Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Method
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
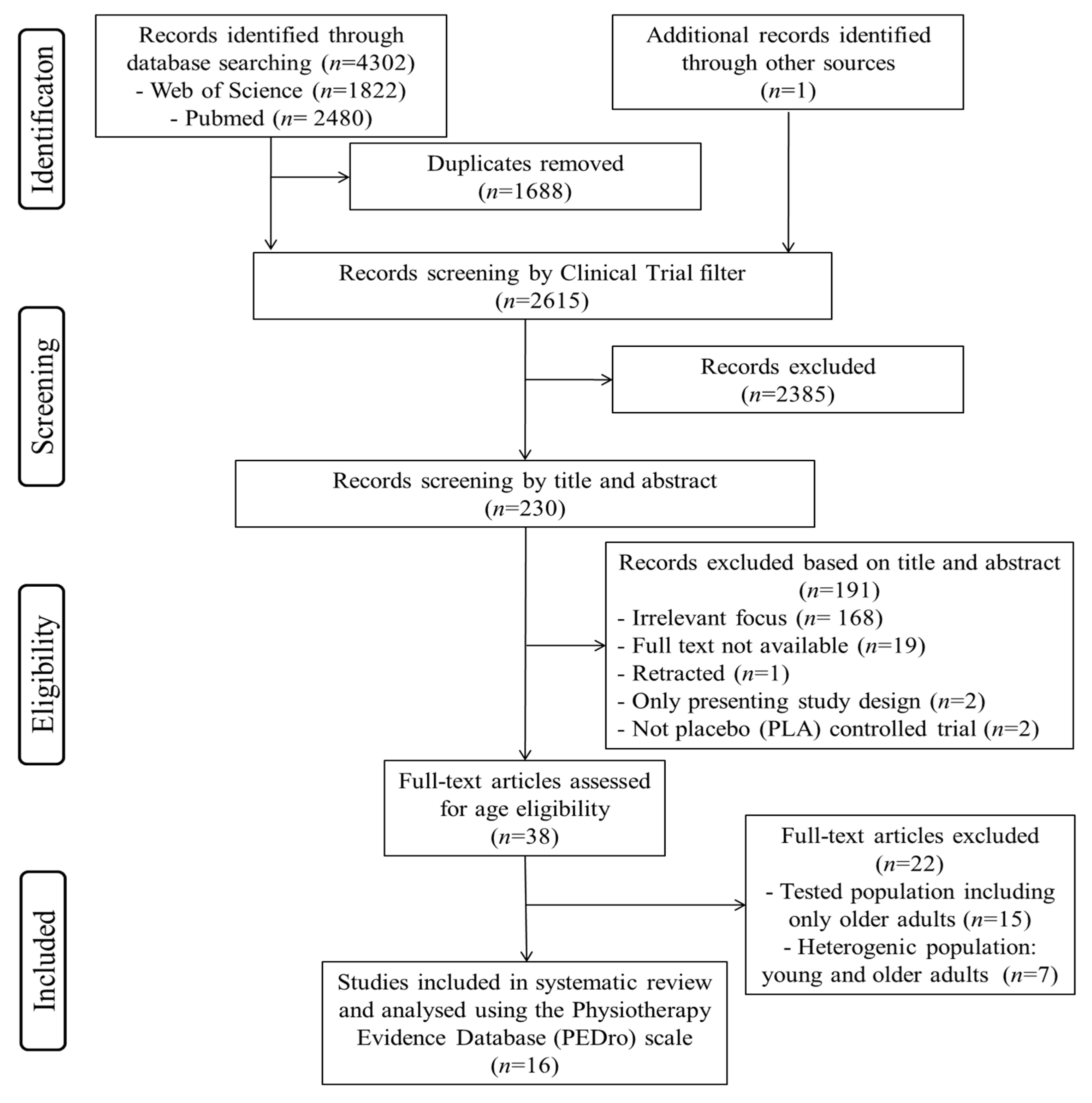
3.1. Study Selection and Characteristics
3.1.1. Study Selection
3.1.2. Study Characteristics
3.2. Subject Characteristics
3.3. Study Design and Supplement Administration
3.4. Methodological Quality of Studies
3.5. Effect of (poly)Phenol Rich Supplementation on Cognitive Functions
3.6. Effect of (poly)Phenol Rich Supplementation on Brain Parameters
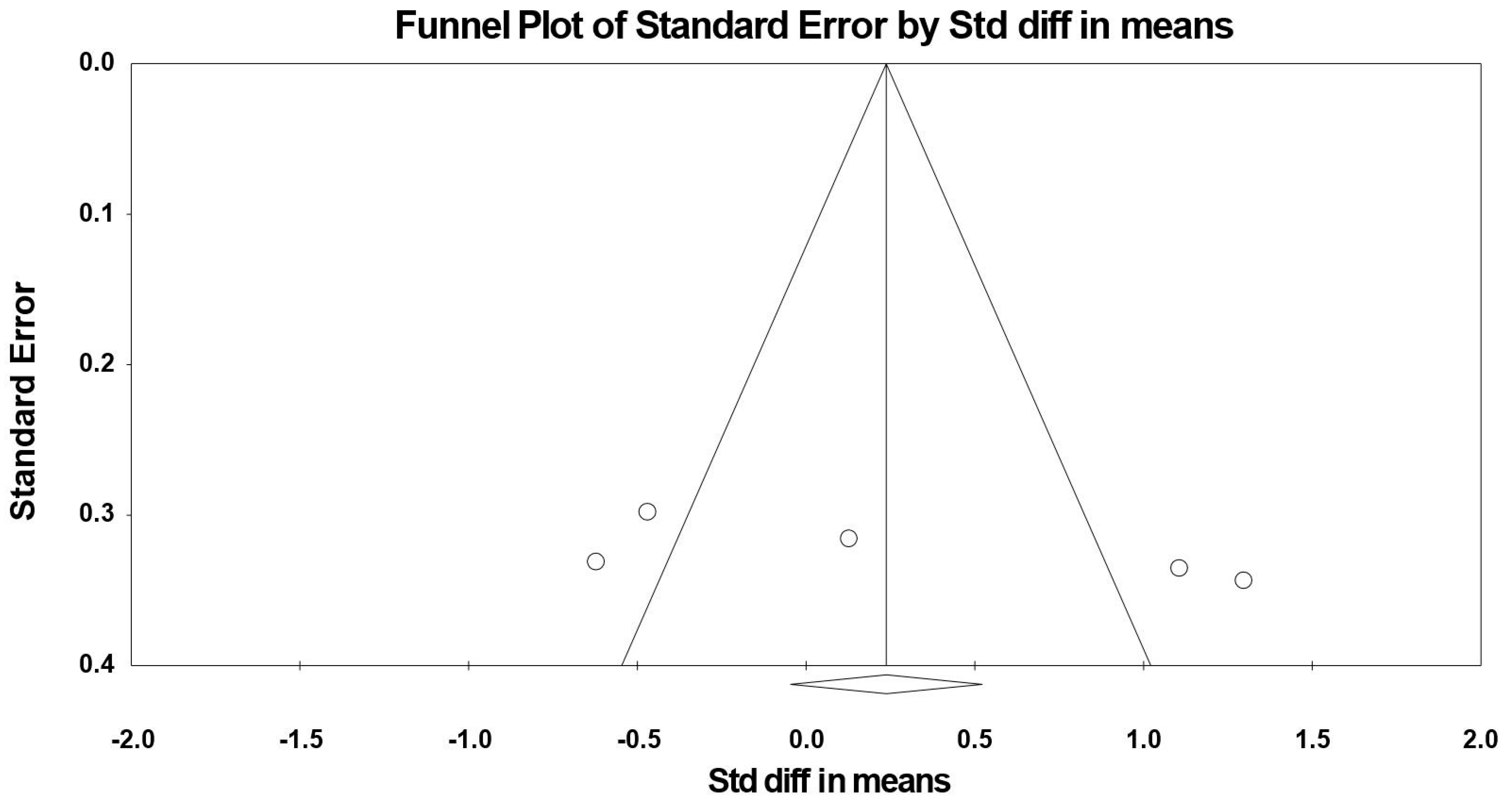
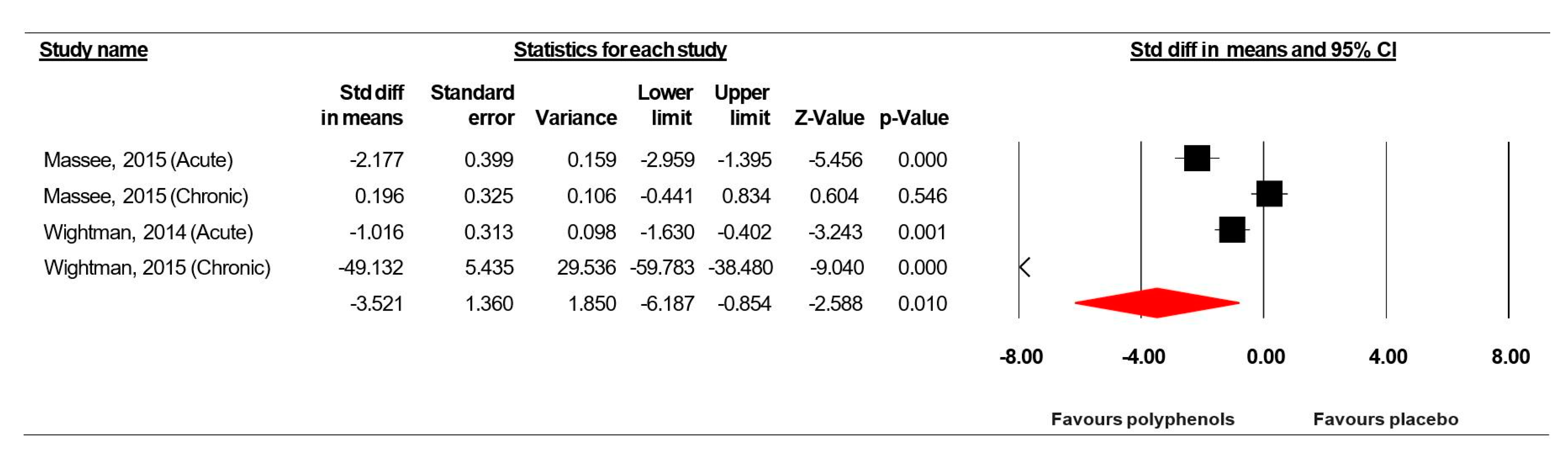
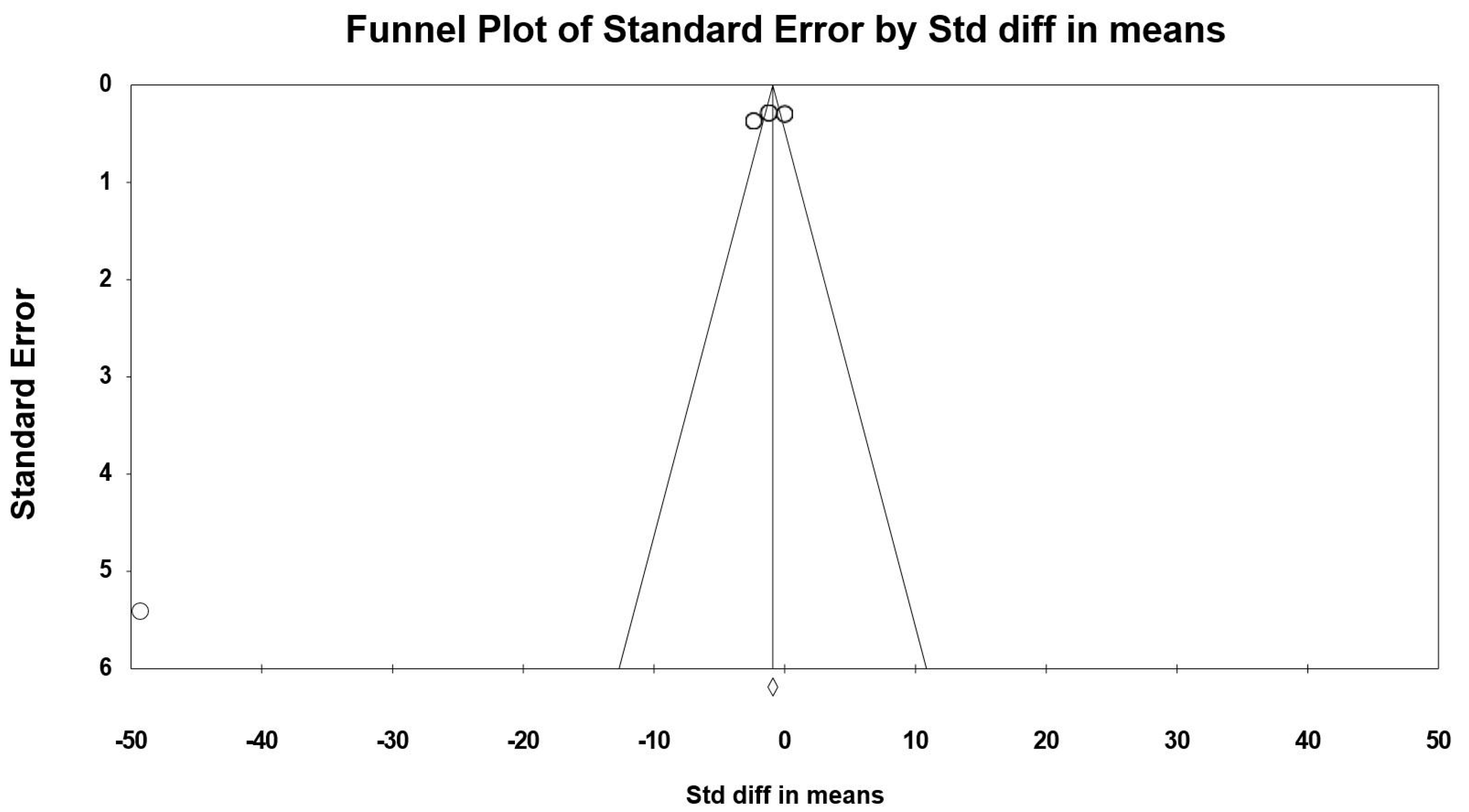
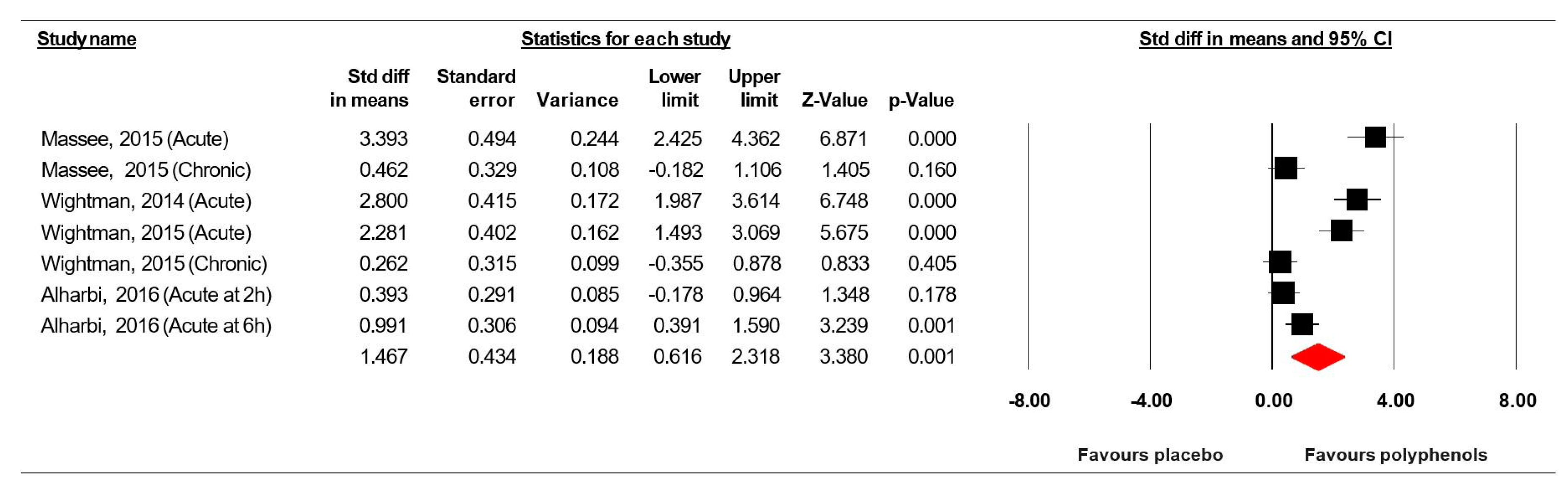
3.7. Meta-Analysis Results
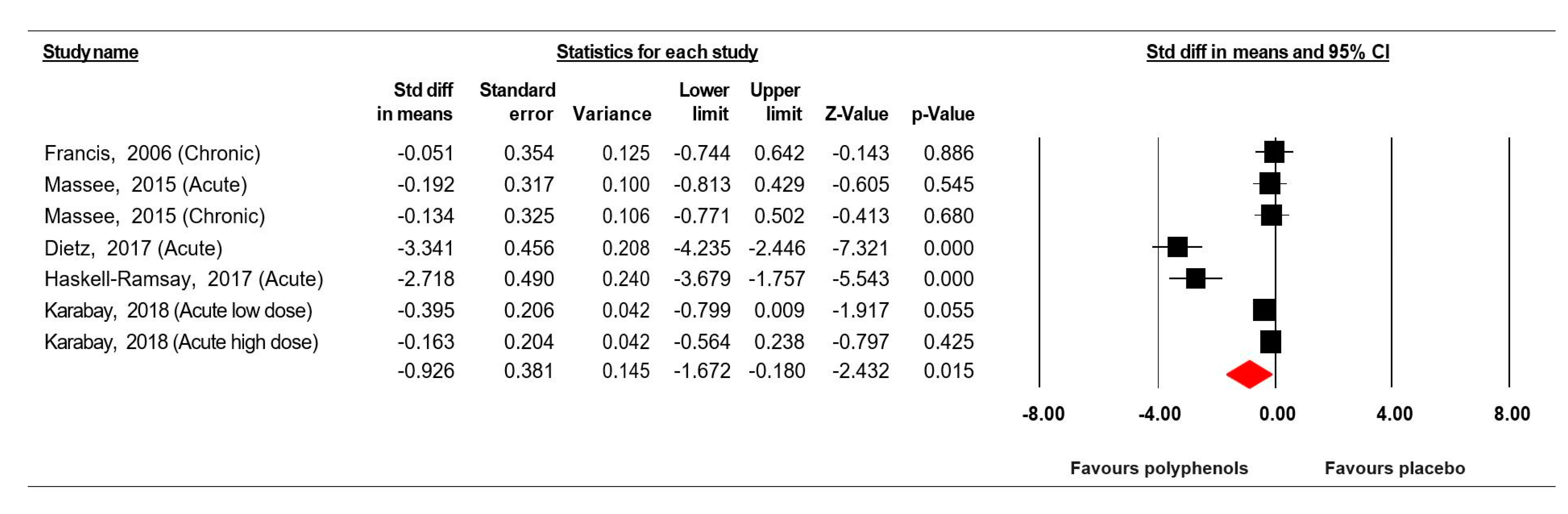
3.7.1. Simple Reaction Time
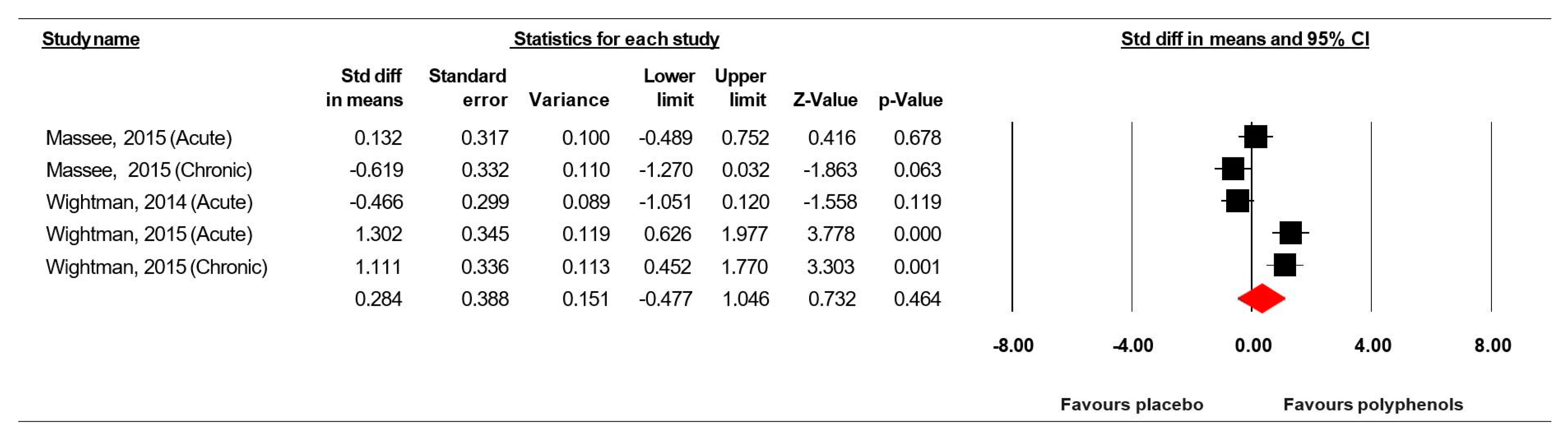
3.7.2. Rapid Visual Information Processing (% correct)
3.7.3. Mental Fatigue
3.7.4. Serial Sevens Subtraction Task (Correct in Number)
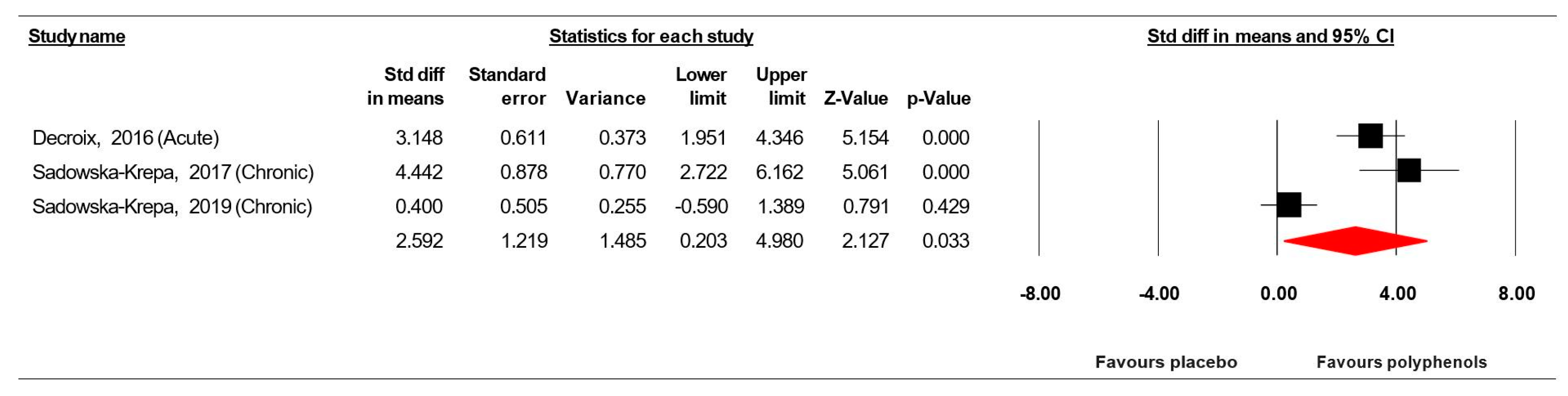
3.7.5. Brain-Derived Neurotrophic Factor
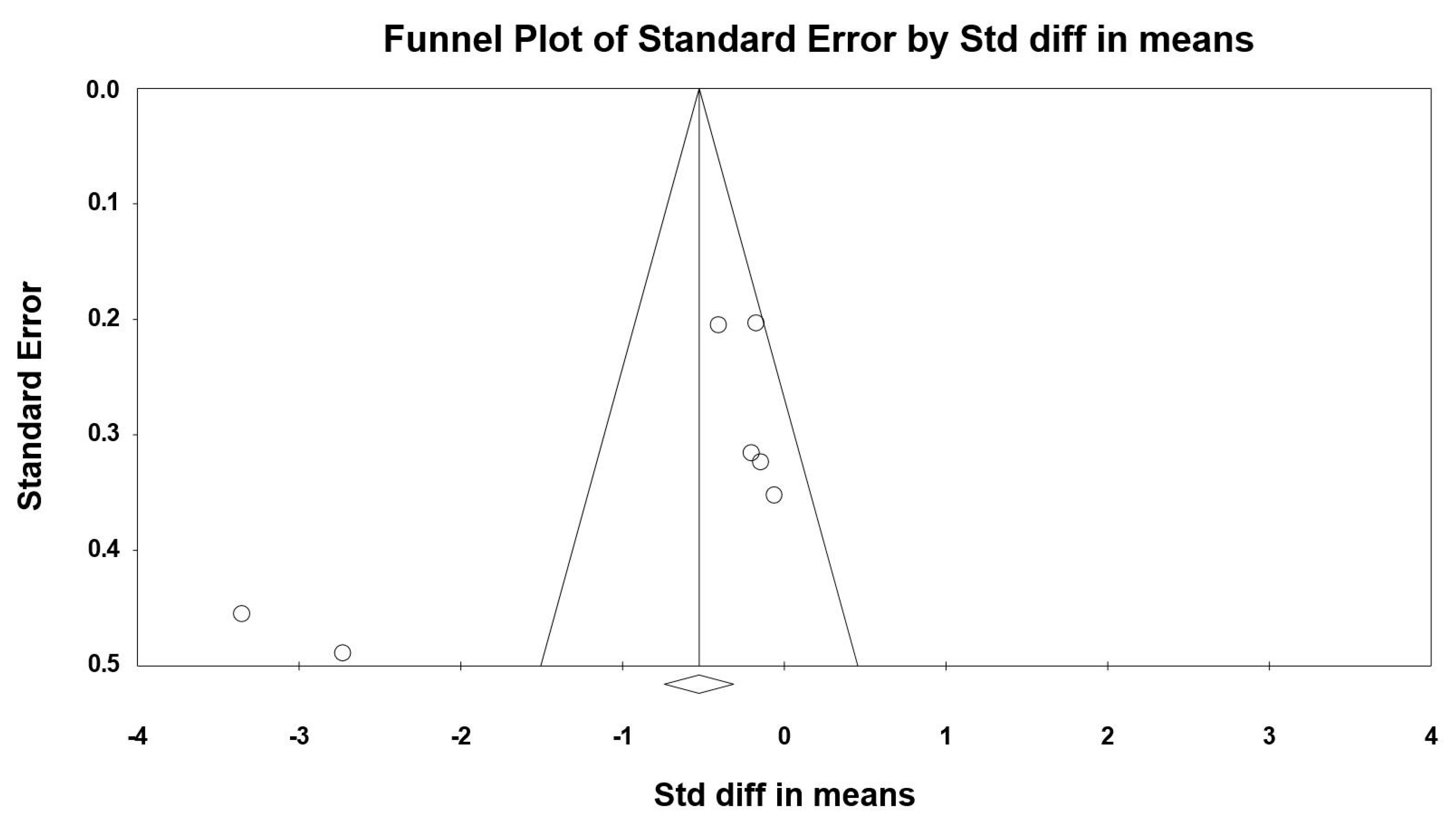
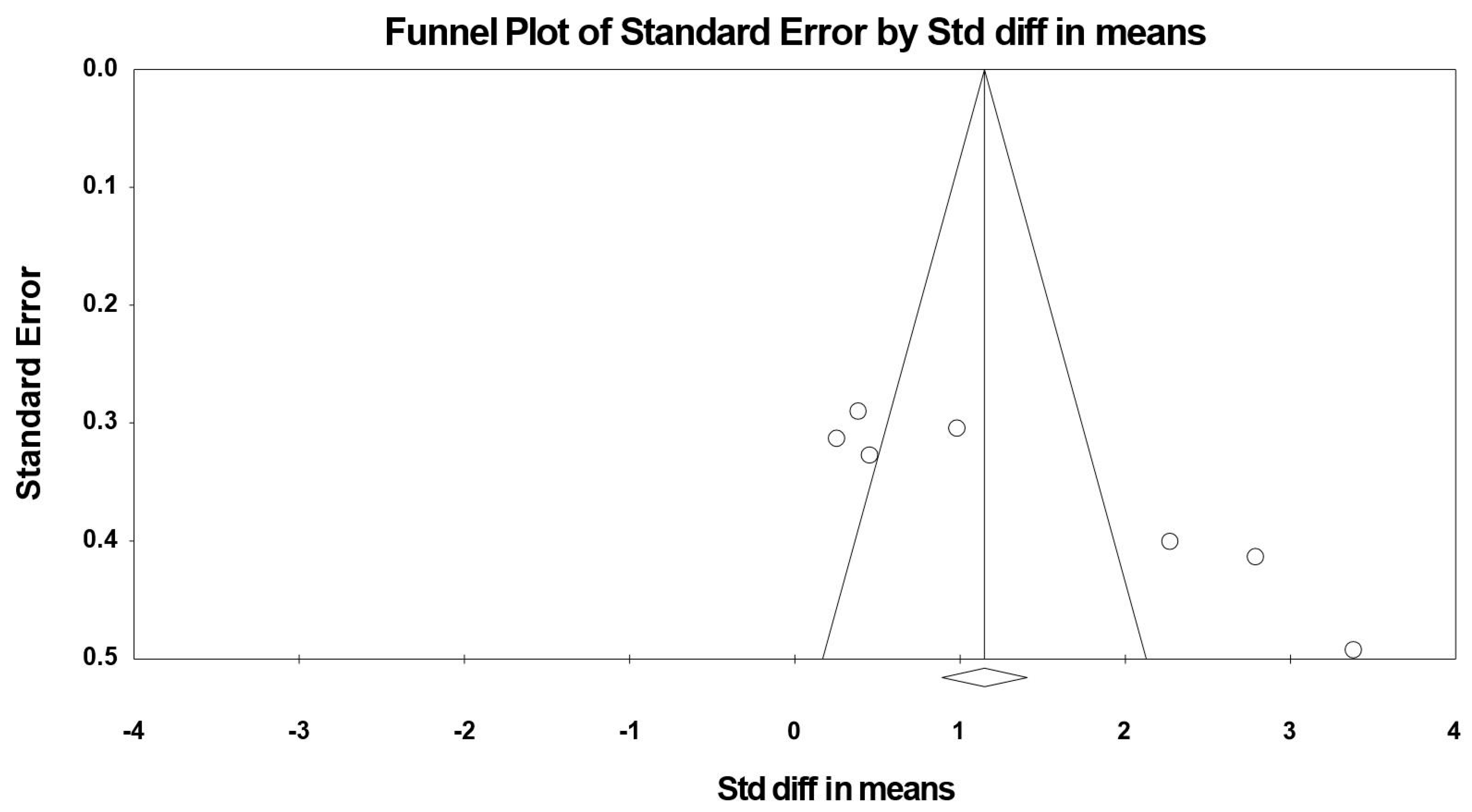
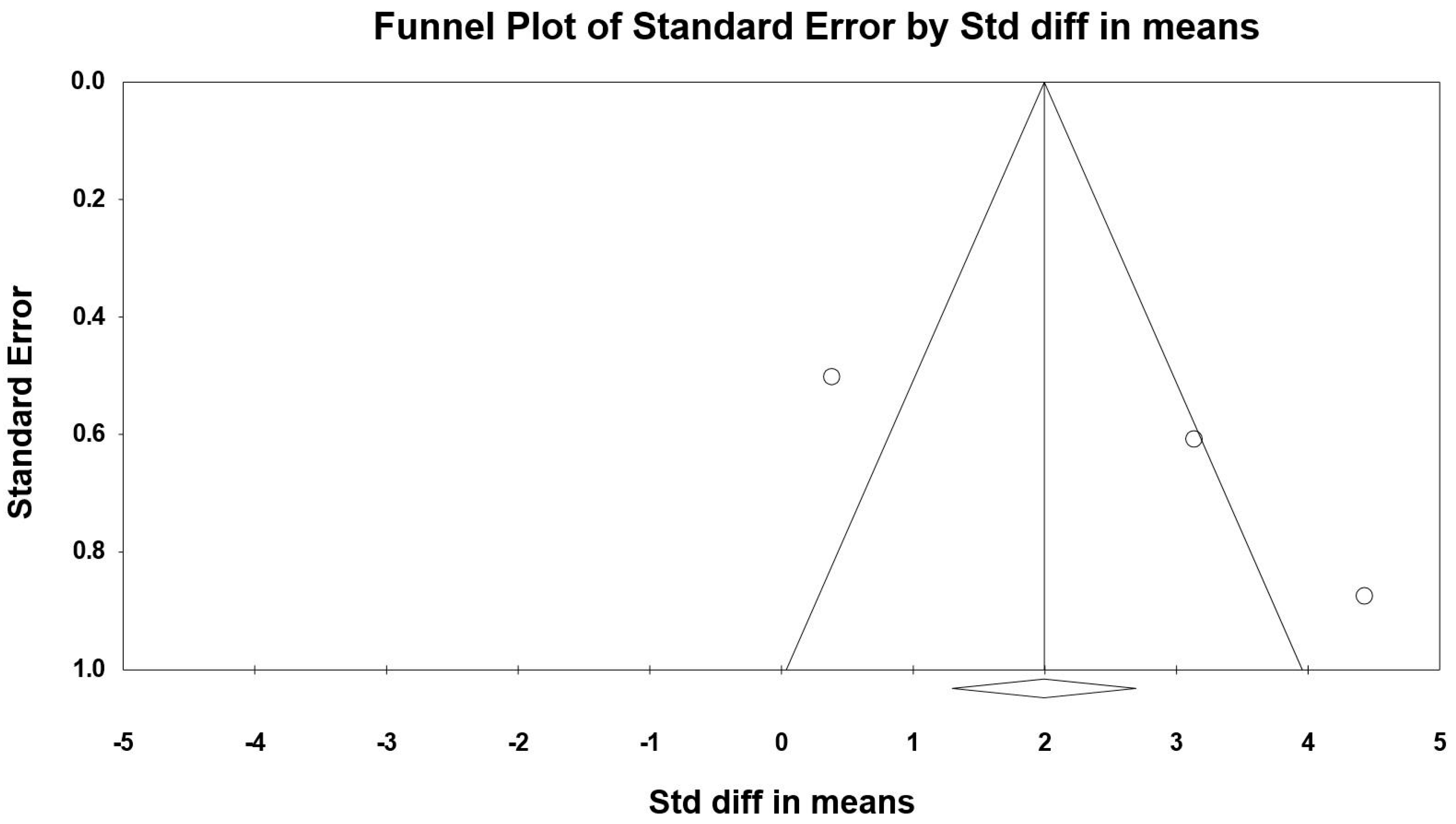
3.8. Sensitivity and Cumulative Meta-Analysis
4. Discussion
- (i)
- (ii)
- (iii)
- and increased spine density and neurogenesis, particularly in the hippocampus [73].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef]
- Hayflick, L. New approaches to old age. Nature 2000, 403, 365. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef]
- Burch, J.B.; Augustine, A.D.; Frieden, L.A.; Hadley, E.; Howcroft, T.K.; Johnson, R.; Khalsa, P.S.; Kohanski, R.A.; Li, X.L.; Macchiarini, F.; et al. Advances in geroscience: Impact on healthspan and chronic disease. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69 (Suppl. 1), S1–S3. [Google Scholar] [CrossRef]
- Crimmins, E.M. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist 2015, 55, 901–911. [Google Scholar] [CrossRef]
- Cheng, S.T. Cognitive Reserve and the Prevention of Dementia: The Role of Physical and Cognitive Activities. Curr. Psychiatry Rep. 2016, 18, 85. [Google Scholar] [CrossRef]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, B.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef]
- Kramer, A.F.; Erickson, K.I.; Colcombe, S.J. Exercise, cognition, and the aging brain. J. Appl. Physiol. 2006, 101, 1237–1242. [Google Scholar] [CrossRef]
- Asha Devi, S.; Satpati, A. Oxidative Stress and the Brain: An Insight into Cognitive Aging. In Topics in Biomedical Gerontology; Rath, P., Sharma, R., Prasad, S., Eds.; Springer: Singapore, 2017; pp. 123–140. [Google Scholar]
- Legdeur, N.; Heymans, M.W.; Comijs, H.C.; Huisman, M.; Maier, A.B.; Visser, P.J. Age dependency of risk factors for cognitive decline. BMC Geriatr. 2018, 18, 187. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstol, L.; Laake, P.; Paur, I.; Kjolsrud-Bohn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappa B activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate Supplementation Accelerates Recovery of Muscle Damage and Soreness and Inflammatory Markers after a Weightlifting Training Session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Hammouda, O.; Chtourou, H.; Trabelsi, K.; Bouaziz, M.; Abdelkarim, O.; Hoekelmann, A.; Ayadi, F.; Souissi, N.; et al. Effects of Pomegranate Juice Supplementation on Oxidative Stress Biomarkers Following Weightlifting Exercise. Nutrients 2017, 9, 819. [Google Scholar] [CrossRef]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of pomegranate supplementation on exercise performance and post-exercise recovery: A systematic review. Br. J. Nutr. 2018, 20, 1201–1216. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Trabelsi, K.; Bragazzi, N.L.; Boukhris, O.; Bouaziz, M.; Ayadi, F.; El Abed, K.; Driss, T.; Souissi, N.; et al. Effects of natural polyphenol-rich pomegranate juice on the acute and delayed response of Homocysteine and steroidal hormones following weightlifting exercises: A double-blind, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 15. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Bailey, S.J.; Turki, M.; Bragazzi, N.L.; Boukhris, O.; El Abed, K.; Bouaziz, M.; Ayadi, F.; Driss, T.; et al. Effects of natural polyphenol-rich pomegranate juice supplementation on plasma ion and lipid profiles following resistance exercise: A placebo-controlled trial. Nutr. Metab. 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Müller, P.; Bouaziz, B.; Boukhris, O.; Glenn, J.M.; Bott, N.; Driss, T.; Chtourou, H.; Müller, N.; et al. The Effect of (Poly)phenol-Rich Interventions on Cognitive Functions and Neuroprotective Measures in Healthy Aging Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 835. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Negri, E.; Talamini, R.; Bosetti, C.; Parpinel, M.; Gnagnarella, P.; Franceschi, S.; Dal Maso, L.; Montella, M.; Giacosa, A.; et al. Flavonoids and colorectal cancer in Italy. Cancer Epidemiol. Prev. Biomark. 2006, 15, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritisscardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.; Butler, L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacology 2015, 232, 3227–3234. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.R.; Fulford, J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study--a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A Randomized, Double-Blinded, Placebo-Controlled Study to Compare the Safety and Efficacy of Low Dose Enhanced Wild Blueberry Powder and Wild Blueberry Extract (ThinkBlue™) in Maintenance of Episodic and Working Memory in Older Adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef]
- Gleason, C.E.; Carlsson, C.M.; Barnet, J.H.; Meade, S.A.; Setchell, K.D.; Atwood, C.S.; Johnson, S.C.; Ries, M.L.; Asthana, S. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing 2009, 38, 86–93. [Google Scholar] [CrossRef]
- Howes, J.B.; Bray, K.; Lorenz, L.; Smerdely, P.; Howes, L.G. The effects of dietary supplementation with isoflavones from red clover on cognitive function in postmenopausal women. Climacteric 2004, 7, 70–77. [Google Scholar] [CrossRef]
- Marsh, C.E.; Carter, H.H.; Guelfi, K.J.; Smith, K.J.; Pike, K.E.; Naylor, L.H.; Green, D.J. Brachial and cerebrovascular functions are enhanced in postmenopausal women after ingestion of chocolate with a high concentration of cocoa. J. Nutr. 2017, 147, 1686–1692. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Houts, R.; Cohen, H.J.; Corcoran, D.L.; Danese, A.; Harrington, H.; Israel, S.; Levine, M.E.; Schaefer, J.D.; et al. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci. USA 2015, 112, E4104–E4110. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Kennedy, B.K.; Longo, V.D.; Seals, D.; Melov, S. Medical research: Treat ageing. Nature 2014, 511, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Timonen, V. Beyond Successful and Active Ageing. In A Theory of Model Ageing; Policy Press: Bristol, UK, 2016; p. 25. [Google Scholar]
- Hayden, E. Anti-ageing pill pushed as bona fide drug. Nature 2015, 522, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef]
- Lamport, D.J.; Pal, D.; Macready, A.L.; Barbosa-Boucas, S.; Fletcher, J.M.; Williams, C.M.; Butler, L.T. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br. J. Nutr. 2016, 116, 2160–2168. [Google Scholar] [CrossRef]
- Dietz, C.; Dekker, M.; Piqueras-Fiszman, B. An intervention study on the effect of matcha tea, in drink and snack bar formats, on mood and cognitive performance. Food Res. Int. 2017, 99, 72–83. [Google Scholar] [CrossRef]
- Karabay, A.; Saija, J.D.; Field, D.T.; Akyürek, E.G. The acute effects of cocoa flavanols on temporal and spatial attention. Psychopharmacology 2018, 235, 1497–1511. [Google Scholar] [CrossRef]
