Association between Periodontitis and High Blood Pressure: Results from the Study of Periodontal Health in Almada-Seixal (SoPHiAS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Blood Pressure Assessment
2.3. Periodontal Examination
2.4. Additional Study Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Group
3.2. Association Between Periodontitis and Hypertension
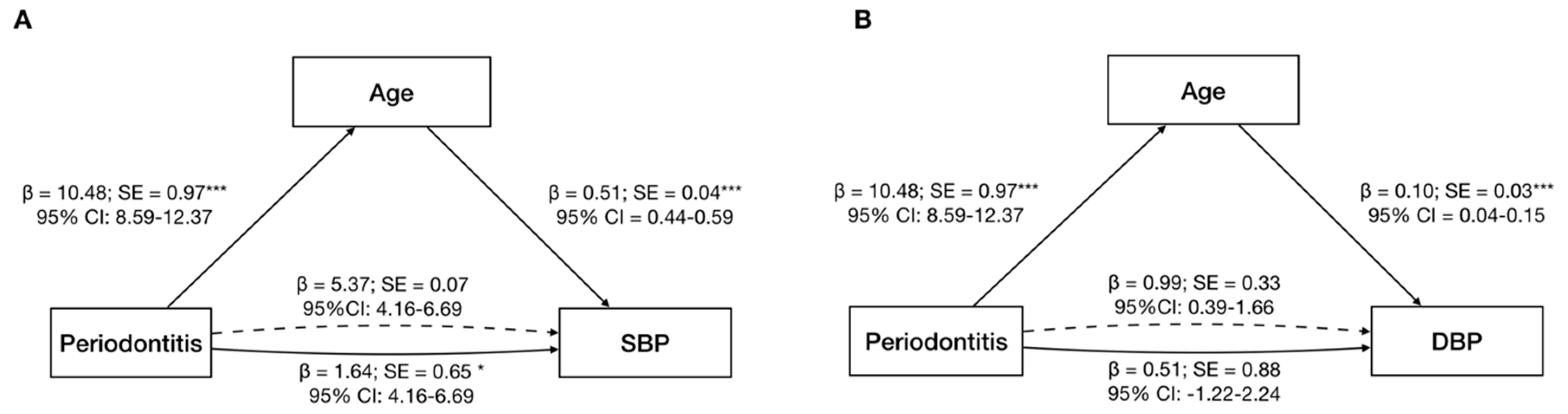
3.3. Age Effect on the Periodontitis–Hypertension Link
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA J. Am. Med. Assoc. 2017, 317, 165–182. [Google Scholar] [CrossRef]
- Padwal, R.S.; Bienek, A.; McAlister, F.A.; Campbell, N.R.C. Epidemiology of Hypertension in Canada: An Update. Can. J. Cardiol. 2015, 32, 687–694. [Google Scholar] [CrossRef]
- Poulter, N.R.; Prabhakaran, D.; Caulfield, M. Hypertension. Lancet 2015, 386, 801–812. [Google Scholar] [CrossRef]
- Cheng, S.; Claggett, B.; Correia, A.W.; Shah, A.M.; Gupta, D.K.; Skali, H.; Ni, H.; Rosamond, W.D.; Heiss, G.; Folsom, A.R.; et al. Temporal trends in the population attributable risk for cardiovascular disease: The atherosclerosis risk in communities study. Circulation 2014, 130, 820–828. [Google Scholar] [CrossRef]
- Willey, J.Z.; Moon, Y.P.; Kahn, E.; Rodriguez, C.J.; Rundek, T.; Cheung, K.; Sacco, R.L.; Elkind, M.S.V. Population attributable risks of hypertension and diabetes for cardiovascular disease and stroke in the Northern Manhattan study. J. Am. Heart Assoc. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Drummond, G.R.; Vinh, A.; Guzik, T.J.; Sobey, C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019, 19, 517–532. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Leung, A.A.; Daskalopoulou, S.S.; Dasgupta, K.; McBrien, K.; Butalia, S.; Zarnke, K.B.; Nerenberg, K.; Harris, K.C.; Nakhla, M.; Cloutier, L.; et al. Hypertension Canada’s 2017 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults. Can. J. Cardiol. 2017, 33, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; Abyu, G.Y.; Alsharif, U.; et al. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Van Dyke, T.E. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAPWorkshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, L.S.; Cai, C.; Shi, Q.; Wen, N.; Xu, J. Association between periodontitis and peripheral artery disease: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, 1–22. [Google Scholar] [CrossRef]
- Muñoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2020, 116, 28–39. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Mascarenhas, P.; Rua, J.; Alves, R.; Cavacas, M.A.; Delgado, A.; Mendes, J.J. Stress, Salivary Cortisol and Periodontitis: A Systematic Review and Meta-analysis of Observational Studies. Arch. Oral Biol. 2018, 96, 58–65. [Google Scholar] [CrossRef]
- Hussain, S.B.; Botelho, J.; Machado, V.; Zehra, S.A.; Mendes, J.J.; Ciurtin, C.; Orlandi, M.; Aiuto, F.D. Is there a bidirectional association between rheumatoid arthritis and periodontitis? A systematic review and meta-analysis. Semin. Arthritis Rheum. 2020. [Google Scholar] [CrossRef]
