Combined and Isolated Effects of Acute Exercise and Brain Stimulation on Executive Function in Healthy Young Adults
Abstract
1. Introduction
1.1. Acute Exercise and Executive Function
1.2. Single-Session tDCS and Executive Function
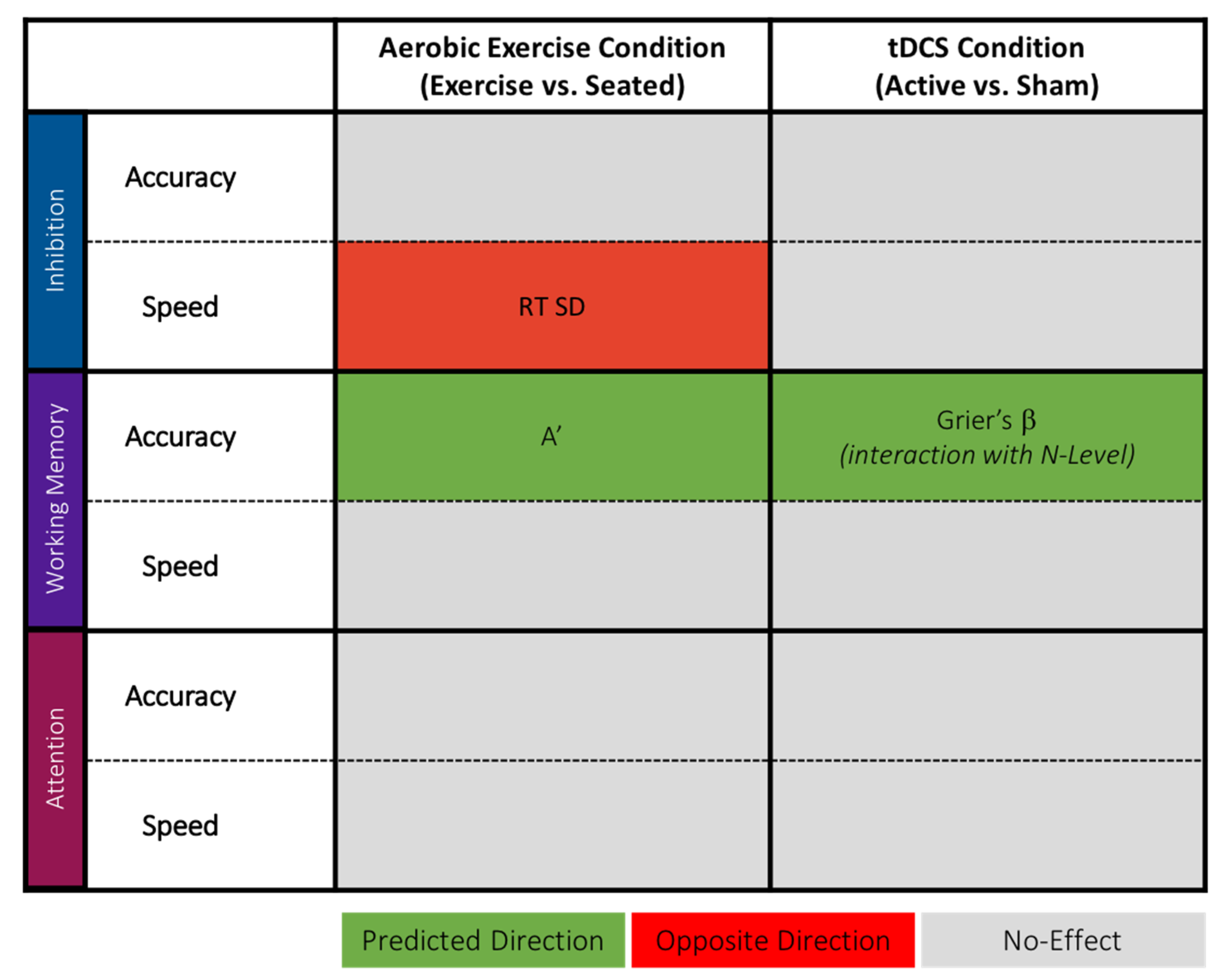
1.3. Current Study Design and Predictions
2. Materials and Methods
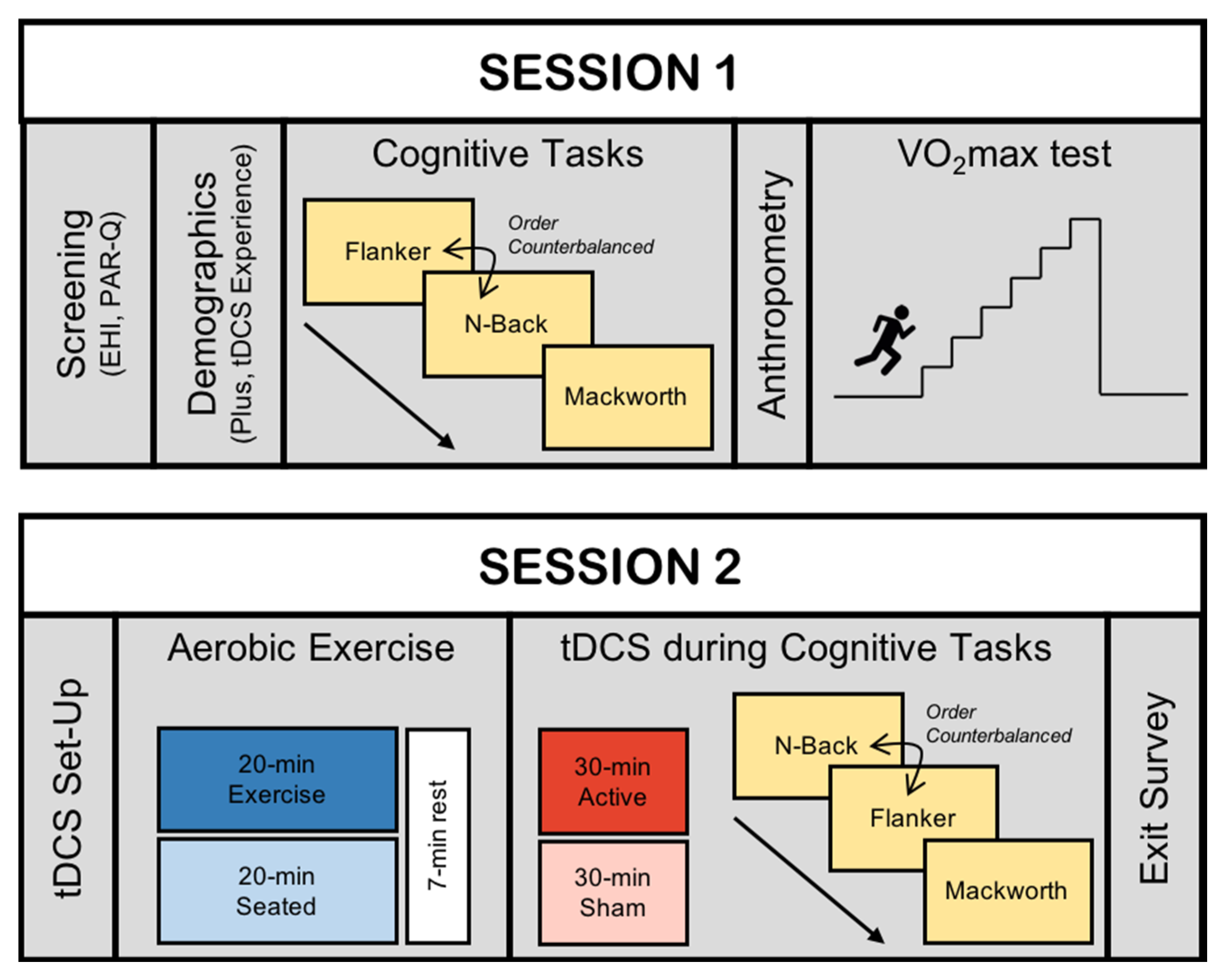
2.1. Study Design
2.2. Participants
2.3. Session 1
2.3.1. Screening Procedures
2.3.2. Demographics
2.3.3. Baseline Cognitive Task Assessment
2.3.4. Cardiorespiratory Fitness Assessment
2.4. Session 2
2.4.1. Aerobic Exercise Manipulation
2.4.2. tDCS Manipulation
2.4.3. Cognitive Assessments
Inhibition (Flanker Task)
Working Memory (n-Back Task)
Sustained Attention (Mackworth Clock Task)
2.4.4. Exit Survey
2.5. Statistical Analysis
2.5.1. Inhibition (Flanker Task)
2.5.2. Working Memory (n-Back Task)
2.5.3. Sustained Attention (Mackworth Clock Task)
3. Results
3.1. tDCS Expectations and Sensations
3.2. Baseline Fitness
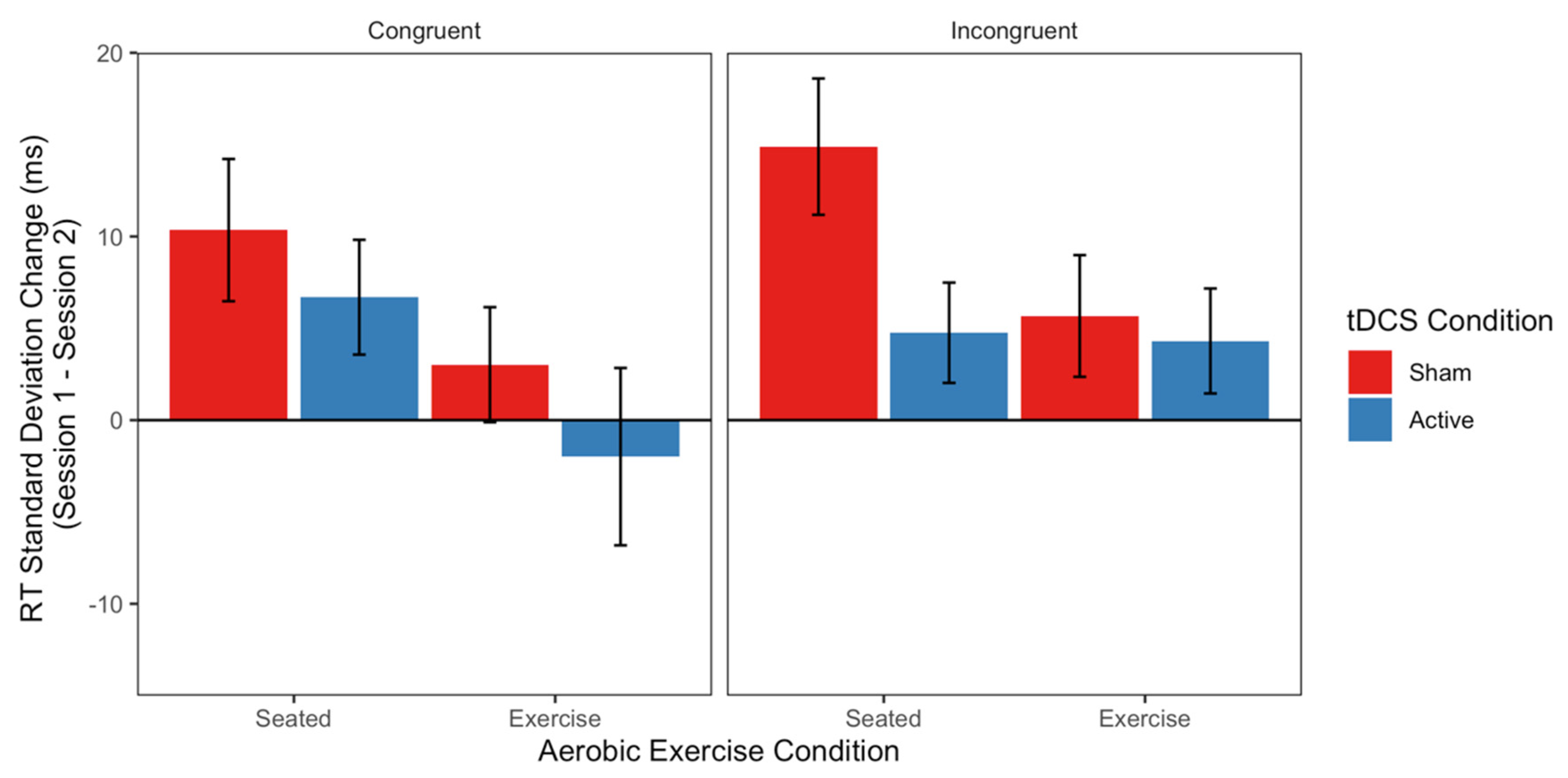
3.3. Inhibition (Flanker Task)
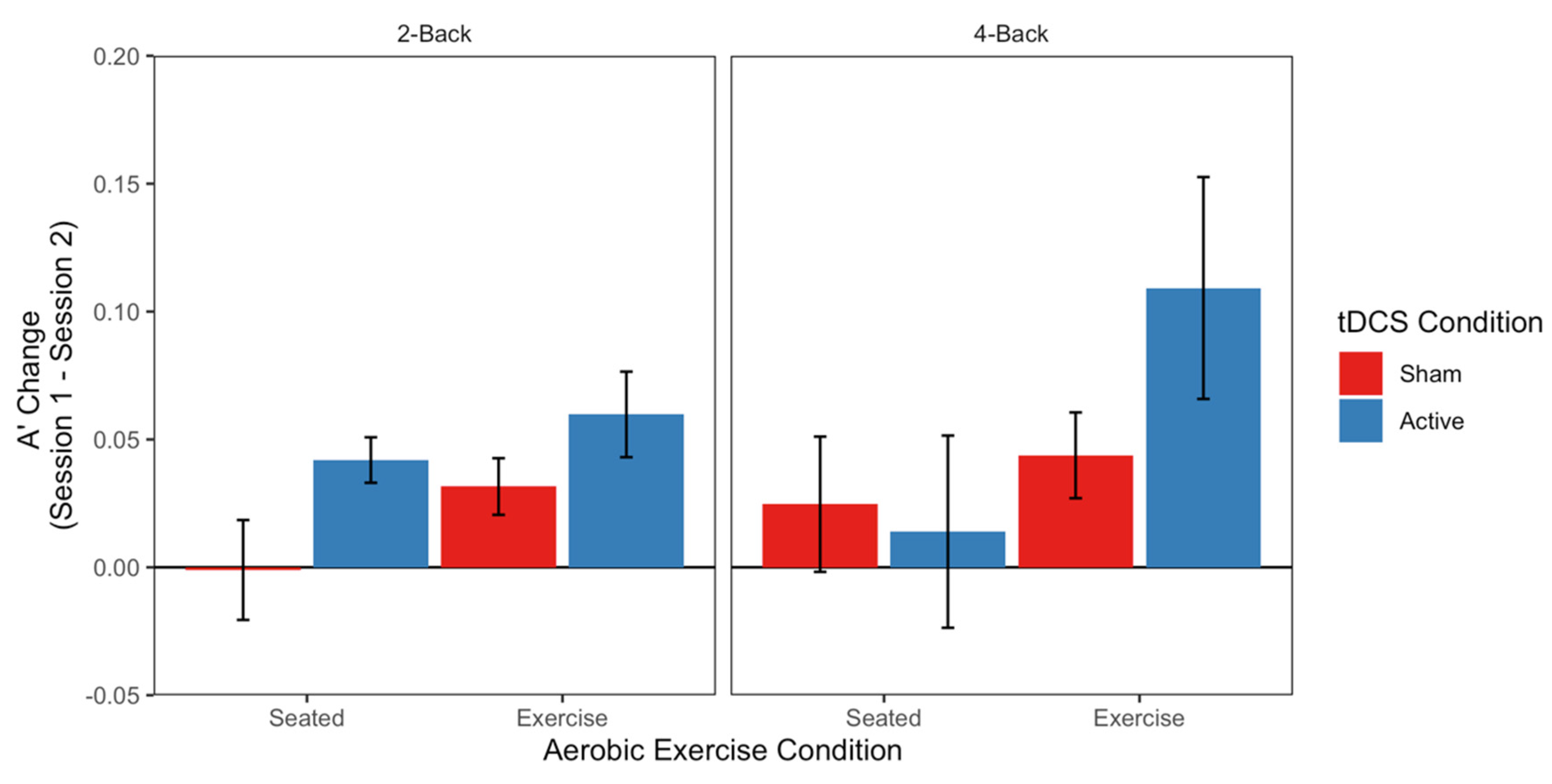
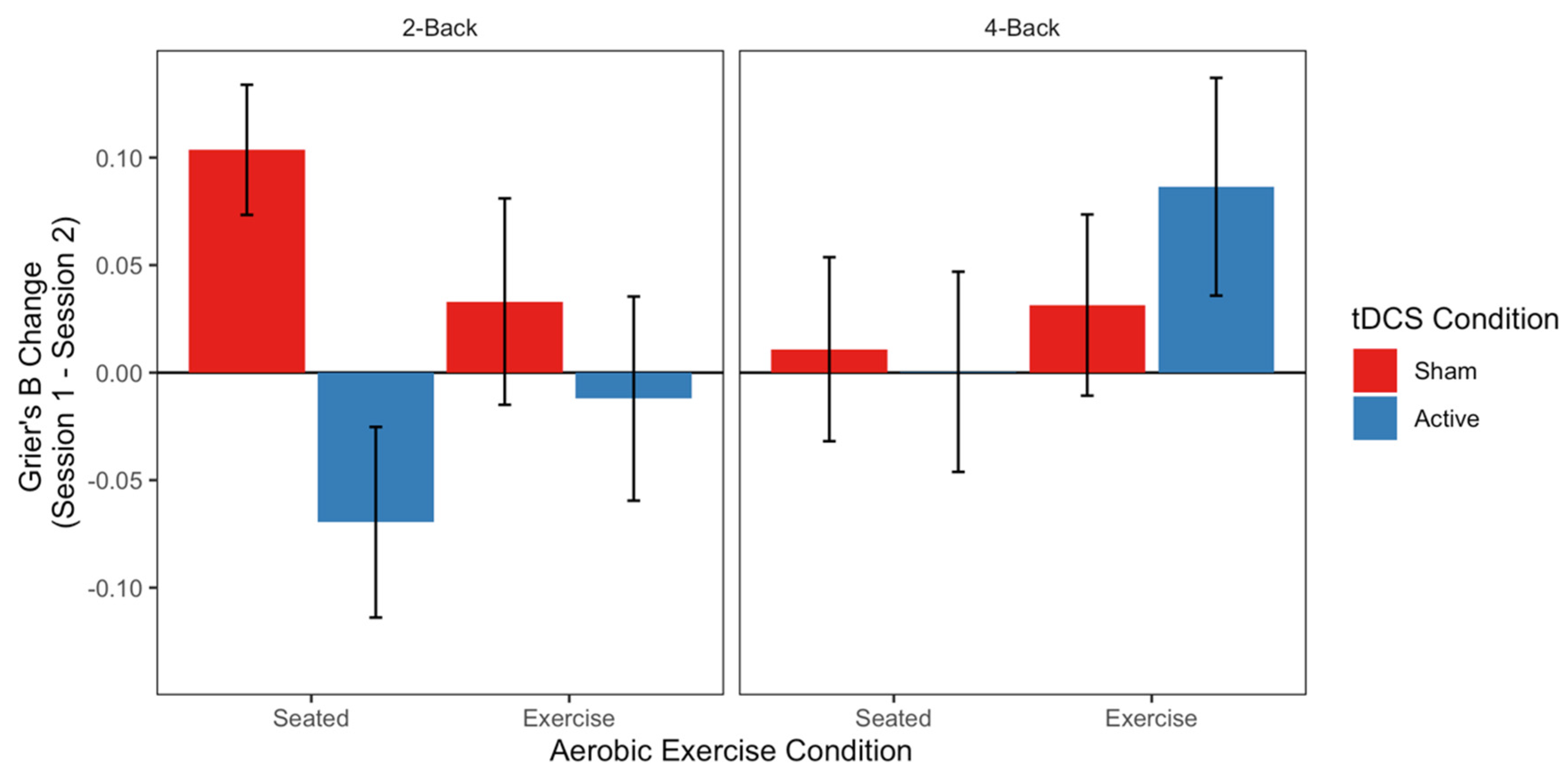
3.4. Working Memory (n-Back Task)
3.5. Sustained Attention (Mackworth Clock Task)
4. Discussion
4.1. Exercise and tDCS for Improved Inhibition
4.2. Exercise and tDCS for Improved Working Memory
4.3. Exercise and tDCS for Improved Sustained Attention
4.4. Combining Exercise and tDCS
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pontifex, M.B.; McGowan, A.L.; Chandler, M.C.; Gwizdala, K.L.; Parks, A.; Fenn, K.; Kamijo, K. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychol. Sport Exerc. 2019, 40, 1–22. [Google Scholar] [CrossRef]
- Oberste, M.; Javelle, F.; Sharma, S.; Joisten, N.; Walzik, D.; Bloch, W.; Zimmer, P. Effects and Moderators of Acute Aerobic Exercise on Subsequent Interference Control: A Systematic Review and Meta-Analysis. Front. Psychol. 2019, 10, 2616. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Vanderhasselt, M.-A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 2014, 86, 1–9. [Google Scholar] [CrossRef]
- Hill, A.T.; Fitzgerald, P.B.; Hoy, K.E.; Information, P.E.K.F.C. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings From Healthy and Neuropsychiatric Populations. Brain Stimul. 2016, 9, 197–208. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Smith, E.E.; Jonides, J. Storage and Executive Processes in the Frontal Lobes. Science 1999, 283, 1657–1661. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Hillman, C.H. The influence of exercise on cognitive abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Giordano, J.; Bikson, M.; Kappenman, E.S.; Clark, V.P.; Coslett, H.B.; Hamblin, M.R.; Hamilton, R.; Jankord, R.; Kozumbo, W.J.; McKinley, R.A.; et al. Mechanisms and Effects of Transcranial Direct Current Stimulation. Dose Response 2017, 15, 1559325816685467. [Google Scholar] [CrossRef] [PubMed]