- File, S.E.; Jarrett, N.; Fluck, E.; Duffy, R.; Casey, K.; Wiseman, H. Eating soya improves human memory. Psychopharmacology 2001, 157, 430–436. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Kłapcińska, B.; Pokora, I.; Domaszewski, P.; Kempa, K.; Podgórski, T. Effects of six-week Ginkgo biloba supplementation on aerobic performance, blood pro/antioxidant balance, and serum brain-derived neurotrophic factor in physically active men. Nutrients 2017, 9, 803. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Domaszewski, P.; Pokora, I.; Żebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47, S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell, C.F.; Forster, J.S.; Veasey, R.C.; Kennedy, D.O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: A double-blind, placebo-controlled, crossover investigation. Human Psychopharmacology Clin. Exp. 2012, 27, 177–186. [Google Scholar] [CrossRef]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef]
- Massee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized, controlled trial. Front. Pharmacol. 2015, 6, 93. [Google Scholar] [CrossRef]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Tagougui, S.; Heyman, E.; Meeusen, R. Acute cocoa flavanol improves cerebral oxygenation without enhancing executive function at rest or after exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 1225–1232. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef]
- Spaak, J.; Merlocco, A.C.; Soleas, G.J.; Tomlinson, G.; Morris, B.L.; Picton, P.; Floras, J.S. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H605–H612. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus. J. Clin. Epidemiol. 1998, 51, 1235. [Google Scholar] [CrossRef]
- Moseley, A.M.; Herbert, R.D.; Maher, C.G.; Sherrington, C.; Elkins, M.R. Reported quality of randomized controlled trials of physiotherapy interventions has improved over time. J. Clin. Epidemiol. 2011, 64, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. Available online: www.cochrane-handbook.orb (accessed on 11 March 2020).
- Hadi, A.; Najafgholizadeh, A.; Aydenlu, E.S.; Shafiei, Z.; Pirivand, F.; Golpour, S.; Pourmasoumi, M. Royal jelly is an effective and relatively safe alternative approach to blood lipid modulation: A meta-analysis. J. Funct. Foods 2018, 41, 202–209. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.B. Estimating Effect Sizes FromPretest-Posttest-Control Group Designs. Organ. Res. Methods 2008, 11, 364–386. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Dye, L.; Wightman, J.D.; Lawton, C.L. The effects of flavonoid and other polyphenol consumption on cognitive performance: A systematic research review of human experimental and epidemiological studies. Nutr. Aging 2012, 1, 5–25. [Google Scholar] [CrossRef]
- Lamport, D.J.; Saunders, C.; Butler, L.T.; Spencer, J.P. Fruits, vegetables, 100% juices, and cognitive function. Nutr. Rev. 2014, 72, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Passamonti, S.; Tramer, F.; Masuero, D.; Guella, G.; Mattivi, F.; Vrhovsek, U. Fate of microbial metabolites of dietary polyphenols in rats: Is the brain their target destination? ACS Chem. Neurosci. 2015, 6, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Valdes, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; Gonzalez, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Aspects Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Lamport, D.J.; Lawton, C.L.; Mansfield, M.W.; Dye, L. Impairments in glucose tolerance can have a negative impact on cognitive function: A systematic research review. Neurosci. Biobehav. Rev. 2009, 33, 394–413. [Google Scholar] [CrossRef]
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015, 89, 126–139. [Google Scholar] [CrossRef]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.G.; Smith, M.A.; Joseph, J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef]