- MacHado, V.; Botelho, J.; Lopes, J.; Patrão, M.; Alves, R.; Chambrone, L.; Alcoforado, G.; Mendes, J.J. Periodontitis impact in interleukin-6 serum levels in solid organ transplanted patients: A systematic review and meta-analysis. Diagnostics 2020, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cabezas, R.; Seelam, N.; Petit, C.; Agossa, K.; Gaertner, S.; Tenenbaum, H.; Davideau, J.L.; Huck, O. Association between periodontitis and arterial hypertension: A systematic review and meta-analysis. Am. Heart J. 2016, 180, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Paizan, M.; Vilela-Martin, J. Is There an Association between Periodontitis and Hypertension? Curr. Cardiol. Rev. 2014, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res. Int. 2014, 2014, 406960. [Google Scholar] [CrossRef] [PubMed]
- Czesnikiewicz-Guzik, M.; Nosalski, R.; Mikolajczyk, T.P.; Vidler, F.; Dohnal, T.; Dembowska, E.; Graham, D.; Harrison, D.G.; Guzik, T.J. Th1-type immune responses to Porphyromonas gingivalis antigens exacerbate angiotensin II-dependent hypertension and vascular dysfunction. Br. J. Pharmacol. 2019, 176, 1922–1931. [Google Scholar] [CrossRef]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Alves, R.; Cavacas, M.A.; Amaro, L.; Mendes, J.J. Study of Periodontal Health in Almada-Seixal (SoPHiAS): A cross-sectional study in the Lisbon Metropolitan Area. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- König, J.; Holtfreter, B.; Kocher, T. Periodontal health in Europe: Future trends based on treatment needs and the provision of periodontal services - position paper 1. Eur. J. Dent. Educ. 2010, 14, 4–24. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Gaio, V.; Kislaya, I.; Graff-Iversen, S.; Cordeiro, E.; Silva, A.C.; Namorado, S.; Barreto, M.; Gil, A.P.; Antunes, L.; et al. Prevalência de hipertensão arterial em Portugal: Resultados do Primeiro Inquérito Nacional com Exame Físico (INSEF 2015). Bol. Epidemiológico Obs. 2017, 8, 11–14. [Google Scholar]
- Zhou, Y.; Jia, L.; Lu, B.; Gu, G.; Hu, H.; Zhang, Z.; Bai, L.; Cui, W. Updated hypertension prevalence, awareness, and control rates based on the 2017ACC/AHA high blood pressure guideline. J. Clin. Hypertens. 2019, 21, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Kintiraki, E.; Papakatsika, S.; Kotronis, G.; Goulis, D.G.; Kotsis, V. Pregnancy-Induced Hypertension. Hormones 2015, 14, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Topouchian, J.; Hakobyan, Z.; Asmar, J.; Gurgenian, S.; Zelveian, P.; Asmar, R. Clinical accuracy of the omron M3 comfort® and the omron evolv® for self-blood pressure measurements in pregnancy and pre-eclampsia—Validation according to the universal standard protocol. Vasc. Health Risk Manag. 