- Cespón, J.; Miniussi, C.; Pellicciari, M.C. Interventional programmes to improve cognition during healthy and pathological ageing: Cortical modulations and evidence for brain plasticity. Ageing Res. Rev. 2018, 43, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Wang, C.-H.; Tseng, P.; Juan, C.-H. Blending transcranial direct current stimulations and physical exercise to maximize cognitive improvement. Front. Psychol. 2015, 6, 678. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K.C.; Pontifex, M.B.; Scudder, M.R.; Brown, M.L.; Hillman, C.H. The effects of single bouts of aerobic exercise, exergaming, and videogame play on cognitive control. Clin. Neurophysiol. 2011, 122, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.; Paul, E.; Watson, P.; Cooke, G.E.; Hillman, C.H.; Cohen, N.J.; Kramer, A.F.; Barbey, A.K. Enhanced Learning through Multimodal Training: Evidence from a Comprehensive Cognitive, Physical Fitness, and Neuroscience Intervention. Sci. Rep. 2017, 7, 5808. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, F.; Pixa, N.H.; Fregni, F. A Review of Acute Aerobic Exercise and Transcranial Direct Current Stimulation Effects on Cognitive Functions and Their Potential Synergies. Front. Hum. Neurosci. 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.B.; Rosselli, M. The Elusive Nature of Executive Functions: A Review of our Current Understanding. Neuropsychol. Rev. 2007, 17, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D.; Craik, F.I.M.; Booth, L. Executive function across the life span. Acta Psychol. 2004, 115, 167–183. [Google Scholar] [CrossRef]
- Chang, Y.; Labban, J.; Gapin, J.; Etnier, J. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef]
- Li, L.; Men, W.-W.; Chang, Y.-K.; Fan, M.-X.; Ji, L.; Wei, G.-X. Acute Aerobic Exercise Increases Cortical Activity during Working Memory: A Functional MRI Study in Female College Students. PLoS ONE 2014, 9, e99222. [Google Scholar] [CrossRef]
- Kao, S.-C.; Westfall, D.R.; Soneson, J.; Gurd, B.; Hillman, C.H. Comparison of the acute effects of high-intensity interval training and continuous aerobic walking on inhibitory control. Psychophysiology 2017, 54, 1335–1345. [Google Scholar] [CrossRef]
- Hillman, C.H.; Snook, E.M.; Jerome, G.J. Acute cardiovascular exercise and executive control function. Int. J. Psychophysiol. 2003, 48, 307–314. [Google Scholar] [CrossRef]
