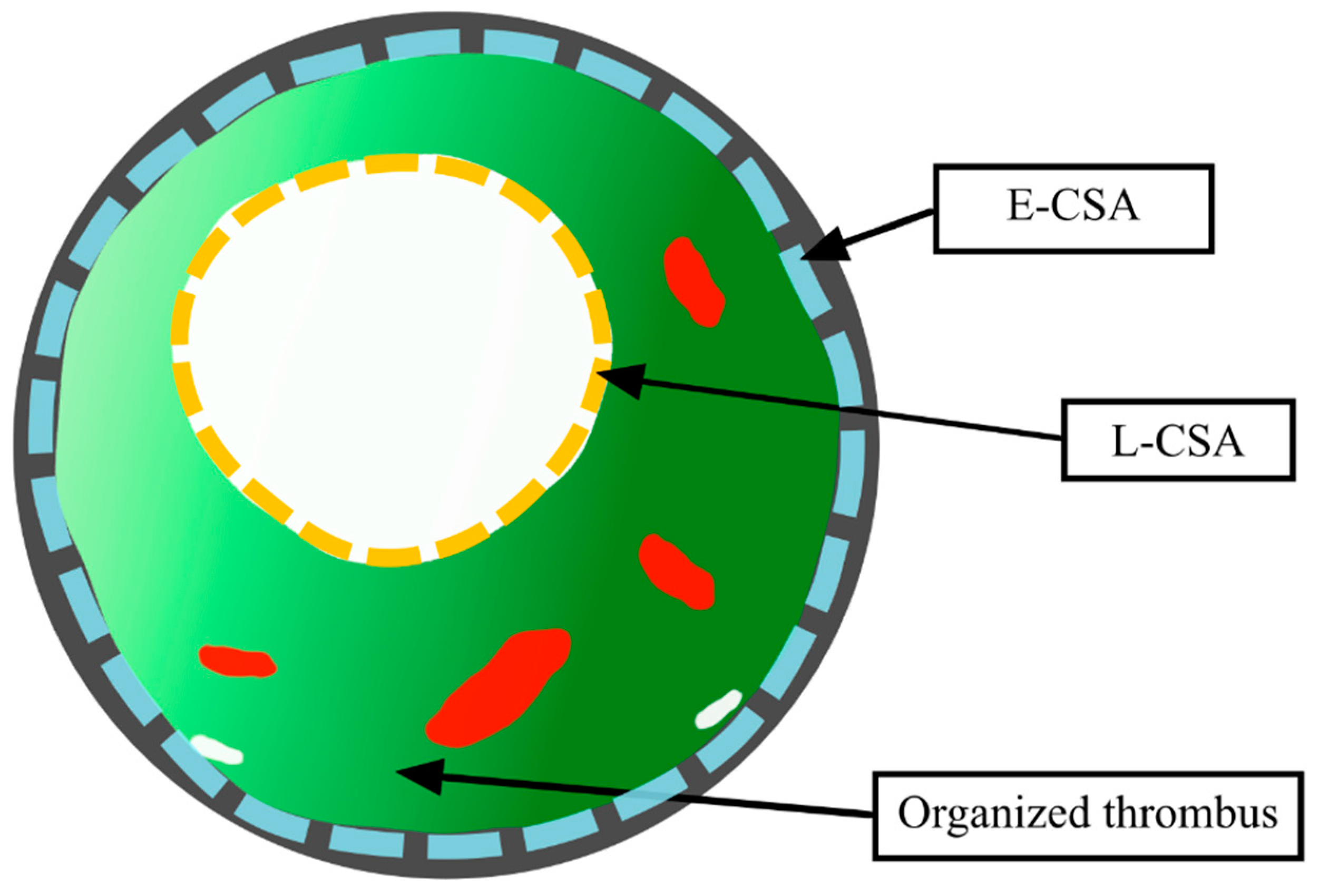
2.4. Identification of Stenoses of PA Segments
2.5. IVUS Evaluation of PA Stenoses
2.6. Mechanisms of PA Lumen Enlargement in Response to BPA
- BPA mechanism Type A (dominant vessel stretching)—defined as the ∆ E-CSA ≥ the median value; here the presumed main mechanism of BPA was vessel stretching;
- BPA mechanism Type B (non-dominant vessel stretching)—defined as the ∆ E-CSA < the median value; the presumed main mechanism of BPA was different from that of vessel stretching.