2018, 14, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Shimbo, D.; Carey, R.M.; Charleston, J.B.; Gaillard, T.; Misra, S.; Myers, M.G.; Ogedegbe, G.; Schwartz, J.E.; Townsend, R.R.; et al. Measurement of Blood Pressure in Humans: A scientific Statement from the American Heart Association. Hypertension 2019, 73, e35–e66. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of Arterial Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Nesse, W.; Abbas, F.; Van Der Ploeg, I.; Spijkervet, F.K.L.; Dijkstra, P.U.; Vissink, A. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, 149–161. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S44–S67. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetesd2016. Diabetes Care 2016, 39, S13–S22. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Heart J. 2019, 40, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Ferri, C. Hypertension Management at Older Age: An Update. High Blood Press. Cardiovasc. Prev. 2019, 26, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Tsakos, G.; Sabbah, W.; Hingorani, A.D.; Netuveli, G.; Donos, N.; Watt, R.G.; D’Aiuto, F. Is periodontal inflammation associated with raised blood pressure? Evidence from a National US survey. J. Hypertens. 2010, 28, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, D.; Del Pinto, R.; Ferri, C.; Marzo, G.; Giannoni, M.; Ortu, E.; Monaco, A. Association between periodontal inflammation and hypertension using periodontal inflamed surface area and bleeding on probing. J. Clin. Periodontol. 2020, 47, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Paraskevas, S.; Huizinga, J.D.; Loos, B.G. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J. Clin. Periodontol. 2008, 35, 277–290. [Google Scholar] [CrossRef]
- Houcken, W.; Teeuw, W.J.; Bizzarro, S.; Alvarez Rodriguez, E.; Mulders, T.A.; Van Den Born, B.J.H.; Loos, B.G. Arterial stiffness in periodontitis patients and controls. J. Hum. Hypertens. 2016, 30, 24–29. [Google Scholar] [CrossRef]
- Vidal, F.; Cordovil, I.; Figueredo, C.M.S.; Fischer, R.G. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: A pilot study. J. Clin. Periodontol. 2013, 40, 681–687. [Google Scholar] [CrossRef]
- Zhou, Q.-B.; Xia, W.-H.; Ren, J.; Yu, B.-B.; Tong, X.-Z.; Chen, Y.-B.; Chen, S.; Feng, L.; Dai, J.; Tao, J.; et al. Effect of Intensive Periodontal Therapy on Blood Pressure and Endothelial Microparticles in Patients With Prehypertension and Periodontitis: A Randomized Controlled Trial. J. Periodontol. 2017, 88, 711–722. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Parkar, M.; Nibali, L.; Suvan, J.; Lessem, J.; Tonetti, M.S. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: Results from a randomized controlled clinical trial. Am. Heart J. 2006, 151, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Offenbacher, S. Relationships among clinical measures of periodontal disease and their associations with systemic markers. Ann. Periodontol. 2002, 7, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Demmer, R.T.; Kocher, T.; Schwahn, C.; Völzke, H.; Jacobs, D.R.; Desvarieux, M. Refining exposure definitions for studies of periodontal disease and systemic disease associations. Community Dent. Oral Epidemiol. 