- Roig, M.; Nordbrandt, S.; Geertsen, S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Hogan, C.L.; Mata, J.; Carstensen, L.L. Exercise holds immediate benefits for affect and cognition in younger and older adults. Psychol. Aging 2013, 28, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.B.; Pierce, G.L.; Darling, W.G.; Voss, M.W. Differential Effects of Acute Exercise on Distinct Aspects of Executive Function. Med. Sci. Sports Exerc. 2015, 47, 1460–1469. [Google Scholar] [CrossRef]
- Aks, D.J. Influence of Exercise on Visual Search: Implications for Mediating Cognitive Mechanisms. Percept. Mot. Skill 1998, 87, 771–783. [Google Scholar] [CrossRef]
- Bullock, T.; Giesbrecht, B. Acute exercise and aerobic fitness influence selective attention during visual search. Front. Psychol. 2014, 5. [Google Scholar] [CrossRef]
- Bard, C.; Fleury, M. Influence of Imposed Metabolic Fatigue on Visual Capacity Components. Percept. Mot. Skill 1978, 47, 1283–1287. [Google Scholar] [CrossRef]
- Brunyé, T.T.; Hussey, E.K.; Fontes, E.B.; Ward, N. Modulating Applied Task Performance via Transcranial Electrical Stimulation. Front. Hum. Neurosci. 2019, 13, 140. [Google Scholar] [CrossRef]
- Kuo, M.-F.; Nitsche, M.A. Exploring prefrontal cortex functions in healthy humans by transcranial electrical stimulation. Neurosci. Bull. 2015, 31, 198–206. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Reinhart, R.M.G.; Cosman, J.D.; Fukuda, K.; Woodman, G.F. Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Atten. Percept. Psychophys. 2017, 79, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.; Valero-Cabré, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2011, 5, 175–195. [Google Scholar] [CrossRef]
- Gbadeyan, O.; McMahon, K.L.; Steinhauser, M.; Meinzer, M. Stimulation of Dorsolateral Prefrontal Cortex Enhances Adaptive Cognitive Control: A High-Definition Transcranial Direct Current Stimulation Study. J. Neurosci. 2016, 36, 12530–12536. [Google Scholar] [CrossRef] [PubMed]
- Karuza, E.A.; Balewski, Z.Z.; Hamilton, R.H.; Medaglia, J.D.; Tardiff, N.; Thompson-Schill, S.L. Mapping the Parameter Space of tDCS and Cognitive Control via Manipulation of Current Polarity and Intensity. Front. Hum. Neurosci. 2016, 10, 2687. [Google Scholar] [CrossRef] [PubMed]
- Denis, G.; Zory, R.; Radel, R. Testing the role of cognitive inhibition in physical endurance using high-definition transcranial direct current stimulation over the prefrontal cortex. Hum. Mov. Sci. 2019, 67, 102507. [Google Scholar] [CrossRef] [PubMed]
- DeDoncker, J.; Brunoni, A.R.; Baeken, C.; Vanderhasselt, M.-A. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimul. 2016, 9, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.C.; Forte, J.D.; Carter, O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimul. 2015, 8, 535–550. [Google Scholar] [CrossRef]
- Mancuso, L.E.; Ilieva, I.P.; Hamilton, R.H.; Farah, M.J. Does Transcranial Direct Current Stimulation Improve Healthy Working Memory?: A Meta-analytic Review. J. Cogn. Neurosci. 2016, 2, 1–27. [Google Scholar] [CrossRef]