2.7. Statistics
3. Results
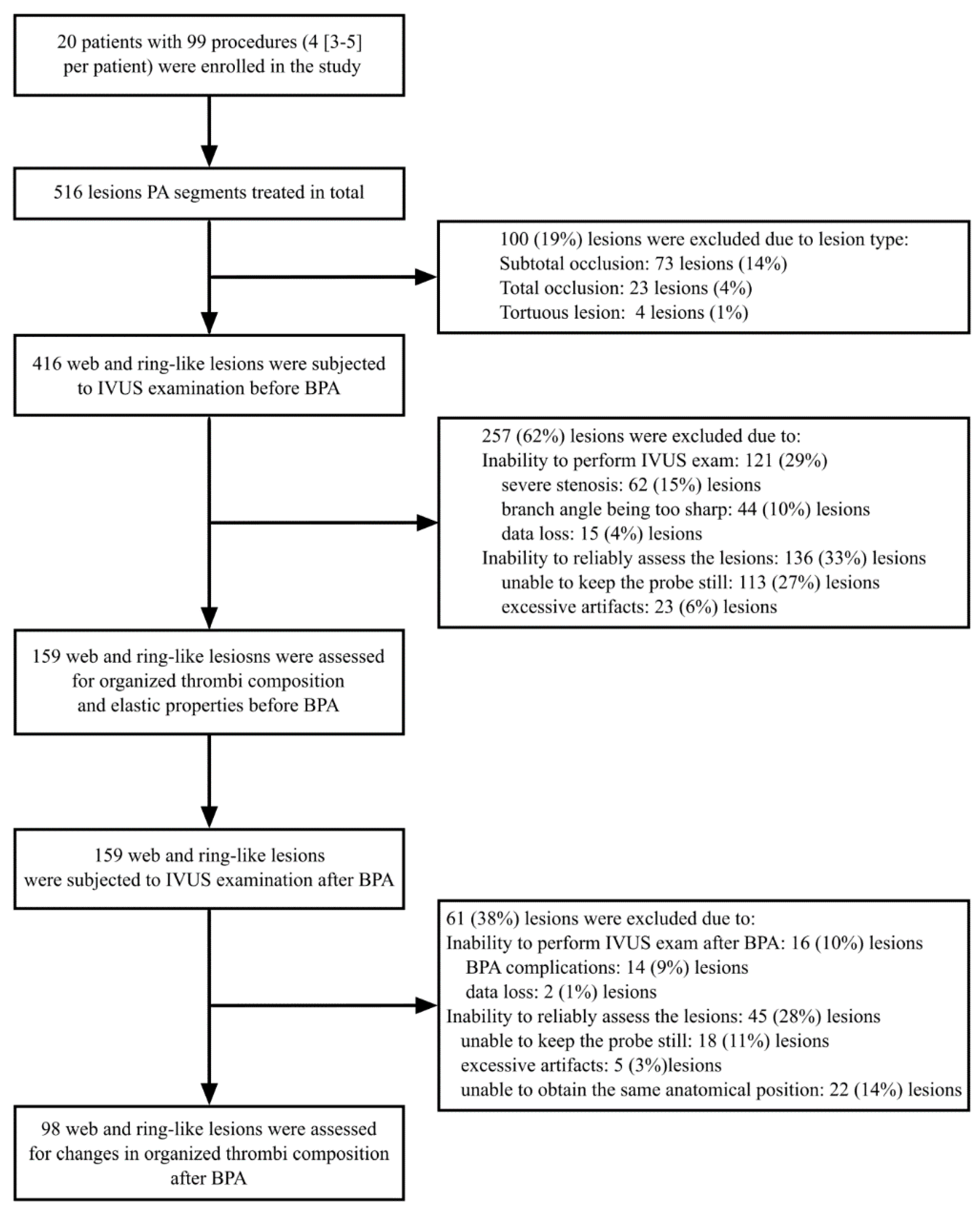
3.1. Study Group
3.2. Baseline Morphology of Stenosed PA Segments and Organized Thrombi
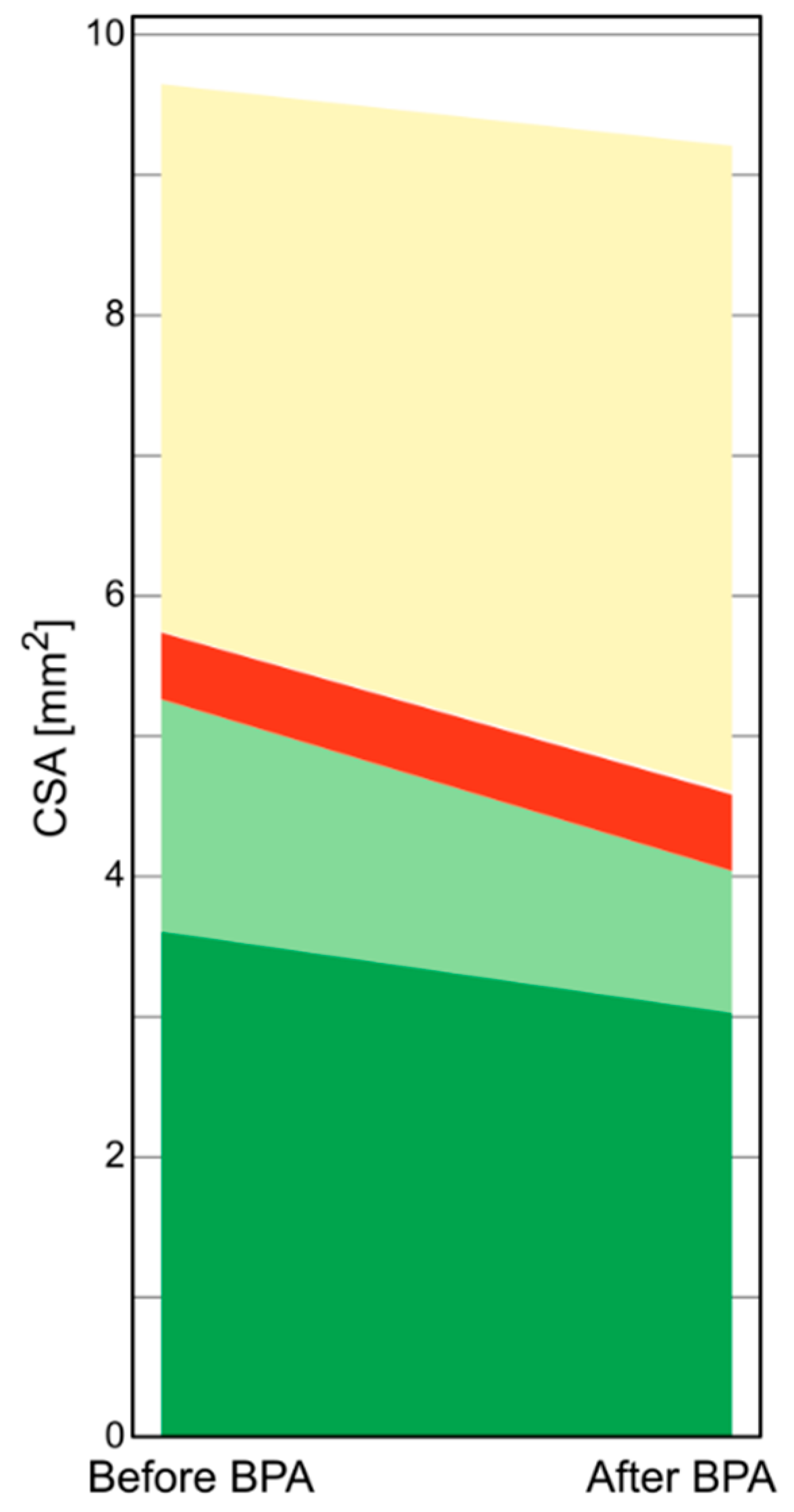
3.3. BPA Induced Changes of the Stenosed PA Segments
3.4. Characterization of Type A and Type B Mechanisms of BPA
3.5. Changes in the Structure of Organized Thrombi after BPA
4. Discussion
Strengths
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koudstaal, T.; Boomars, K.A.; Kool, M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: An immunological perspective. J. Clin. Med. 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Yi, E.S. Pulmonary thromboendarterectomy: A clinicopathologic study of 200 consecutive pulmonary thromboendarterectomy cases in one institution. Hum. Pathol. 2007, 38, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Thistlethwaite, P.A.; Mo, M.; Madani, M.M.; Deutsch, R.; Blanchard, D.; Kapelanski, D.P.; Jamieson, S.W. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J. Thorac. Cardiovasc. Surg. 2002, 124, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Skoro-Sajer, N.; Marta, G.; Gerges, C.; Hlavin, G.; Nierlich, P.; Taghavi, S.; Sadushi-Kolici, R.; Klepetko, W.; Lang, I.M. Surgical specimens, haemodynamics and long-term outcomes after pulmonary endarterectomy. Thorax 2014, 69, 116–122. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef]
- Kopeć, G.; Stępniewski, J.; Waligóra, M.; Kurzyna, M.; Biederman, A.; Podolec, P. Staged treatment of central and peripheral lesions in chronic thromboembolic pulmonary hypertension. Pol. Arch. Med. Wewn. 2016, 126, 97–99. [Google Scholar] [CrossRef]
- Kopeć, G.; Magoń, W.; Stępniewski, J.; Waligóra, M.; Jonas, K.; Podolec, P. Pregnancy in a patient with chronic thromboembolic pulmonary hypertension after successful treatment with balloon pulmonary angioplasty. Can. J. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Shimokawahara, H.; Ogawa, A.; Mizoguchi, H.; Yagi, H.; Ikemiyagi, H.; Matsubara, H. Vessel stretching is a cause of lumen enlargement immediately after balloon pulmonary angioplasty: Intravascular ultrasound analysis in patients with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2018, 11, e006010. [Google Scholar] [CrossRef] [PubMed]