2008, 36, 493–502. [Google Scholar] [CrossRef]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA J. Am. Med. Assoc. 2013, 310, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.; Girerd, N.; Zannad, F.; Rossignol, P.; Boivin, J.M. Health-related determinants of undiagnosed arterial hypertension: A population-based study. Fam. Pract. 2018, 36, 276–283. [Google Scholar] [CrossRef]
- Melgarejo, J.D.; Maestre, G.E.; Thijs, L.; Asayama, K.; Boggia, J.; Casiglia, E.; Hansen, T.W.; Imai, Y.; Jacobs, L.; Jeppesen, J.; et al. Prevalence, Treatment, and Control Rates of Conventional and Ambulatory Hypertension Across 10 Populations in 3 Continents. Hypertension 2017, 70, 50–58. [Google Scholar] [CrossRef]
- Scholes, S.; Conolly, A.; Mindell, J.S. Income-based inequalities in hypertension and in undiagnosed hypertension. J. Hypertens. 2020, 38, 912–924. [Google Scholar] [CrossRef]
- Sogunuru, G.P.; Mishra, S. Asian managemet of hypertension: Current status, home blood pressure, and specific concerns in India. J. Clin. Hypertens. 2020, 22, 479–482. [Google Scholar] [CrossRef]
- Darnaud, C.; Thomas, F.; Pannier, B.; Danchin, N.; Bouchard, P. Oral health and blood pressure: The IPC cohort. Am. J. Hypertens. 2015, 28, 1257–1261. [Google Scholar] [CrossRef]
- Caillon, A.; Paradis, P.; Schiffrin, E.L. Role of immune cells in hypertension. Br. J. Pharmacol. 2019, 176, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Tanoue, T.; Yamashita, T.; Yodoi, K.; Matsumoto, T.; Emoto, T.; Mizoguchi, T.; Hayashi, T.; Kitano, N.; Sasaki, N.; et al. Commensal bacteria at the crossroad between cholesterol homeostasis and chronic inflammation in atherosclerosis. J. Lipid Res. 2017, 58, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, T.P.; Nosalski, R.; Szczepaniak, P.; Budzyn, K.; Osmenda, G.; Skiba, D.; Sagan, A.; Wu, J.; Vinh, A.; Marvar, P.J.; et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J. 2016, 30, 1987–1999. [Google Scholar] [CrossRef]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of Periodontitis and Endothelial Function. N. Engl. J. Med. 2007, 336, 911–920. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.D.; et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: A 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Mendes, J.J. The 2018 periodontitis case definition improves accuracy performance of full-mouth partial diagnostic protocols. Sci. Rep. 2020, 10, 7093. [Google Scholar] [CrossRef]

| No Periodontitis (n = 423) | Periodontitis (n = 634) | p-Value # | |
|---|---|---|---|
| Age, mean (SD) | 54.8 (17.8) | 65.2 (13.5) | <0.001 |
| Gender, n (%) | |||
| Female (n = 610) | 283 (46.4) | 327 (53.6) | <0.001 |
| Male (n = 447) | 140 (31.3) | 307 (68.7) | |
| Race, n (%) | |||
| Caucasian (n = 916) | 359 (39.2) | 557 (60.8) | 0.164 |
| Black (n = 130) | 57 (43.8) | 73 (56.2) | |
| Asian (n = 11) | 7 (63.6) | 4 (36.4) | |
| Education Level, n (%) | |||