- McKinley, R.A. Transcranial Direct Current Stimulation for Fatigue and Attentional Disorders. In Neuromodulation; Academic Press: London, UK, 2018; pp. 1637–1648. [Google Scholar]
- Reteig, L.C.; Talsma, L.J.; Van Schouwenburg, M.R.; Slagter, H.A. Transcranial Electrical Stimulation as a Tool to Enhance Attention. J. Cogn. Enhanc. 2017, 2016, 1–25. [Google Scholar] [CrossRef]
- McIntire, L.K.; McKinley, R.A.; Goodyear, C.; Nelson, J. A Comparison of the Effects of Transcranial Direct Current Stimulation and Caffeine on Vigilance and Cognitive Performance During Extended Wakefulness. Brain Stimul. 2014, 7, 499–507. [Google Scholar] [CrossRef]
- McIntire, L.K.; McKinley, R.A.; Nelson, J.M.; Goodyear, C. Transcranial direct current stimulation versus caffeine as a fatigue countermeasure. Brain Stimul. 2017, 10, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Seo, J.-H.; Kim, Y.-H.; Ko, M.-H. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. NeuroReport 2014, 25, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Hussey, E.K.; Ward, N.; Christianson, K.; Kramer, A.F. Language and Memory Improvements following tDCS of Left Lateral Prefrontal Cortex. PLoS ONE 2015, 10, e0141417. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Han, S.J. Improvement of the Working Memory and Naming by Transcranial Direct Current Stimulation. Ann. Rehabil. Med. 2012, 36, 585–595. [Google Scholar] [CrossRef]
- Oldfield, R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychology 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Schade, S.; Moliadze, V.; Paulus, W.; Antal, A. Modulating neuronal excitability in the motor cortex with tDCS shows moderate hemispheric asymmetry due to subjects’ handedness: A pilot study. Restor. Neurol. Neurosci. 2012, 30, 191–198. [Google Scholar] [CrossRef]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can. J. Sport Sci. J. Can. Sci. Sport 1992, 17, 338–345. [Google Scholar]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- DaSilva, A.F.; Volz, M.S.; Bikson, M.; Fregni, F. Electrode positioning and montage in transcranial direct current stimulation. J. Vis. Exp. 2011, e2744. [Google Scholar] [CrossRef]
- McKinley, R.A.; McIntire, L.; Bridges, N.; Goodyear, C.; Bangera, N.B.; Weisend, M.P. Acceleration of image analyst training with transcranial direct current stimulation. Behav. Neurosci. 2013, 127, 936–946. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Deveau, J.; Jaeggi, S.M.; Zordan, V.; Phung, C.; Seitz, A.R. How to build better memory training games. Front. Syst. Neurosci. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E.; Bullmore, E.T. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Hussey, E.K.; Harbison, J.I.; Teubner-Rhodes, S.; Mishler, A.; Velnoskey, K.; Novick, J.M. Memory and Language Improvements Following Cognitive Control Training. J. Exp. Psychol. Learn. Mem. Cogn. 2017, 43, 23–58. [Google Scholar] [CrossRef] [PubMed]