- Kitani, M.; Ogawa, A.; Sarashina, T.; Yamadori, I.; Matsubara, H. Histological changes of pulmonary arteries treated by balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2014, 7, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Ogawa, A.; Miyaji, K.; Mizoguchi, H.; Shimokawahara, H.; Naito, T.; Oka, T.; Yunoki, K.; Munemasa, M.; Matsubara, H. Novel angiographic classification of each vascular lesion in chronic thromboembolic pulmonary hypertension based on selective angiogram and results of balloon pulmonary angioplasty. Circ. Cardiovasc. Interv. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Stępniewski, J.; Magoń, W.; Waligóra, M.; Podolec, P. Prolonged catheter balloon inflation for the treatment of hemoptysis complicating balloon pulmonary angioplasty. Pol. Arch. Intern. Med. 2017, 127, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Waligóra, M.; Stępniewski, J.; Zmudka, K.; Podolec, P.; Matsubara, H. In vivo characterization of changes in composition of organized thrombus in patient with chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Int. J. Cardiol. 2015, 186, 279–281. [Google Scholar] [CrossRef]
- Kopeć, G.; Kurzyna, M.; Mroczek, E.; Chrzanowski, Ł.; Mularek-Kubzdela, T.; Skoczylas, I.; Kuśmierczyk, B.; Pruszczyk, P.; Błaszczak, P.; Lewicka, E.; et al. Database of pulmonary hypertension in the polish population (BNP-PL): Design of the registry. Kardiol. Pol. 2019, 77, 972–974. [Google Scholar] [CrossRef]
- Kopeć, G.; Kurzyna, M.; Mroczek, E.; Chrzanowski, Ł.; Mularek-Kubzdela, T.; Skoczylas, I.; Kuśmierczyk, B.; Pruszczyk, P.; Błaszczak, P.; Lewicka, E.; et al. Characterization of patients with pulmonary arterial hypertension: Data from the polish registry of pulmonary hypertension (BNP-PL). J. Clin. Med. 2020, 9, 173. [Google Scholar] [CrossRef]
- Kurzyna, M.; Araszkiewicz, A.; Błaszczak, P.; Grabka, M.; Hawranek, M.; Kopec, G.; Mroczek, E.; Zembala, M.; Torbicki, A.; Ochała, A. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the polish cardiac society’s Working group on pulmonary circulation and association of cardiovascular interventions. Kardiol. Pol. 2015, 73, 63–68. [Google Scholar] [CrossRef]
- Kubiak, G.M.; Ciarka, A.; Biniecka, M.; Ceranowicz, P. Right heart catheterization—Background, physiological basics, and clinical implications. J. Clin. Med. 2019, 8, 1331. [Google Scholar] [CrossRef]