- Katusic, Z.S.; Austin, S.A. Endothelial nitric oxide: Protector of a healthy mind. Eur. Heart J. 2014, 35, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Hellsten, Y.; Nyberg, M.; Jensen, L.G.; Mortensen, S.P. Vasodilator interactions in skeletal muscle blood flow regulation. J. Physiol. 2012, 590, 6297–6305. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Byrns, R.E.; Sumi, D.; de Nigris, F.; Napoli, C. Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide. Nitric Oxide 2006, 15, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. The interactions of flavonoids within neuronal signaling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef]
- Calver, A.; Collier, J.; Vallance, P. Nitric oxide and blood vessels: Physiological role and clinical implications. Biochem. Educ. 1992, 20, 130–135. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Garthwaite, J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991, 14, 60–67. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Hashimoto, E.; Shimizu, E.; Iyo, M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Rev. 2004, 45, 104–114. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Nguyen, T.T. Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef]
- Gundimeda, U.; McNeill, T.H.; Fan, T.K.; Deng, R.; Rayudu, D.; Chen, Z. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: Role of 67-kDa laminin receptor and hydrogen peroxide. Biochem. Biophys. Res. Commun. 2014, 445, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Glassman, G. Understanding CrossFit. CrossFit J. 2007, 56, 1–2. [Google Scholar]
- Murawska-Cialowicz, E.; Wojna, J.; Zuwala-Jagiello, J. Crossfit training changes brain-derived neurotrophic factor and irisin levels at rest, after Wingate and progressive tests, and improves aerobic capacity and body composition of young physically active men and women. J. Physiol. Pharmacol. 2015, 66, 811–821. [Google Scholar] [PubMed]
- Abd El Mohsen, M.M.; Kuhnle, G.; Rechner, A.R.; Schroeter, H.; Rose, S.; Jenner, P.; Rice-Evans, C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef]
- Rendeiro, C.; Vauzour, D.; Rattray, M.; Waffo-Téguo, P.; Mérillon, J.M.; Butler, L.T.; Williams, C.M.; Spencer, J.P.E. Dietary levels of pure flavonoids improve spatial memory performance and increase hippocampal brain-derived neurotrophic factor. PLoS ONE 2013, 8, e63535. [Google Scholar] [CrossRef]
- Rosenberg, A.; Mangialasche, F.; Ngandu, T.; Solomon, A.; Kivipelto, M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease, and Dementia: From FINGER to World-Wide FINGERS. J. Prev. Alzheimers Dis. 2020, 7, 29–36. [Google Scholar] [CrossRef]
| Authors | Study Design | Treatment | Phenolyc Content | Dose | Duration | Washout Period | Study Population | Effect on Cognition | Used Tools |
|---|---|---|---|---|---|---|---|---|---|
| File et al. [41] | Randomized, double blind, parallel-groups study | High or low soya diet | A high soya (100 mg total isoflavones/day) or a low soya (0.5 mg total isoflavones/day) | One per day | 10 weeks | N/A | Twenty-seven student volunteers (15 men and 12 women) | ↔ non-significant effects on tests of attention or semantic memory; ↑ significantly improve short-term and long-term memory and mental flexibility (rule shifting and reversal) in males and females; ↑ significantly improve performance in a test of planning (Stockings of Cambridge) and in a letter fluency test only in females. | The digit-symbol substitution test (DSST); The digit cancellation (DC); The paced auditory serial addition test (PASAT); test of immediate memory, a short story (from the revised Weschler Memory Scale) with 25 units of information was read at the rate of one unit per second; the Cambridge Neuropsychological Test Automated Battery (CANTAB; CeNeS Ltd., Cambridge); Long-term episodic memory was measured by presenting a set of 22 pictures of common objects – each picture was shown for 5 s and then 20 min later; Letter fluency tests; A test of rule shifting and reversal (IDED). |