- Stanislaw, H.; Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999, 31, 137–149. [Google Scholar] [CrossRef]
- Balota, D.A.; Yap, M.J. Moving Beyond the Mean in Studies of Mental Chronometry. Curr. Dir. Psychol. Sci. 2011, 20, 160–166. [Google Scholar] [CrossRef]
- Flehmig, H.C.; Steinborn, M.; Langner, R.; Scholz, A.; Westhoff, K. Assessing intraindividual variability in sustained attention: Reliability, relation to speed and accuracy, and practice effects. Psychol. Sci. 2007, 49, 132–149. [Google Scholar]
- Heathcote, A.; Popiel, S.J.; Mewhort, D.J. Analysis of response time distributions: An example using the Stroop task. Psychol. Bull. 1991, 109, 340–347. [Google Scholar] [CrossRef]
- Spieler, D.H.; Balota, D.A.; Faust, M.E. Levels of selective attention revealed through analyses of response time distributions. J. Exp. Psychol. Hum. Percept. Perform. 2000, 26, 506–526. [Google Scholar] [CrossRef]
- Moore, R.D.; Wu, C.-T.; Pontifex, M.B.; O’Leary, K.C.; Scudder, M.R.; Raine, L.B.; Johnson, C.R.; Hillman, C.H. Aerobic fitness and intra-individual variability of neurocognition in preadolescent children. Brain Cogn. 2013, 82, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Raine, L.B.; Kao, S.-C.; Pindus, D.; Westfall, D.R.; Shigeta, T.T.; Logan, N.; Cadenas-Sanchez, C.; Li, J.; Drollette, E.S.; Pontifex, M.B.; et al. A Large-Scale Reanalysis of Childhood Fitness and Inhibitory Control. J. Cogn. Enhanc. 2018, 2, 170–192. [Google Scholar] [CrossRef]
- Henseler, I.; Mädebach, A.; Kotz, S.; Jescheniak, J.D. Modulating Brain Mechanisms Resolving Lexico-semantic Interference during Word Production: A Transcranial Direct Current Stimulation Study. J. Cogn. Neurosci. 2014, 26, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Penolazzi, B.; Pastore, M.; Mondini, S. Electrode montage dependent effects of transcranial direct current stimulation on semantic fluency. Behav. Brain Res. 2013, 248, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Brysbaert, M.; Stevens, M. Power Analysis and Effect Size in Mixed Effects Models: A Tutorial. J. Cogn. 2018, 1, 9. [Google Scholar] [CrossRef]
- Baayen, R.H.; Davidson, D.J.; Bates, D. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008, 59, 390–412. [Google Scholar] [CrossRef]
- Luke, S.G. Evaluating significance in linear mixed-effects models in R. Behav. Res. Methods 2016, 49, 1494–1502. [Google Scholar] [CrossRef]
- Brown, V.A. An Approachable Introduction to Linear Mixed Effects Modeling with Implementation in R 2020. Available online: https://psyarxiv.com/9vghm/ (accessed on 26 April 2020).