- Magoń, W.; Stępniewski, J.; Waligóra, M.; Jonas, K.; Podolec, P.; Kopeć, G. Pulmonary Artery Elastic Properties after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Can. J. Cardiol. 2019, 35, 422–429. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Wagenvoort, C.A. Pathology of pulmonary thromboembolism. Chest 1995, 107, 10S–17S. [Google Scholar] [CrossRef]
- Lang, I.M.; Dorfmüller, P.; Noordegraaf, A.V. The pathobiology of chronic thromboembolic pulmonary hypertension. Ann. Am. Thorac. Soc. 2016, 13, S215–S221. [Google Scholar] [CrossRef]
- Quarck, R.; Wynants, M.; Verbeken, E.; Meyns, B.; Delcroix, M. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2015, 46, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Morbini, P.; D’Armini, A.M.; Repetto, A.; Minzioni, G.; Piovella, F.; Viganò, M.; Tavazzi, L. Plaque composition in plexogenic and thromboembolic pulmonary hypertension: The critical role of thrombotic material in pultaceous core formation. Heart 2002, 88, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Kuban, B.D.; Tuzcu, E.M.; Schoenhagen, P.; Nissen, S.E.; Vince, D.G. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002, 106, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Margolis, M.P.; Kuban, B.D.; Vince, D.G. Automated coronary plaque characterisation with intravascular ultrasound backscatter: Ex vivo validation. EuroIntervention 2007, 3, 113–120. [Google Scholar] [PubMed]
- Kubo, T.; Nakamura, N.; Matsuo, Y.; Okumoto, Y.; Wu, X.; Choi, S.-Y.; Komukai, K.; Tanimoto, T.; Ino, Y.; Kitabata, H.; et al. Virtual histology intravascular ultrasound compared with optical coherence tomography for identification of thin-cap fibroatheroma. Int. Heart J. 2011, 52, 175–179. [Google Scholar] [CrossRef][Green Version]
- Wood, F.O.; Badhey, N.; Garcia, B.; Abdel-karim, A.R.; Maini, B.; Banerjee, S.; Brilakis, E.S. Analysis of saphenous vein graft lesion composition using near-infrared spectroscopy and intravascular ultrasonography with virtual histology. Atherosclerosis 2010, 212, 528–533. [Google Scholar] [CrossRef]
- Diethrich, E.B.; Margolis, M.P.; Reid, D.B.; Burke, A.; Ramaiah, V.; Rodriguez-Lopez, J.A.; Wheatley, G.; Olsen, D.; Virmani, R. Virtual histology intravascular ultrasound assessment of carotid artery disease: The carotid artery plaque virtual histology evaluation (CAPITAL) study. J. Endovasc. Ther. 2007, 14, 676–686. [Google Scholar] [CrossRef]