| No education (n = 42) | 11 (26.2) | 31 (73.8) | <0.001 |
| Basic (n = 410) | 134 (32.7) | 276 (67.3) | |
| Medium (n = 490) | 205 (41.8) | 285 (58.2) | |
| Higher (n = 115) | 73 (63.5) | 42 (36.5) | |
| Smoking Habits, n (%) | |||
| Never (n = 624) | 294 (47.1) | 330 (52.9) | <0.001 |
| Former (n = 288) | 83 (28.8) | 205 (71.2) | |
| Current (n = 145) | 46 (31.7) | 99 (68.3) | |
| Income, mean (SD) (€) | 1097.0 (767.3) | 981.5 (667.9) | 0.023 |
| Clinical Variables | |||
| Hypertension, n (%) | |||
| No (n = 357) | 188 (52.7) | 169 (47.3) | <0.001 |
| Yes (n = 700) | 235 (33.6) | 465 (66.4) | |
| SBP, mean (SD) | 129.5 (20.1) | 136.5 (20.4) | <0.001 |
| DBP, mean (SD) | 77.9 (13.2) | 79.6 (13.6) | 0.082 |
| SBP ≥ 140 mmHg, n (%) | |||
| No (n = 681) | 308 (45.2) | 373 (54.8) | <0.001 |
| Yes (n = 376) | 115 (30.6) | 261 (69.4) | |
| Taking Antihypertensive Medication, n (%) | |||
| Yes (n = 532) | 172 (32.3) | 360 (77.7) | <0.001 |
| No (n = 525) | 251 (47.8) | 274 (52.2) | |
| Number of medical conditions, mean (SD) | 1.89 (1.6) | 2.36 (1.53) | <0.001 |
| Diabetes Mellitus, n (%) | |||
| Yes (n = 204) | 53 (26.0) | 151 (74.0) | <0.001 |
| No (n = 853) | 370 (43.4) | 483 (56.6) | |
| BMI, mean (SD) | 27.1 (4.9) | 27.5 (4.7) | 0.051 |
| Periodontal Clinical Parameters, Mean (SD) | |||
| Missing Teeth (n) | 6.6 (6.1) | 10.8 (7.1) | <0.001 |
| Mean PPD (mm) | 1.51 (0.30) | 2.22 (0.83) | <0.001 |
| PPD ≥ 3 mm (%) | 7.2 (8.1) | 29.9 (24.0) | <0.001 |
| PPD ≥ 4 mm (%) | 6.1 (16.0) | 13.0 (17.8) | <0.001 |
| PPD ≥ 5 mm (%) | 1.1 (4.5) | 8.8 (15.1) | <0.001 |
| PPD ≥ 6 mm (%) | 0.1 (0.2) | 2.7 (6.7) | <0.001 |
| PPD ≥ 7 mm (%) | 0.0 (0.1) | 1.3 (4.2) | <0.001 |
| Mean CAL (mm) | 1.72 (0.35) | 3.39 (1.52) | <0.001 |
| CAL ≥ 3 mm (%) | 15.2 (12.3) | 56.6 (26.0) | <0.001 |
| CAL ≥ 4 mm (%) | 3.9 (5.3) | 37.8 (27.9) | <0.001 |
| CAL ≥ 5 mm (%) | 0.8 (2.0) | 25.2 (26.0) | <0.001 |
| CAL ≥ 6 mm (%) | 0.3 (1.1) | 15.2 (21.9) | <0.001 |
| CAL ≥ 7 mm (%) | 0.1 (0.7) | 9.0 (16.9) | <0.001 |
| Mean Rec (mm) | 0.22 (0.27) | 1.18 (1.15) | <0.001 |
| PISA (mm2) | 12.2 (26.3) | 65.9 (137.1) | <0.001 |
| PESA (mm2) | 177.7 (76.8) | 218.8 (170.8) | 0.002 |
| PI (%) | 12.3 (21.1) | 30.6 (33.2) | <0.001 |
| BoP (%) | 5.6 (9.3) | 20.9 (23.6) | <0.001 |
| Periodontitis OR (95%CI) | Stage 1 (Mild) OR (95%CI) | Stage 2 (Moderate) OR (95%CI) | Stage 3 (Severe) OR (95%CI) | |
|---|---|---|---|---|
| All Participants (n = 1057) | ||||
| Model 1 | 2.20 (1.70–2.86) *** | 1.72 (1.10–2.57) ** | 2.60 (1.82–3.72) *** | 2.20 (1.57–3.08) *** |
| Model 2 | 2.14 (1.64–2.78) *** | 1.69 (1.13–2.52) * | 2.54 (1.77–3.63) *** | 2.12 (1.51–2.98) *** |
| Model 3 | 2.36 (1.64–2.78) *** | 1.79 (1.19–2.70) ** | 2.87 (1.97–4.17) *** | 2.36 (1.66–3.36) *** |
| Model 4 | 2.31 (1.75–3.04) *** | 1.75 (1.15–2.66) ** | 2.78 (1.90–4.01) *** | 2.32 (1.62–3.32) *** |
| Model 5 | 1.24 (0.90–1.71) | 1.36 (0.84–2.18) | 1.41 (0.92–2.15) | 1.04 (0.69–1.55) |