- Enders, C.K.; Tofighi, D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychol. Methods 2007, 12, 121–138. [Google Scholar] [CrossRef]
- Hillman, C.H.; Pontifex, M.B.; Raine, L.B.; Castelli, D.M.; Hall, E.E.; Kramer, A.F. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience 2009, 159, 1044–1054. [Google Scholar] [CrossRef]
- Themanson, J.; Hillman, C.H. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience 2006, 141, 757–767. [Google Scholar] [CrossRef]
- Mehren, A.; Özyurt, J.; Lam, A.P.; Brandes, M.; Müller, H.H.O.; Thiel, C.M.; Philipsen, A. Acute Effects of Aerobic Exercise on Executive Function and Attention in Adult Patients With ADHD. Front. Psychol. 2019, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Tamnes, C.K.; Fjell, A.M.; Westlye, L.T.; Østby, Y.; Walhovd, K.B. Becoming Consistent: Developmental Reductions in Intraindividual Variability in Reaction Time Are Related to White Matter Integrity. J. Neurosci. 2012, 32, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.C.; Zacks, R.T.; Slade, J.M. Brain activation during interference resolution in young and older adults: An fMRI study. NeuroImage 2010, 50, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Zanão, T.A.; Valiengo, L.; Lotufo, P.A.; Benseñor, I.M.; Fregni, F.; Brunoni, A.R. Acute working memory improvement after tDCS in antidepressant-free patients with major depressive disorder. Neurosci. Lett. 2013, 537, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.T.; Coombes, S.A.; Armstrong, N.B.; Higgins, T.J.; Janelle, C.M. Evaluating attentional and affective changes following an acute exercise bout using a modified dot-probe protocol. J. Sports Sci. 2010, 28, 1065–1076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Llorens, F.; Sanabria, D.; Huertas, F. The Influence of Acute Intense Exercise on Exogenous Spatial Attention Depends on Physical Fitness Level. Exp. Psychol. 2015, 62, 20–29. [Google Scholar] [CrossRef]
- Bue-Estes, C.L.; Willer, B.; Burton, H.; Leddy, J.J.; Wilding, G.E.; Horvath, P.J. Short-Term Exercise to Exhaustion and its Effects on Cognitive Function in Young Women. Percept. Mot. Skill 2008, 107, 933–945. [Google Scholar] [CrossRef]
- Fritz, K.M.; O’Connor, P.J. Acute Exercise Improves Mood and Motivation in Young Men with ADHD Symptoms. Med. Sci. Sports Exerc. 2016, 48, 1153–1160. [Google Scholar] [CrossRef]
- Nelson, J.; McKinley, R.A.; Golob, E.J.; Warm, J.S.; Parasuraman, R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS). NeuroImage 2014, 85, 909–917. [Google Scholar] [CrossRef]
- Axelrod, V.; Rees, G.; Lavidor, M.; Bar, M. Increasing propensity to mind-wander with transcranial direct current stimulation. Proc. Natl. Acad. Sci. USA 2015, 112, 3314–3319. [Google Scholar] [CrossRef]
- Edwards, D.; Cortes, M.; Wortman-Jutt, S.; Putrino, D.; Bikson, M.; Thickbroom, G.; Pascual-Leone, A. Transcranial Direct Current Stimulation and Sports Performance. Front. Hum. Neurosci. 2017, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Angius, L.; Hopker, J.G.; Mauger, A.R. The Ergogenic Effects of Transcranial Direct Current Stimulation on Exercise Performance. Front. Physiol. 2017, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.P.; Coffman, B.A.; Mayer, A.R.; Weisend, M.P.; Lane, T.D.; Calhoun, V.D.; Raybourn, E.M.; Garcia, C.M.; Wassermann, E.M. TDCS guided using fMRI significantly accelerates learning to identify concealed objects. NeuroImage 2010, 59, 117–128. [Google Scholar] [CrossRef]
- Angius, L.; Pageaux, B.; Hopker, J.G.; Marcora, S.; Mauger, A.R. Transcranial direct current stimulation improves isometric time to exhaustion of the knee extensors. Neurosci. 2016, 339, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Angius, L.; Santarnecchi, E.; Pascual-Leone, A.; Marcora, S. Transcranial Direct Current Stimulation over the Left Dorsolateral Prefrontal Cortex Improves Inhibitory Control and Endurance Performance in Healthy Individuals. Neuroscience 2019, 419, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Okano, A.H.; Fontes, E.B.; Montenegro, R.; Farinatti, P.; Cyrino, E.S.; Min, L.L.; Bikson, M.; Noakes, T.D. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br. J. Sports Med. 2013, 49, 1213–1218. [Google Scholar] [CrossRef]
- Hwang, J.; Castelli, D.M.; Gonzalez-Lima, F. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med. Sci. 2016, 31, 1151–1160. [Google Scholar] [CrossRef]
- Nieratschker, V.; Kiefer, C.; Giel, K.; Krüger, D.M.R.; Plewnia, C. The COMT Val/Met Polymorphism Modulates Effects of tDCS on Response Inhibition. Brain Stimul. 2015, 8, 283–288. [Google Scholar] [CrossRef]
- Labelle, V.; Bosquet, L.; Mekary, S.; Vu, T.T.M.; Smilovitch, M.; Bherer, L. Fitness Level Moderates Executive Control Disruption During Exercise Regardless of Age. J. Sport Exerc. Psychol. 2014, 36, 258–270. [Google Scholar] [CrossRef]