- Mahmud, E.; Madani, M.M.; Kim, N.H.; Poch, D.; Ang, L.; Behnamfar, O.; Patel, M.P.; Auger, W.R. Chronic thromboembolic pulmonary hypertension: Evolving therapeutic approaches for operable and inoperable disease. J. Am. Coll. Cardiol. 2018, 71, 2468–2486. [Google Scholar] [CrossRef]
- Townsley, M.I. Structure and composition of pulmonary arteries, capillaries, and veins. Compr. Physiol. 2012, 2, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Hunter, K.S.; Lammers, S.R.; Shandas, R. Pulmonary vascular stiffness: Measurement, modeling, and implications in normal and hypertensive pulmonary circulations. Compr. Physiol. 2011, 1, 1413–1435. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Podolec, P.; Podolec, J.; Rubiś, P.; Zmudka, K.; Tracz, W. Atherosclerosis progression affects the relationship between endothelial function and aortic stiffness. Atherosclerosis 2009, 204, 250–254. [Google Scholar] [CrossRef] [PubMed]

| Variable | ||||
|---|---|---|---|---|
| Age [years] | 67 | 65–75 | ||
| Male sex [n,%] | 6 | 30 | ||
| Time from onset of symptoms to CTEPH diagnosis [months] | 10 | 5–22 | ||
| Before BPA | 6-months after final BPA | |||
| NYHA class [n, %] | ||||
| III | 20 | 100 | 2 | 10 |
| II | 0 | 0 | 12 | 60 |
| I | 0 | 0 | 6 | 30 |
| 6-min walking distance [m] | 330 | 260–380 | 393 | 34–450 |
| NT-proBNP [pg/mL] | 1726 | 521–2678 | 236 | 144–722 |
| mPAP [mmHg] | 39 | 37–50 | 29 | 25–31 |
| RAP [mmHg] | 4 | 3–7 | 4 | 3–6 |
| CI [L/min/m2] | 2.31 | 1.95–2.62 | 2.49 | 2.32–3.00 |
| PVR [WU] | 8.9 | 6.3–11.1 | 3.9 | 3.5–5.7 |
| Type A n = 49 | Type B n = 49 | p | ||
|---|---|---|---|---|
| Lesion type [n, %] | ring | 7 [14] | 6 [12] | 0.74 |
| web | 42 [86] | 43 [88] | ||
| Lesion location [n, %] | segmental | 21 [43] | 15 [31] | 0.34 |
| subsegmental | 28 [57] | 34 [69] | ||
| Balloon to segment ratio [%] | 80.5 [59.8–88.3] | 82.7 [68.4–93.0] | 0.4 | |
| CSA [mm2]: | L-CSA | 5.25 [3.8–6.2] | 3.85 [2.7–5.3] | 0.03 |
| E-CSA | 15.25 [11.7–23.3] | 12.6 [8.1–18.4] | 0.01 | |
| T-CSA | 11.2 [7.3–17.3] | 8.3 [5.1–13.8] | 0.01 | |
| Components of the organized thrombus in IVUS-VH [%] | Dark-green | 55.75 [47.8–59.8] | 59.6 [53.9–66.8] | 0.02 |
