Prevalence of Elbow Joint Arthritis and Enthesitis in Rheumatoid Arthritis
Abstract
1. Introduction
2. Patients & Methods
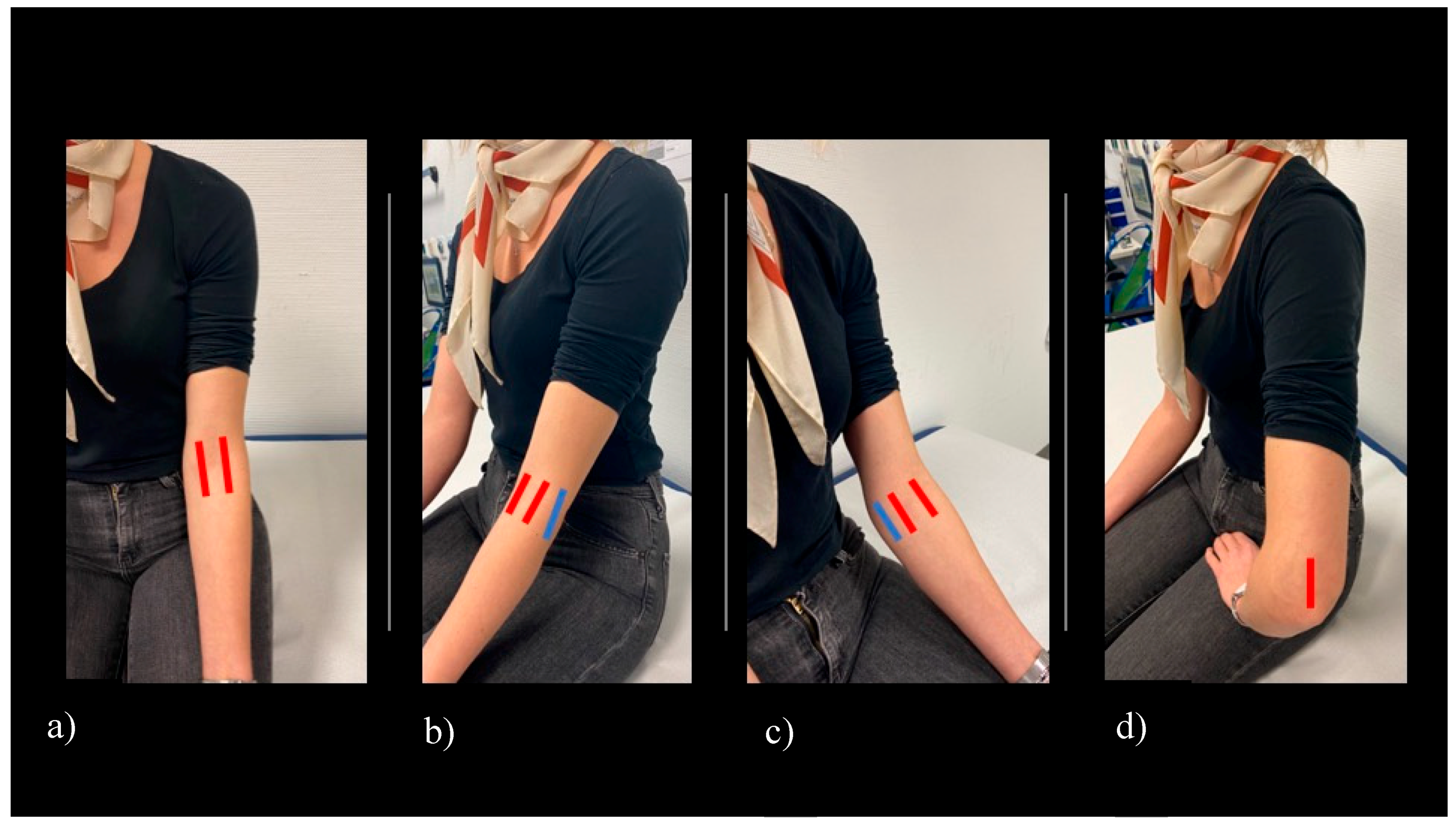
2.1. Clinical Assessment
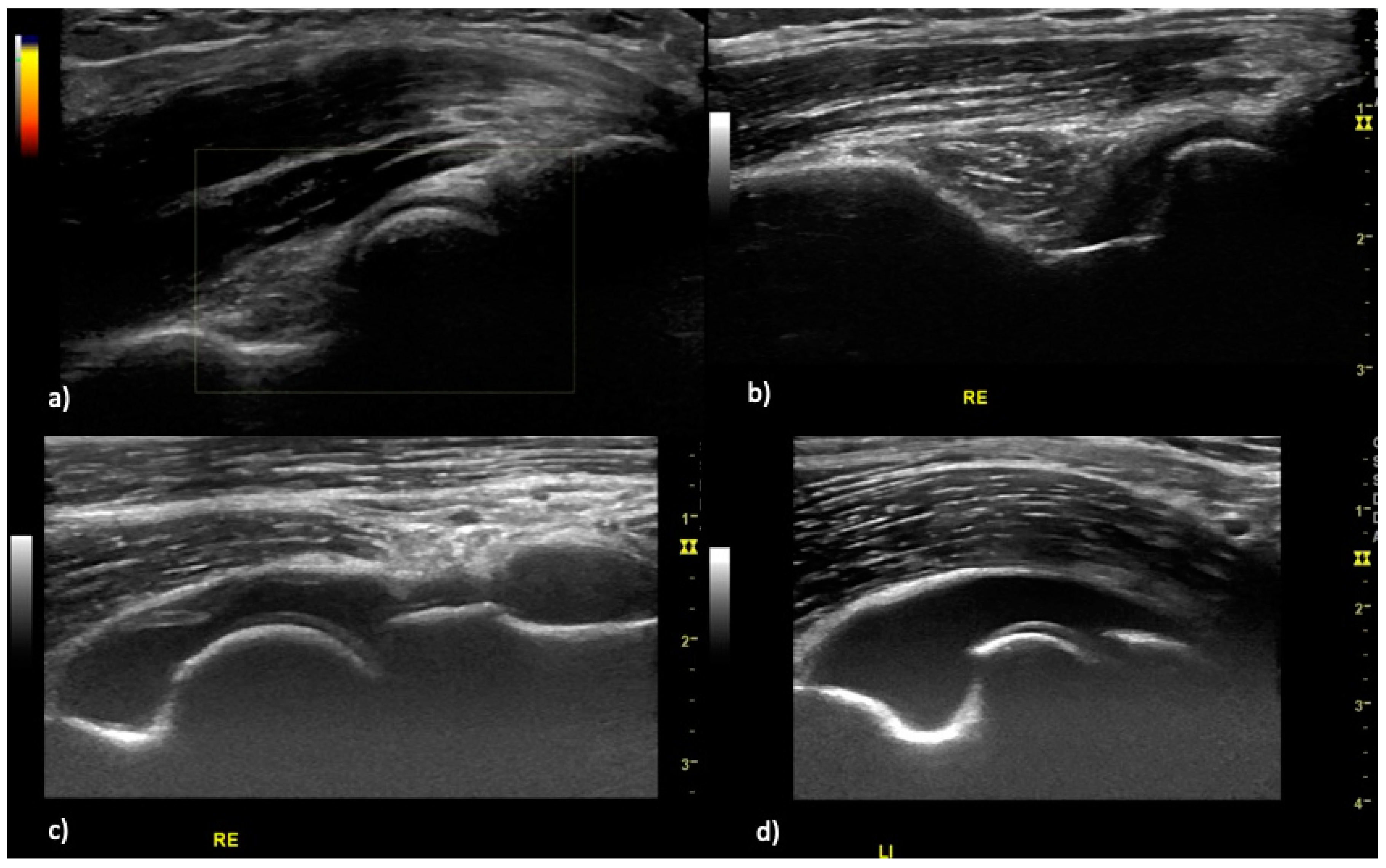
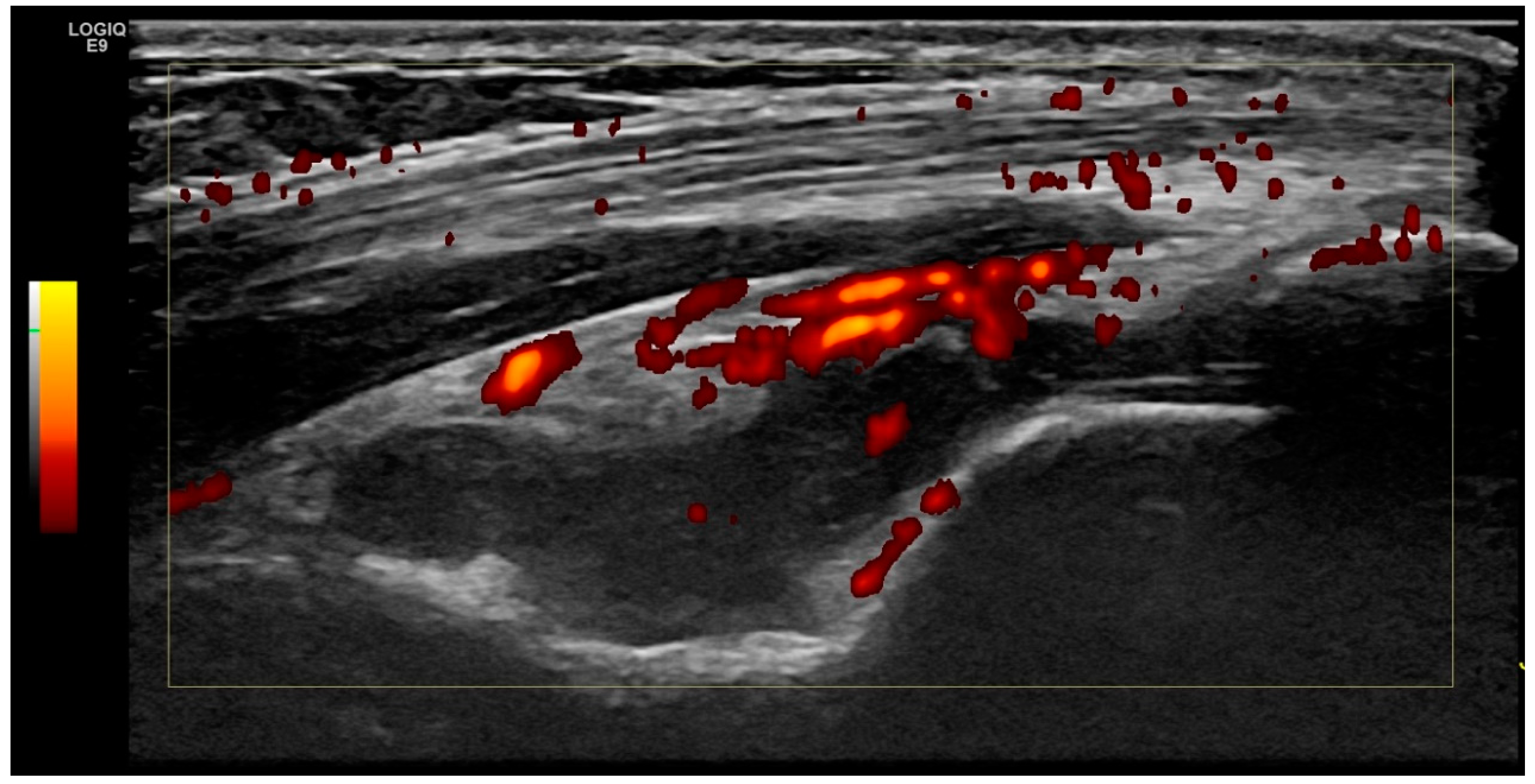
2.2. Ultrasound Examination
2.3. Scoring System
2.4. Definition of Elbow Joint Arthritis and Enthesitis
2.5. Inter- and Intra-Reader Reliability
2.6. Statistical Analysis
2.7. Ethical Approval
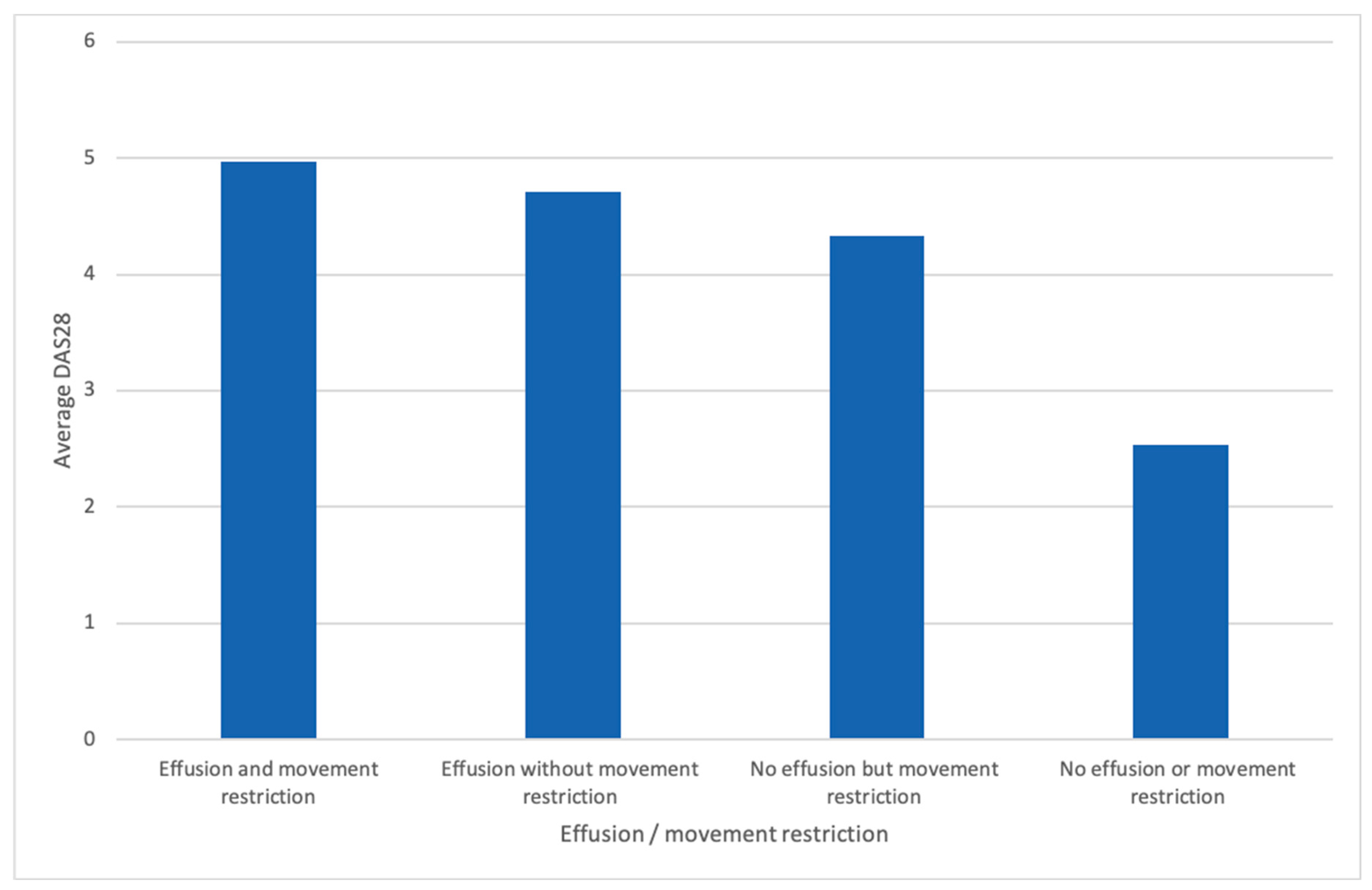
3. Results
3.1. Patient Characteristics
3.2. Intra- and Interreader Reliability
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| DEGUM | Deutsche Gesellschaft für Ultraschall in der Medizin (German Society for Ultrasound in Medicine) |
| DV | Diana Vossen |
| EFSUMB | European Federation of Societies for Ultrasound and Biology |
| MSUS | Musculoskeletal ultrasound |
| VSS | Valentin Sebastian Schäfer |
| WH | Wolfgang Hartung |
Appendix A
| Rater 1: VSS | Rater 2: DV | |||
|---|---|---|---|---|
| Observed Agreement | Significance | Observed Agreement | Significance | |
| Kendall tau (GSUS) | 0.787 | 0.000 | 0.813 | 0.000 |
| Kappa value (PDUS) | 1.000 | 0.000 | 0.459 | 0.015 |
References
- Gibofsky, A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am. J. Manag. Care 2012, 18, S295–S302. [Google Scholar] [PubMed]