- Labelle, V.; Bosquet, L.; Mekary, S.; Bherer, L. Decline in executive control during acute bouts of exercise as a function of exercise intensity and fitness level. Brain Cogn. 2013, 81, 10–17. [Google Scholar] [CrossRef]
- Hendy, A.; MacPherson, H.; Nuzum, N.; Della Gatta, P.A.; Alexander, S.E.; Hoy, K.E.; Enticott, P.G.; Teo, W.-P. High intensity aerobic exercise does not prime the brain for anodal transcranial direct current stimulation. Brain Stimul. 2019, 12, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
| Demographics and Anthropometry (Session 1) | |||||||
| Exercise | tDCS | Age (Years) | Sex (No. Females) | Height (cm) | Weight (kg) | ||
| Exercise | Active | 22.58 | 12 | 169.2 ± 12.0 | 71.2 ± 21.0 | ||
| Exercise | Sham | 22.21 | 18 | 165.2 ± 10.2 | 65.4 ± 18.8 | ||
| Seated | Active | 23.54 | 12 | 170.4 ± 9.7 | 72.7 ± 27.5 | ||
| Seated | Sham | 20.83 | 18 | 167.0 ± 10.3 | 66.1 ± 10.0 | ||
| Cardiorespiratory Fitness Baseline (Session 1) | |||||||
| Exercise | tDCS | VO2 Peak (mL/kg/min) | Baseline HR (bpm) | Max HR (bpm) | Grade (% incline) | Speed (mph) | Duration (s) |
| Exercise | Active | 50.3 ± 10.0 | 77.6 ± 13.3 | 185.3 ± 15.4 | 11 +/− 3.1 | 5.5 +/− 0.6 | 610.8 +/− 156.7 |
| Exercise | Sham | 46.6 ± 9.9 | 77.0 ± 17.1 | 186.8 ± 8.1 | 10.5 +/− 3.6 | 5.4 +/− 0.5 | 572.8 +/− 190 |
| Seated | Active | 49.8 ± 8.0 | 76.6 ± 9.3 | 189.9 ± 9.2 | 10.8 +/− 3.5 | 5.7 +/− 0.6 | 601.9 +/− 152.4 |
| Seated | Sham | 45.1 ± 10.5 | 81.3 ± 13.6 | 191.5 ± 9.8 | 11 +/− 1.8 | 5.6 +/− 0.5 | 609.1 +/− 80 |
| tDCS Expectations (Sessions 1 and 2) | |||||||
| Exercise | tDCS | Before Session 1 | After Session 2 | ||||
| (Out of 10) | (Out of 10) | ||||||
| Exercise | Active | 4.50 | 5.33 | ||||
| Exercise | Sham | 4.29 | 4.25 | ||||
| Seated | Active | 4.71 | 5.57 | ||||
| Seated | Sham | 4.33 | 5.08 | ||||
| tDCS Sensations (Session 2) | |||||||
| Exercise | tDCS | Distracting | Itchy | Pain | Heat | Discomfort | |
| (Yes = 1/No = 0) | (Out of 10) | (Out of 10) | (Out of 10) | (Out of 10) | |||
| Exercise | Active | 0.33 | 2.88 | 1.33 | 1.42 | 2.38 | |
| Exercise | Sham | 0.17 | 2.38 | 1.75 | 1.29 | 2.08 | |
| Seated | Active | 0.22 | 3.43 | 1.26 | 2.09 | 2.52 | |
| Seated | Sham | 0.21 | 2.79 | 1.83 | 1.71 | 2.38 | |
| Predictor | Fixed Effects | Random Effects | ||
|---|---|---|---|---|
| Coefficient | SE | t-Value | By-Subject Variance | |
| Accuracy | ||||
| Intercept | −0.001 | 0.007 | −0.161 | 0.003074 |
| Aerobic Exercise | −0.015 | 0.013 | −1.149 | |
| tDCS | −0.014 | 0.013 | −1.082 | |
| Congruency | −0.003 | 0.006 | −0.481 | |
| Aerobic Exercise x tDCS | −0.011 | 0.026 | −0.405 | |
| Aerobic Exercise x Congruency | 0.001 | 0.013 | 0.078 | |
| tDCS x Congruency | −0.007 | 0.013 | −0.503 | |
| Aerobic Exercise x tDCS x Congruency | −0.014 | 0.026 | −0.525 | |
| Correct Median Response Time (ms) | ||||
| Intercept | 4.208 | 2.773 | 1.518 | 690.07 |
| Aerobic Exercise | −4.865 | 5.546 | −0.877 | |
| tDCS | −7.010 | 5.546 | −1.264 | |
| Congruency | −1.292 | 1.415 | −0.913 | |
| Aerobic Exercise x tDCS | 0.625 | 11.091 | 0.056 | |
| Aerobic Exercise x Congruency | −2.938 | 2.830 | −1.038 | |
| tDCS x Congruency | −0.938 | 2.830 | −0.331 | |
| Aerobic Exercise x tDCS x Congruency | 0.500 | 5.659 | 0.088 | |
| Correct Response Time Standard Deviation (ms) | ||||
| Intercept | 5.963 | 1.513 | 3.941 | 144.8 |
| Aerobic Exercise | −6.420 | 3.026 | −2.122 | |
| tDCS | −5.039 | 3.026 | −1.665 | |
| Congruency | −2.890 | 1.767 | −1.635 | |
| Aerobic Exercise x tDCS | 3.711 | 6.052 | 0.613 | |
| Aerobic Exercise x Congruency | −3.171 | 3.535 | −0.897 | |
| tDCS x Congruency | 1.419 | 3.535 | 0.401 | |
| Aerobic Exercise x tDCS x Congruency | −10.121 | 7.070 | −1.431 | |
| ex-Gaussian μ | ||||
| Intercept | 2.267 | 2.524 | 0.898 | 484.4 |
| Aerobic Exercise | −1.371 | 5.048 | −0.272 | |
| tDCS | −5.969 | 5.048 | −1.183 | |
| Congruency | −0.288 | 2.302 | −0.125 | |
| Aerobic Exercise x tDCS | −2.284 | 10.096 | −0.226 | |
| Aerobic Exercise x Congruency | 0.315 | 4.603 | 0.068 | |
| tDCS x Congruency | −1.864 | 4.603 | −0.405 | |
| Aerobic Exercise x tDCS x Congruency | 1.132 | 9.207 | 0.123 | |
| ex-Gaussian σ | ||||
| Intercept | 6.167 | 1.073 | 5.746 | 49.56 |
| Aerobic Exercise | −3.748 | 2.147 | −1.746 | |
| tDCS | −4.275 | 2.147 | −1.991 | |
| Congruency | −2.173 | 1.595 | −1.362 | |
| Aerobic Exercise x tDCS | −1.581 | 4.293 | −0.368 | |
| Aerobic Exercise x Congruency | 2.482 | 3.189 | 0.778 | |
| tDCS x Congruency | −0.829 | 3.189 | −0.260 | |
| Aerobic Exercise x tDCS x Congruency | −8.989 | 6.379 | −1.409 | |
| ex-Gaussian τ | ||||
| Intercept | 1.556 | 1.782 | 0.873 | 165.5 |
| Aerobic Exercise | −3.934 | 3.563 | −1.104 | |
| tDCS | −1.643 | 3.563 | −0.461 | |
| Congruency | −0.566 | 2.409 | −0.235 | |
| Aerobic Exercise x tDCS | 3.420 | 7.127 | 0.480 | |
| Aerobic Exercise x Congruency | −3.365 | 4.817 | −0.699 | |
| tDCS x Congruency | 2.148 | 4.817 | 0.446 | |