| Francis et al. [45] | A double blind counterbalanced manner | Flavanol-rich cocoa | High flavanol cocoa drink (172 mg flavanols per drink), low flavanol cocoa drink (13 mg flavanols per drink) | One drink/day | 5 days | 2 weeks | Sixteen young female subjects between the ages of 18 and 30 years | ↔ non-significant effects on behavioral reaction times and switch cost | The letter-digit task |
| Kennedy et al. [46] | Randomized, double-blind, placebo-controlled, counterbalanced order, crossover design | Resveratrol | Not specified | Two doses (250 and 500 mg) | Acute | 7 days | Twenty-four healthy adults (4 men, 20 women; mean age: 20.17 years; age range: 18–25 years) | ↔ non-significant effect on cognitive task performance and mental fatigue | The 9-min battery consists of 4-min Serial Subtraction, 5-min rapid visual information processing (RVIP) and a Mental Fatigue Visual Analogue Scale. |
| Wightman et al. [47] | Double-blind, placebo-controlled, counterbalanced order, crossover design | Green tea polyphenol epigallocatechin gallate (EGCG) | Two capsules each containing either 135 mg or 270 mg EGCG (94% pure EGCG plus 6% excipients) | Two doses (135 and 270 mg) of EGCG | Acute | 7 days | Twenty-seven healthy adults (11 men, 16 women, mean age 22 years, range 18–30 years) | ↔ non-significant effect on cognitive performance | Serial subtractions; Oddball reaction time task; rapid visual information processing task (RVIP); Stroop task; simple reaction time. |
| Wightman et al. [48] | Randomized, double-blind, placebo-controlled, counterbalanced order cross-over | Resveratrol | 250 mg of trans-resveratrol | Two capsules | Acute | At least a week | Twenty-three healthy adults (four males and nineteen females, mean age 21 years, range 19–34 years, SD 3·2 years) | ↔ non-significant effects on cognitive function | Serial subtractions; rapid visual information processing; N-back task. |
| Massee et al. [49] | Randomized, placebo-controlled, double-blind, parallel design | Cocoa flavanols | 3058 mg T. cacao seed extract standardized to contain 250 mg catechin polyphenols and 5.56 mg caffeine | One tablet daily (250 mg) | Acute and chronic (4 weeks) | N/A | 38 young, healthy participants aged 18–40 years (M = 24.13, SD = 4.47) | ↑ significantly improved performance acutely on the Serial component of the Cognitive Demand Battery (CDB). ↓ significantly decrease participants’ self-reported mental fatigue prior to commencing the CDB testing battery, ↔ non-significant effects significant effects were found for cognition measured with the SUCCAB. | Swinburne University Computerized Cognitive Assessment Battery (SUCCAB) [(1) Simple reaction time; (2) Choice reaction time; (3) Immediate recognition; (4) Congruent Stroop color word; (5) Incongruent Stroop color word; (6) Spatial working memory; (7) Contextual memory; (8) Delayed recognition]; Cognitive Demand Battery (CDB) [(1) Mental fatigue scales; (2) Serial Threes subtraction task; (3) Serial Sevens subtraction task;(4) Rapid Visual Information Processing Task (RVIP); (5) Mental fatigue scales] |
| Wightman et al. [36] | Randomized, double-blind, placebo-controlled, parallel-groups study | Resveratrol | TransmaxTM by BiotiviaTM with a guaranteed purity of 98%, also containing 10 mg of piperine/capsule | 500 mg once day | Acute and chronic (28 days) | N/A | Sixty adults aged between 18 and 30 years | ↑ significantly improve accuracy during serial subtraction task performance as acute effect. ↑ significantly improve accuracy during the 3-Back task before treatment consumption with ↔ non-significant effect on the remaining functions. | Serial subtractions; rapid visual information processing (RVIP); 3-Back |
| Alharbi et al. [37] | Randomized, double-blind, placebo-controlled, counterbalanced order, crossover design | Flavonoid-rich orange juice | 272 mg flavonoids | 240-mL FR orange juice (272 mg flavonoids) | Acute | 2 weeks | Twenty-four healthy males (mean age: 51 ± 6, 6 years old) | ↑ significantly improve cognitive function (z score) and subjective alertness | Digit Symbol Substitution Test (seconds); Serial Sevens (number correct); Immediate Verbal Recall (words); Delayed Verbal Recall (words); Continuous Performance Task (errors); Simple Finger Tapping (correct responses); Complex Finger Tapping (correct responses); Contrast Sensitivity (Michelson Contrast) |