- Conaghan, P.G.; O’Connor, P.; McGonagle, D.; Astin, P.; Wakefield, R.J.; Gibbon, W.W.; Quinn, M.; Karim, Z.; Green, M.J.; Proudman, S.; et al. Elucidation of the relationship between synovitis and bone damage: A randomized magnetic resonance imaging study of individual joints in patients with early rheumatoid arthritis. Arthritis Rheum. 2003, 48, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Boutry, N.; Morel, M.; Flipo, R.-M.; Demondion, X.; Cotten, A. Early rheumatoid arthritis: A review of MRI and sonographic findings. AJR Am. J. Roentgenol. 2007, 189, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, M.; Court-Payen, M.; Jacobsen, S.; Klarlund, M.; Thomsen, H.S.; Østergaard, M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 955–962. [Google Scholar] [CrossRef]
- Drossaers-Bakker, K.W.; Kroon, H.M.; Zwinderman, A.H.; Breedveld, F.C.; Hazes, J.M.W. Radiographic damage of large joints in long-term rheumatoid arthritis and its relation to function. Rheumatology 2000, 39, 998–1003. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Kang, T.; Horton, L.; Emery, P.; Wakefield, R.J. Value of ultrasound in rheumatologic diseases. J. Korean Med. Sci. 2013, 28, 497–507. [Google Scholar] [CrossRef]
- D’Agostino, M.A.; Terslev, L.; Wakefield, R.; Østergaard, M.; Balint, P.; Naredo, E.; Iagnocco, A.; Backhaus, M.; Grassi, W.; Emery, P. Novel algorithms for the pragmatic use of ultrasound in the management of patients with rheumatoid arthritis: From diagnosis to remission. Ann. Rheum. Dis. 2016, 75, 1902–1908. [Google Scholar] [CrossRef]