| Light-green | 35.15 [27.3–44.1] | 27.4 [19.5–42] | 0.03 | |
| Red | 6.2 [3.4–11.7] | 7.85 [4.7–17] | 0.11 | |
| White | 0.35 [0–1.7] | 0.15 [0–0.7] | 0.1 | |
| Change (∆) in CSA of the stenosed PA after BPA [mm2]: | ∆ L-CSA | 1.9 [1.2–3.4] | 0.8 [0.6–1.7] | 0.001 |
| ∆ E-CSA | 2.8 [1.7–4.6] | 0.1 [−0.4–0.7] | 0.001 | |
| ∆ T-CSA | 0.7 [−0.1–2.0] | −0.9 [−2.0–−0.1] | 0.001 | |
| Percentage change (∆) in CSA of the stenosed PA after BPA [%]: | ∆ L-CSA | 37.4 [21.4–64.1] | 27.8 [15.0–39.4] | 0.05 |
| ∆ E-CSA | 20.4 [10.1–30.4] | 0.7 [−2.1–4.5] | 0.001 | |
| ∆ T-CSA | 7.0 [−1.3–28.9] | −13.4 [−28.3–−1.9] | 0.001 | |
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Dark-green CSA (%) | 0.98 | 0.91–1.06 | 0.68 |
| Light-green CSA (%) | 1.01 | 0.95–1.07 | 0.70 |
| E-CSA (mm2) | 1.09 | 1.01–1.17 | 0.03 |
| Organized thrombus CSA (%) | 0.98 | 0.94–1.02 | 0.40 |
| Balloon to vessel diameter ratio (%) | 1.01 | 0.99–1.03 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magoń, W.; Stępniewski, J.; Waligóra, M.; Jonas, K.; Przybylski, R.; Sikorska, M.; Podolec, P.; Kopeć, G. Virtual Histology to Evaluate Mechanisms of Pulmonary Artery Lumen Enlargement in Response to Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2020, 9, 1655. https://doi.org/10.3390/jcm9061655
Magoń W, Stępniewski J, Waligóra M, Jonas K, Przybylski R, Sikorska M, Podolec P, Kopeć G. Virtual Histology to Evaluate Mechanisms of Pulmonary Artery Lumen Enlargement in Response to Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine. 2020; 9(6):1655. https://doi.org/10.3390/jcm9061655
Chicago/Turabian StyleMagoń, Wojciech, Jakub Stępniewski, Marcin Waligóra, Kamil Jonas, Roman Przybylski, Martyna Sikorska, Piotr Podolec, and Grzegorz Kopeć. 2020. "Virtual Histology to Evaluate Mechanisms of Pulmonary Artery Lumen Enlargement in Response to Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension" Journal of Clinical Medicine 9, no. 6: 1655. https://doi.org/10.3390/jcm9061655
APA StyleMagoń, W., Stępniewski, J., Waligóra, M., Jonas, K., Przybylski, R., Sikorska, M., Podolec, P., & Kopeć, G. (2020). Virtual Histology to Evaluate Mechanisms of Pulmonary Artery Lumen Enlargement in Response to Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine, 9(6), 1655. https://doi.org/10.3390/jcm9061655