| Decroix et al. [50] | Randomized, double-blind, placebo-controlled, counterbalanced order, crossover design | Cocoa flavanol | High CF-content chocolate milk (CF, 903.75 mg flavanol, Acticoa) or a PLA that contained low-CF chocolatemilk (PLA, 15 mg flavanol) | 900 mg | Acute | 7 days | Twelve well-trained men of 30 ± 3 years old | ↔ non-significant effect on cognitive performance | Reaction time (RT) and accuracy on neutral, congruent and incongruent stimuli and Stroop interference |
| Lamport et al. [38] | Randomized, single-blind, placebo-controlled, counterbalanced order, crossover design | Flavanone-rich citrus juice | 70.5-mg flavonoids | 500-mL citrus juice containing 70.5-mg flavonoids | Acute | 1-week | Sixteen healthy young adults aged 18–30 years | ↑ significantly improve performance on the Digit Symbol Substitution Test at 2 h relative to baseline and the control drink, ↔ non-significant effect on any other behavioral cognitive tests. | Freiburg Vision Test (version 3.6.3), Word Recall (immediate), Logical Memory (immediate recall), Sequence Learning Task, Digit Symbol Substitution Test (DSST), Stroop Test, Letter Memory Test, Go-No Go Task, Spatial Delayed Recall, Word Recall (delayed) and Logical Memory (delayed). |
| Dietz et al. [39] | Randomized, single-blind, placebo-controlled, counterbalanced order, crossover design | Matcha tea, matcha tea bar | 4.0 g of matcha tea powder, equivalent to two average portions of matcha tea (2 × 2 g powder in 100 mL water) | 4 g of matcha tea | Acute | 24 h | Nineteen females and four males (mean age 24.7 years, age range 20–35 years) | ↑ significantly improve tasks measuring basic attention abilities and psychomotor speed in response to stimuli over a defined period of time. ↔ non-significant effect on other tasks of the cognitive test battery. | Immediate word recall task; Simple reaction time task; Digit vigilance task; Choice reaction task; Spatial working memory task; Numeric working memory task; Delayed word recall task; Delayed word recognition task; Delayed picture recognition task; Speed of attention; Accuracy of attention; Episodic secondary memory; Working memory; Quality of memory; Speed of memory |
| Haskell-Ramsay et al. [51] | Randomized, placebo-controlled, double-blind, counterbalanced-crossover design | Purple grape juice | Phenolic content: 1504.5 μg/mL; Anthocyanin content: 138.3 mg/L | 200 mL Welch’s™ purple grape juice | Acute | Between 6 and 7 days | Twenty participants (7 males; mean age 21.05 years, SD 0.89) | ↑ significantly improve overall speed on attention tasks | Word presentation; Immediate word recall; Picture presentation; Simple reaction time; Digit vigilance; Choice reaction time; Numeric working memory; Delayed word recall; Delayed word recognition; Delayed picture recognition |
| Karabay et al. [40] | Randomized, double-blind, placebo and baseline-controlled counterbalanced, crossover design | Cocoa flavanols | 374 mg in the low-dose condition and 747 mg in high-dose condition | 300 mL | Acute | 1-week | Forty-eight (24 female) healthy (mean age = 22.15 years, range = 18–29, SEM = 0.01) | ↑ significantly improve visual search efficiency, reflected by reduced reaction time. ↔ non-significant effect on temporal attention nor integration | Attentional blink/integration task (RSVP); Visual search task (VS) |