- Terslev, L.; Naredo, E.; Aegerter, P.; Wakefield, R.J.; Backhaus, M.; Balint, P.; Bruyn, G.A.W.; Iagnocco, A.; Jousse-Joulin, S.; Schmidt, W.A.; et al. Scoring ultrasound synovitis in rheumatoid arthritis: A EULAR-OMERACT ultrasound taskforce-Part 2: Reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open 2017, 3, e000427. [Google Scholar] [CrossRef]
- Bruyn, G.A.W.; Siddle, H.J.; Hanova, P.; Costantino, F.; Iagnocco, A.; Sedie, A.D.; Gutierrez, M.; Hammer, H.B.; Jernberg, E.; Loeille, D.; et al. Ultrasound of Subtalar Joint Synovitis in Patients with Rheumatoid Arthritis: Results of an OMERACT Reliability Exercise Using Consensual Definitions. J. Rheumatol. 2019, 46, 351–359. [Google Scholar] [CrossRef]
- Dougados, M.; Devauchelle-Pensec, V.; Ferlet, J.F.; Jousse-Joulin, S.; D’Agostino, M.-A.; Backhaus, M.; Bentin, J.; Chalès, G.; Chary-Valckenaere, I.; Conaghan, P.; et al. The ability of synovitis to predict structural damage in rheumatoid arthritis: A comparative study between clinical examination and ultrasound. Ann. Rheum. Dis. 2013, 72, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Naredo, E.; Gamero, F.; Bonilla, G.; Uson, J.; Carmona, L.; Laffon, A. Ultrasonographic assessment of inflammatory activity in rheumatoid arthritis: Comparison of extended versus reduced joint evaluation. Clin. Exp. Rheumatol. 2005, 23, 881–884. [Google Scholar] [PubMed]
- Hartung, W.; Kellner, H.; Strunk, J.; Sattler, H.; Schmidt, W.A.; Ehrenstein, B.; Fleck, M.; Backhaus, M. Development and evaluation of a novel ultrasound score for large joints in rheumatoid arthritis: One year of experience in daily clinical practice. Arthritis Care Res. 2012, 64, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Draghi, F.; Danesino, G.M.; de Gautard, R.; Bianchi, S. Ultrasound of the elbow: Examination techniques and US appearance of the normal and pathologic joint. J. Ultrasound 2007, 10, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Terslev, L.; Hammer, H.B.; Torp-Pedersen, S.; Szkudlarek, M.; Iagnocco, A.; D’Agostino, M.A.; Schmidt, W.A.; Uson, J.; Bruyn, G.A.; Filippucci, E.; et al. EFSUMB Minimum Training Requirements for Rheumatologists Performing Musculoskeletal Ultrasound. Ultraschall Med. Stuttg. Ger. 1980 2013, 34, e11. [Google Scholar] [CrossRef]
- Balint, P.V.; Terslev, L.; Aegerter, P.; Bruyn, G.A.W.; Chary-Valckenaere, I.; Gandjbakhch, F.; Iagnocco, A.; Jousse-Joulin, S.; Möller, I.; Naredo, E.; et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: An OMERACT US initiative. Ann. Rheum. Dis. 2018, 77, 1730–1735. [Google Scholar] [CrossRef]
- Light, R.J. Measures of response agreement for qualitative data: Some generalizations and alternatives. Psychol. Bull. 1971, 76, 365–377. [Google Scholar] [CrossRef]
- Streiner, D.; Norman, G.; Cairney, J. Health Measurement Scales: A Practical Guide to Their Development and Use; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Landis, J.R.; Koch, G.G. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef]
- Bergstra, S.A.; Chopra, A.; Saluja, M.; Vega-Morales, D.; Govind, N.; Huizinga, T.W.J.; van der Helm-van Mil, A. Evaluation of the joint distribution at disease presentation of patients with rheumatoid arthritis: A large study across continents. RMD Open 2017, 3, e000568. [Google Scholar] [CrossRef]