| Aerobic Exercise x tDCS x Congruency | −3.643 | 9.634 | −0.378 | |
| Predictor | Fixed Effects | Random Effects | |||
|---|---|---|---|---|---|
| Coefficient | SE | t-Value | By-Subject Variance | By-Sequence Variance | |
| Average Accuracy | |||||
| Intercept | 0.049 | 0.007 | 7.498 | 0.00160 | 0.00006 |
| Aerobic Exercise | 0.021 | 0.011 | 1.877 | ||
| tDCS | 0.012 | 0.011 | 1.111 | ||
| N-Level | 0.009 | 0.007 | 1.189 | ||
| Aerobic Exercise x tDCS | −0.003 | 0.022 | −0.114 | ||
| Aerobic Exercise x N-Level | −0.008 | 0.015 | −0.515 | ||
| tDCS x N-Level | 0.021 | 0.015 | 1.443 | ||
| Aerobic Exercise x tDCS x N-Level | −0.015 | 0.030 | −0.522 | ||
| Discriminability (A’) | |||||
| Intercept | 0.040 | 0.009 | 4.452 | 0.00067 | |
| Aerobic Exercise | 0.041 | 0.018 | 2.266 | ||
| tDCS | 0.031 | 0.018 | 1.730 | ||
| N-Level | −0.015 | 0.017 | −0.852 | ||
| Aerobic Exercise x tDCS | 0.031 | 0.036 | 0.843 | ||
| Aerobic Exercise x N-Level | −0.032 | 0.035 | −0.919 | ||
| tDCS x N-Level | 0.008 | 0.035 | 0.237 | ||
| Aerobic Exercise x tDCS x N-Level | −0.091 | 0.070 | −1.307 | ||
| Response Bias (Grier’s β) | |||||
| Intercept | 0.023 | 0.038 | 0.612 | 0.007986 | 0.005785 |
| Aerobic Exercise | 0.023 | 0.032 | 0.729 | ||
| tDCS | −0.043 | 0.032 | −1.355 | ||
| N-Level | −0.019 | 0.026 | −0.703 | ||
| Aerobic Exercise x tDCS | 0.097 | 0.064 | 1.510 | ||
| Aerobic Exercise x N-Level | −0.060 | 0.053 | −1.135 | ||
| tDCS x N-Level | −0.131 | 0.053 | −2.493 | ||
| Aerobic Exercise x tDCS x N-Level | 0.063 | 0.105 | 0.593 | ||
| Correct Median Response Time (ms) | |||||
| Intercept | 80.55 | 13.46 | 5.99 | 11888.3 | 147.4 |
| Aerobic Exercise | −9.66 | 24.63 | −0.39 | ||
| tDCS | −22.66 | 24.63 | −0.92 | ||
| N-Level | −4.27 | 10.54 | −0.40 | ||
| Aerobic Exercise x tDCS | −36.05 | 49.25 | −0.73 | ||
| Aerobic Exercise x N-Level | −26.96 | 21.09 | −1.28 | ||
| tDCS x N-Level | 14.45 | 21.09 | 0.69 | ||
| Aerobic Exercise x tDCS x N-Level | −37.32 | 42.17 | −0.89 | ||
| Correct Response Time Standard Deviation | |||||
| Intercept | 31.51 | 6.67 | 4.72 | 2071.88 | 58.28 |
| Aerobic Exercise | −9.73 | 11.47 | −0.85 | ||
| tDCS | −5.05 | 11.47 | −0.44 | ||
| N-Level | −12.60 | 6.72 | −1.87 | ||
| Aerobic Exercise x tDCS | −24.24 | 22.94 | −1.06 | ||
| Aerobic Exercise x N-Level | 24.24 | 13.45 | 1.80 | ||
| tDCS x N-Level | −12.14 | 13.45 | −0.90 | ||
| Aerobic Exercise x tDCS x N-Level | −28.36 | 26.90 | −1.05 | ||
| ex-Gaussian μ | |||||
| Intercept | 57.25 | 12.18 | 4.70 | 10253 | |
| Aerobic Exercise | 2.68 | 24.36 | 0.11 | ||
| tDCS | −15.49 | 24.36 | −0.64 | ||
| N-Level | 7.06 | 12.89 | 0.55 | ||
| Aerobic Exercise x tDCS | −25.64 | 48.72 | −0.53 | ||
| Aerobic Exercise x N-Level | −33.97 | 25.78 | −1.32 | ||
| tDCS x N-Level | 10.71 | 25.78 | 0.42 | ||
| Aerobic Exercise x tDCS x N-Level | 2.92 | 51.55 | 0.06 | ||
| ex-Gaussian σ | |||||
| Intercept | 20.22 | 5.32 | 3.80 | 1464 | |
| Aerobic Exercise | 2.87 | 10.64 | 0.27 | ||
| tDCS | 3.53 | 10.64 | 0.33 | ||
| N-Level | −4.08 | 7.23 | −0.56 | ||
| Aerobic Exercise x tDCS | −11.42 | 21.29 | −0.54 | ||
| Aerobic Exercise x N-Level | 5.73 | 14.46 | 0.40 | ||
| tDCS x N-Level | −6.16 | 14.46 | −0.43 | ||
| Aerobic Exercise x tDCS x N-Level | −36.64 | 28.92 | −1.27 | ||
| ex-Gaussian τ | |||||
| Intercept | 24.83 | 6.42 | 3.86 | 1504 | |
| Aerobic Exercise | −1.25 | 12.85 | −0.10 | ||
| tDCS | 0.41 | 12.85 | 0.03 | ||
| N-Level | −13.00 | 10.12 | −1.28 | ||
| Aerobic Exercise x tDCS | −3.42 | 25.70 | −0.13 | ||
| Aerobic Exercise x N-Level | 8.98 | 20.24 | 0.44 | ||
| tDCS x N-Level | −29.95 | 20.24 | −1.48 | ||
| Aerobic Exercise x tDCS x N-Level | −27.36 | 40.48 | −0.68 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussey, E.K.; Fontes, E.B.; Ward, N.; Westfall, D.R.; Kao, S.-C.; Kramer, A.F.; Hillman, C.H. Combined and Isolated Effects of Acute Exercise and Brain Stimulation on Executive Function in Healthy Young Adults. J. Clin. Med. 2020, 9, 1410. https://doi.org/10.3390/jcm9051410
Hussey EK, Fontes EB, Ward N, Westfall DR, Kao S-C, Kramer AF, Hillman CH. Combined and Isolated Effects of Acute Exercise and Brain Stimulation on Executive Function in Healthy Young Adults. Journal of Clinical Medicine. 2020; 9(5):1410. https://doi.org/10.3390/jcm9051410
Chicago/Turabian StyleHussey, Erika K., Eduardo B. Fontes, Nathan Ward, Daniel R. Westfall, Shih-Chun Kao, Arthur F. Kramer, and Charles H. Hillman. 2020. "Combined and Isolated Effects of Acute Exercise and Brain Stimulation on Executive Function in Healthy Young Adults" Journal of Clinical Medicine 9, no. 5: 1410. https://doi.org/10.3390/jcm9051410
APA StyleHussey, E. K., Fontes, E. B., Ward, N., Westfall, D. R., Kao, S.-C., Kramer, A. F., & Hillman, C. H. (2020). Combined and Isolated Effects of Acute Exercise and Brain Stimulation on Executive Function in Healthy Young Adults. Journal of Clinical Medicine, 9(5), 1410. https://doi.org/10.3390/jcm9051410