| Authors | Study Design | Treatment | Phenolyc Content | Dose | Duration | Washout Period | Study Population | Effect on CBF | Used Techniques/Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Francis et al. [45] | A double blind counterbalanced manner | Flavanol-rich cocoa | High flavanol cocoa drink (172 mg flavanols per drink), low flavanol cocoa drink (13 mg flavanols per drink) | One drink/day | 5 days | 2 weeks | Sixteen young female subjects between the ages of 18 and 30 years | ↑ significantly increase the BOLD signal intensity in response to a cognitive task, ↑ significantly increase the cerebral blood flow to gray matter | Functional magnetic resonance imaging (FMRI) based on blood oxygenation level-dependent (BOLD) contrast to explore the effect of flavanols on the human brain. |
| Spaak et al. [52] | A randomized, single-blind trial counterbalanced order, crossover design | Red wine | Dose 1 = 155 mL; dose 2 = 310 mL; given to a 68-kg man | Acute | 2 weeks | Thirteen volunteers (24–47 years; 7 men, 6 women) | ↑ significantly increase Stroke volume | Stroke volume (SV) was determined by Doppler ultrasound directed above the aortic annulus | |
| Kennedy et al. [46] | Randomized, double-blind, placebo-controlled, counterbalanced order, crossover | Resveratrol | Not specified | Two doses (250 and 500 mg) | Acute | 7 days | Twenty-four healthy adults (4 men, 20 women; mean age: 20.17 years; age range: 18–25 years) | ↑ significantly increase cerebral blood flow (CBF) during task performance, as indexed by total concentrations of hemoglobin and deoxyhemoglobin | Functional NIRS is a brain-imaging technique that is predicated on the intrinsic optical absorption properties of oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) after the introduction of near-infrared light through the intact skull. |
| Wightman et al. [47] | Double-blind, placebo-controlled, counterbalanced order, crossover design | Green tea polyphenol epigallocatechin gallate (EGCG) | Two capsules each containing either 135 mg or 270 mg EGCG (94% pure EGCG plus 6% excipients) | Two doses (135 and 270 mg) of EGCG | Acute | 7 days | Twenty-seven healthy adults (11 men, 16 women, mean age 22 years, range 18–30 years) | ↓ significantly decrease both oxygenated and total hemoglobin, ↔ non-significant effect on deoxygenated hemoglobin. | NIRS is a non-invasive brain imaging technique in which two nominal wavelengths of light (~765 and 855 nm), which are differentially absorbed by oxygenated (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) |
| Wightman et al. [48] | Randomized, double-blind, placebo-controlled, counterbalanced order cross-over | Resveratrol | 250 mg of trans-resveratrol | Two capsules | Acute | At least a week | Twenty-three healthy adults (four males and nineteen females, mean age 21 years, range 19–34 years, SD 3·2 years, all right handed) | ↑ significantly improve CBF during task performance | Near-IR spectroscopy |
| Wightman et al. [36] | Randomized, double-blind, placebo-controlled, parallel-groups study | Resveratrol | TransmaxTM by BiotiviaTM with a guaranteed purity of 98%, also containing 10 mg of piperine/ capsule | 500 mg once day | Acute and chronic (28 days) | N/A | Sixty adults aged between 18 and 30 years | ↑ significantly improve CBF parameters on day 1, as assessed by NIRS | Transcranial Doppler; near-IR spectroscopy (NIRS); Venous blood samples |
| Decroix et al. [50] | Randomized, double-blind, placebo-controlled, counterbalanced order, crossover design | Cocoa flavanol | High CF-content chocolate milk (903.75 mg flavanol) or a PLA contained low-CF chocolate milk (15 mg flavanol) | 900 mg | Acute | 7 days | Twelve well-trained men of 30 ± 3 years old | ↑ significantly increase cerebral oxygenation; ↔ non-significant effect on BDNF | Functional NIRS, a noninvasive optical imaging technique, was used to assess acute changes in local cerebral blood volume (reflecting CBF) and oxygenation (Oxymon continuous-wave NIRS (CW-NIRS) system (Artinis Medical Systems B.V.); Blood parameter (BDNF) |
| Lamport et al. [38] | Randomized, single-blind, placebo-controlled, counterbalanced order, crossover design | Flavanone-rich citrus juice | 70.5-mg flavonoids | 500-mL citrus juice containing 70.5-mg flavonoids | Acute | 1-week | Sixteen healthy young adults aged 18–30 years | ↑ significantly increase regional perfusion in the inferior and middle right frontal gyrus at 2 h relative to baseline and the control drink. | fMRI arterial spin labelling (ASL) |