- Porter, B.B.; Richardson, C.; Vainio, K. Rheumatoid Arthritis of the Elbow: The Results of Synovectomy. J. Bone Jt. Surg. Br. 1974, 56-B, 427–437. [Google Scholar] [CrossRef]
- Cooney, W.P., III; Bryan, R.S. Rheumatoid arthritis in the upper extremity: Treatment of the elbow and shoulder joints. AAOS Instr Course Lect 1979, 28, 247–262. [Google Scholar]
- Lehtinen, J.T.; Kaarela, K.; Ikävalko, M.; Kauppi, M.J.; Belt, E.A.; Kuusela, P.P.; Kautiainen, H.J.; Lehto, M.U. Incidence of elbow involvement in rheumatoid arthritis. A 15 year endpoint study. J. Rheumatol. 2001, 28, 70–74. [Google Scholar] [PubMed]
- Groves, C.; Chandramohan, M.; Chew, N.S.; Aslam, T.; Helliwell, P.S. Clinical Examination, Ultrasound and MRI Imaging of The Painful Elbow in Psoriatic Arthritis and Rheumatoid Arthritis: Which is Better, Ultrasound or MR, for Imaging Enthesitis? Rheumatol. Ther. 2017, 4, 71–84. [Google Scholar] [CrossRef]
- Mera-Varela, A.; Ferreiro-Iglesias, A.; Perez-Pampin, E.; Porto-Silva, M.; Gómez-Reino, J.J.; Gonzalez, A. Ultrasonographic assessment of enthesitis in HLA-B27 positive patients with rheumatoid arthritis, a matched case-only study. PLoS ONE 2013, 8, e58616. [Google Scholar] [CrossRef]
- Ibrahim, G.; Groves, C.; Chandramohan, M.; Beltran, A.; Valle, R.; Reyes, B.; Healy, P.; Harrison, A.; Helliwell, P.S. Clinical and ultrasound examination of the leeds enthesitis index in psoriatic arthritis and rheumatoid arthritis. ISRN Rheumatol. 2011, 2011, 731917. [Google Scholar] [CrossRef][Green Version]