| Participants Not Taking Antihypertensive Medication (n = 525) | ||||
| Model 1 | 1.85 (1.27–2.70) *** | 1.69 (0.96–2.95) | 2.60 (1.61–4.21) *** | 1.36 (0.82–2.70) |
| Model 2 | 1.81 (1.24–2.65) ** | 1.66 (0.95–2.90) | 2.55 (1.57–4,14) *** | 1.32 (0.79–2.22) |
| Model 3 | 1.89 (1.28–2.79) *** | 1.66 (0.94–2.91) | 2.67 (1.64–3.36) *** | 1.44 (0.85– 2.44) |
| Model 4 | 1.86 (1.26–2.75) ** | 1.66 (0.94–2.92) | 2.60 (1.59–4.26) *** | 1.43 (0.85–2.42) |
| Model 5 | 1.24 (0.81–1.90) | 1.36 (0.75–2.45) | 1.62 (0.96–2.75) | 0.82 (0.47–1.46) |
| All Participants (n = 1057) | No Antihypertensive Use (n = 525) | Antihypertensive Use (n = 532) | ||||
|---|---|---|---|---|---|---|
| ß Coefficient (SE) | p | ß Coefficient (SE) | p | ß Coefficient (SE) | p | |
| SBP (mmHg) | ||||||
| Age (years) | 0.44 (0.04) | <0.001 | 0.44 (0.04) | <0.001 | 0.40 (0.09) | <0.001 |
| BMI (kg/m2) | 0.52 (0.12) | <0.001 | 0.40 (0.17) | 0.018 | 0.54 (0.18) | 0.003 |
| %PPD ≥ 6 mm | 45.47 (12.37) | <0.001 | - | - | - | - |
| PESA (Total) | −0.02 (0.01) | 0.001 | - | - | - | - |
| BoP (%) | 7.86 (3.14) | 0.012 | 7.42 (3.75) | 0.048 | - | - |
| %PPD ≥ 7 mm | - | - | - | - | 78.36 (0.18) | <0.001 |
| Missing teeth (n) | - | - | - | - | 0.28 (0.13) | 0.031 |
| DBP (mmHg) | ||||||
| Age (years) | 0.07 (0.03) | 0.009 | 0.09 (0.04) | 0.009 | - | - |
| BMI (kg/m2) | 0.33 (0.09) | <0.001 | 0.42 (0.13) | 0.002 | - | - |
| %PPD ≥ 6 mm | 40.73 (9.93) | <0.001 | - | - | 22.97 (9.76) | 0.019 |
| PESA (Total) | −0.01 (0.00) | 0.030 | - | - | - | - |
| BoP (%) | 10.10 (2.44) | <0.001 | - | - | 10.88 (2.97) | <0.001 |
| Mean CAL (mm) | −0.79 (0.39) | 0.045 | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, V.; Aguilera, E.M.; Botelho, J.; Hussain, S.B.; Leira, Y.; Proença, L.; D’Aiuto, F.; Mendes, J.J. Association between Periodontitis and High Blood Pressure: Results from the Study of Periodontal Health in Almada-Seixal (SoPHiAS). J. Clin. Med. 2020, 9, 1585. https://doi.org/10.3390/jcm9051585
Machado V, Aguilera EM, Botelho J, Hussain SB, Leira Y, Proença L, D’Aiuto F, Mendes JJ. Association between Periodontitis and High Blood Pressure: Results from the Study of Periodontal Health in Almada-Seixal (SoPHiAS). Journal of Clinical Medicine. 2020; 9(5):1585. https://doi.org/10.3390/jcm9051585
Chicago/Turabian StyleMachado, Vanessa, Eva Muñoz Aguilera, João Botelho, Syed Basit Hussain, Yago Leira, Luís Proença, Francesco D’Aiuto, and José João Mendes. 2020. "Association between Periodontitis and High Blood Pressure: Results from the Study of Periodontal Health in Almada-Seixal (SoPHiAS)" Journal of Clinical Medicine 9, no. 5: 1585. https://doi.org/10.3390/jcm9051585
APA StyleMachado, V., Aguilera, E. M., Botelho, J., Hussain, S. B., Leira, Y., Proença, L., D’Aiuto, F., & Mendes, J. J. (2020). Association between Periodontitis and High Blood Pressure: Results from the Study of Periodontal Health in Almada-Seixal (SoPHiAS). Journal of Clinical Medicine, 9(5), 1585. https://doi.org/10.3390/jcm9051585