| Sadowska-Kr˛epa et al. [42] | Randomized, double-blind, placebo-controlled, parallel-groups study | Ginkgo biloba Supplementation | 80 mg EGb capsules containing 19.2 mg flavonoid glycosides (24%) | Two capsules once a day (160 mg/day) | Six weeks | N/A | 18healthy, physically active young men, age category: 18–25 years | ↔ non-significant effect on basal BDNF content; ↑ significantly increase serum BDNF concentration immediately post-test | Blood parameter (BDNF) |
| Sadowska-Krępa et al. [43] | Randomized, double-blind, placebo-controlled, parallel-groups study | Green tea extract (GTE) | One 250 mg GTE capsule contained 245 mg polyphenols, including 200 mg catechins | Two capsules once daily | Six weeks | N/A | 16 healthy, physicallyactive young men, age category (18–25 years) | ↔ non-significant effect on BDNF | Blood parameter (BDNF) |
| Items | File et al. [41] | Francis et al. [45] | Spaak et al. [52] | Kennedy et al. [46] | Wightman et al. [47] | Wightman et al. [48] | Massee et al. [49] | Wightman et al. [36] | Alharbi et al. [37] | Decroix et al. [50] | Lamport et al. [38] | Dietz et al. [39] | Haskell-Ramsay et al. [51] | Sadowska-Krepa et al. [42] | Karabay et al. [40] | Sadowska-Krepa et al. [43] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Eligibility criteria were specified | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 2 | Subjects were randomly allocated to groups (in a crossover study, subjects were randomly allocated an order in which treatments were received) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 3 | Allocation was concealed | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 4 | The groups were similar at baseline regarding the most important prognostic indicators | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 5 | There was blinding of all subjects | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 6 | There was blinding of all therapists who administered the therapy | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 7 | There was blinding of all assessors who measured at least one key outcome | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 8 | Measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 9 | All subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analyzed by “intention to treat” | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 10 | The results of between-group statistical comparisons are reported for at least one key outcome | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 11 | The study provides both point measures and measures of variability for at least one key outcome | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Total score | 9 | 9 | 7 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, A.; Trabelsi, K.; Boukhris, O.; Bouaziz, B.; Müller, P.; M Glenn, J.; Bott, N.T.; Müller, N.; Chtourou, H.; Driss, T.; et al. Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1598. https://doi.org/10.3390/jcm9051598
Ammar A, Trabelsi K, Boukhris O, Bouaziz B, Müller P, M Glenn J, Bott NT, Müller N, Chtourou H, Driss T, et al. Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2020; 9(5):1598. https://doi.org/10.3390/jcm9051598
Chicago/Turabian StyleAmmar, Achraf, Khaled Trabelsi, Omar Boukhris, Bassem Bouaziz, Patrick Müller, Jordan M Glenn, Nicholas T. Bott, Notger Müller, Hamdi Chtourou, Tarak Driss, and et al. 2020. "Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 9, no. 5: 1598. https://doi.org/10.3390/jcm9051598
APA StyleAmmar, A., Trabelsi, K., Boukhris, O., Bouaziz, B., Müller, P., M Glenn, J., Bott, N. T., Müller, N., Chtourou, H., Driss, T., & Hökelmann, A. (2020). Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 9(5), 1598. https://doi.org/10.3390/jcm9051598