- Frediani, B.; Falsetti, P.; Storri, L.; Allegri, A.; Bisogno, S.; Baldi, F.; Marcolongo, R. Ultrasound and clinical evaluation of quadricipital tendon enthesitis in patients with psoriatic arthritis and rheumatoid arthritis. Clin. Rheumatol. 2002, 21, 203–206. [Google Scholar] [CrossRef]
- Ebstein, E.; Coustet, B.; Masson-Behar, V.; Forien, M.; Palazzo, E.; Dieudé, P.; Ottaviani, S. Enthesopathy in rheumatoid arthritis and spondyloarthritis: An ultrasound study. Jt. Bone Spine Rev. Rhum. 2018, 85, 577–581. [Google Scholar] [CrossRef]
- Micu, M.C.; Alcalde, M.; Sáenz, J.I.; Crespo, M.; Collado, P.; Bolboacă, S.D.; Naredo, E. Impact of musculoskeletal ultrasound in an outpatient rheumatology clinic. Arthritis Care Res. 2013, 65, 615–621. [Google Scholar] [CrossRef]
- Mangnus, L.; van Steenbergen, H.W.; Reijnierse, M.; van der Helm-van Mil, A.H.M. Magnetic Resonance Imaging-Detected Features of Inflammation and Erosions in Symptom-Free Persons From the General Population: Mri-Detected Features in Symptom-Free Individuals. Arthritis Rheumatol. 2016, 68, 2593–2602. [Google Scholar] [CrossRef]
- EL-Melegy, D.N.; El-Khouly, R.M.; Mwafi, M.E.E.-D.; Zyton, H.A.E.-H. Magnetic resonance imaging versus musculoskeletal ultrasound in the evaluation of temporomandibular joint in rheumatoid arthritis patients. Egypt. Rheumatol. 2017, 39, 207–211. [Google Scholar] [CrossRef]
- Mathew, A.J.; Danda, D.; Conaghan, P.G. MRI and ultrasound in rheumatoid arthritis. Curr. Opin. Rheumatol. 2016, 28, 323–329. [Google Scholar] [CrossRef] [PubMed]
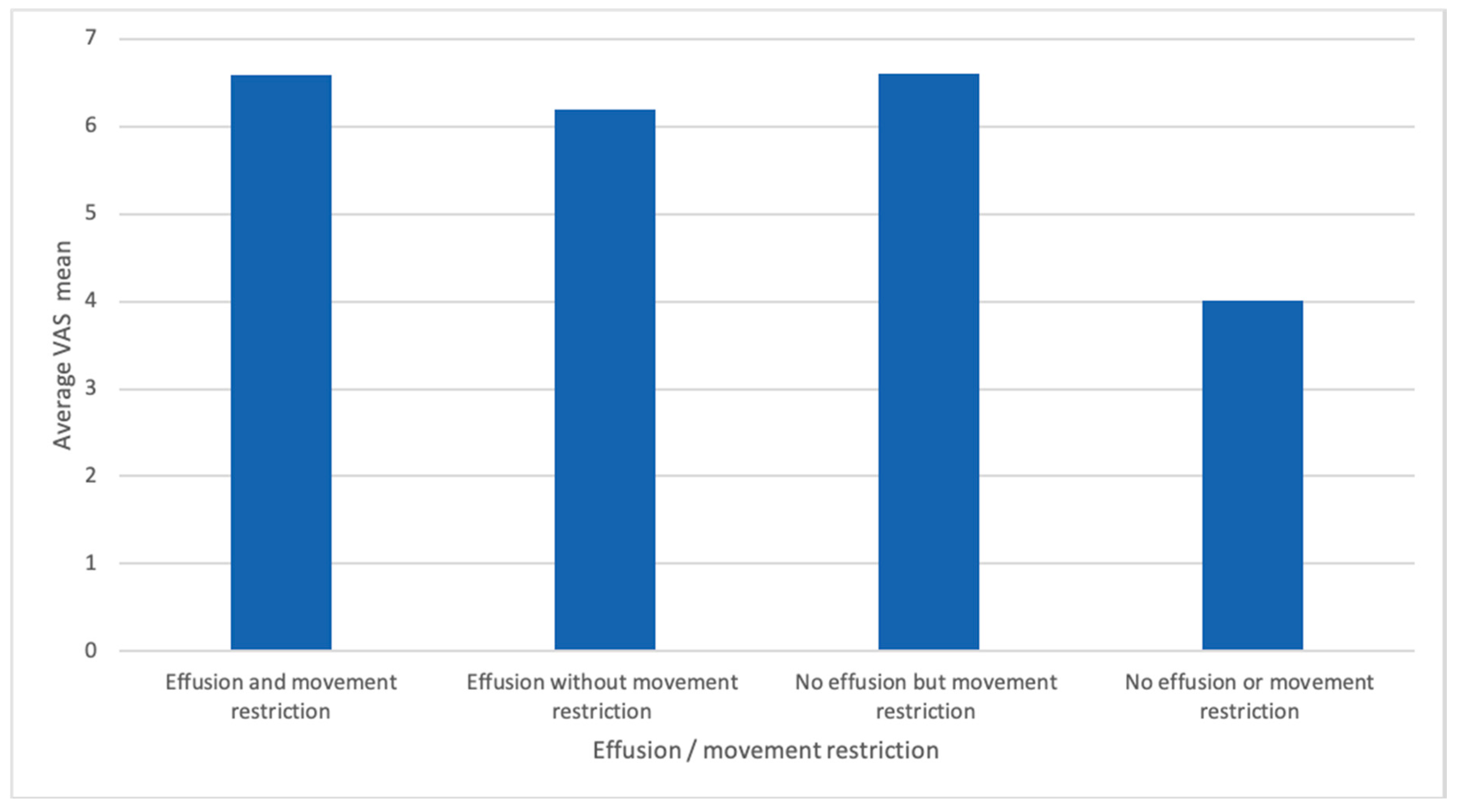
| Rheumatoid Arthritis Group | Control Group | |
|---|---|---|
| Age | 60 (±SD 13) | 48 (±SD 16) |
| Gender male/female | 34 (33 %)/68 (67%) | 9 (18%)/41 (82%) |
| DAS 28 | 3.57 | n.a. |
| high activity (>5.1) | high activity: 58 (56.90%) | |
| moderate activity (3.2–5.1) | moderate: 42 (41.20%) | |
| low activity (2.6–3.2) | low: 1 (1.00%) | |
| remission (<2.6) | clinical remission: 1 (1.00%) | |
| VAS pain (0–10) | 6.41 (SD 2.30) | 2.42 (SD 2.90) |
| RA Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Right Side | Left Side | Both Sides | Right Side | Left Side | Both Sides | |
| Movement restriction | 26 (25.50%) | 22 (21.60%) | 16 (15.68%) | 3 (6.00%) | 3 (6.00%) | 1 (2.00 %) |
| Any Joint effusion | 42 (41.20%) | 40 (39.20) | 26 (25.00%) | 6 (12.00%) | 4 (8.00%) | 2 (4.00%) |
| Hypervascularisation | 4 (3.90 %) | 3 (2.90 %) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Ultrasound arthritis definition (Effusion ≥Grade 2 and/or hypervascularisation) | 27 (26.47%) (grade > II: 25, hypervascularisation: 2) | 25 (24.51) (grade >II: 24, hypervascularisation: 1) | 16 (15.68%) | 0 (0%) | 0 (0.00%) | 0 (0.00%) |
| Enthesitis | 1 (1.00 %) | 3 (2.00%) | 0 (0.00%) | 1 (2.00%) | 0 (0.00%) | 0 (0.00%) |
| Prevalence (Mean) | Observed Agreement | K (Kappa) | Standard Deviation | |
|---|---|---|---|---|
| B-mode (0 to 3) | Grade 0: 42% (17/40) | DV/VSS: 92.5% (37/40) DV/WH: 85% (34/40) VSS/WH: 85% (34/40) | DV/VSS: 0.896 DV/WH: 0.791 VSS/WH: 0.787 | DV/VSS: 0.058 DV/WH: 0.077 VSS/WH: 0.079 |
| Grade I: 20% (8/40) | ||||
| Grade II: 18% (7/40) | ||||
| Grade III: 20% (8/40) | ||||
| PD-mode (y/n) | Grade 0: 87.5% (35/40) | DV/VSS: 95% (38/40) DV/WH: 95% (38/40) VSS/WH: 90% (36/40) | DV/VSS: 0.724 DV/WH: 0.805 VSS/WH: 0.553 | DV/VSS: 0.183 DV/WH: 0.132 VSS/WH: 0.190 |
| Grade I: 12.5% (5/40) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, V.S.; Recker, F.; Vossen, D.; Geffken, I.; Matuschek, E.; Hartung, W. Prevalence of Elbow Joint Arthritis and Enthesitis in Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 1590. https://doi.org/10.3390/jcm9051590
Schäfer VS, Recker F, Vossen D, Geffken I, Matuschek E, Hartung W. Prevalence of Elbow Joint Arthritis and Enthesitis in Rheumatoid Arthritis. Journal of Clinical Medicine. 2020; 9(5):1590. https://doi.org/10.3390/jcm9051590
Chicago/Turabian StyleSchäfer, Valentin Sebastian, Florian Recker, Diana Vossen, Isabelle Geffken, Eva Matuschek, and Wolfgang Hartung. 2020. "Prevalence of Elbow Joint Arthritis and Enthesitis in Rheumatoid Arthritis" Journal of Clinical Medicine 9, no. 5: 1590. https://doi.org/10.3390/jcm9051590
APA StyleSchäfer, V. S., Recker, F., Vossen, D., Geffken, I., Matuschek, E., & Hartung, W. (2020). Prevalence of Elbow Joint Arthritis and Enthesitis in Rheumatoid Arthritis. Journal of Clinical Medicine, 9(5), 1590. https://doi.org/10.3390/jcm9051590






