Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Outcomes
2.4. Data Collection
2.4.1. Clinical Data: Mothers
2.4.2. Clinical Data: Children
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Lin, J.; Fu, W.; Liu, S.; Gong, C.; Dai, J. Mediterranean diet during pregnancy and childhood for asthma in children: A systematic review and meta-analysis of observational studies. Pediatr. Pulmonol. 2019, 54, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L. What are the effects of a mediterranean diet on allergies and asthma in children? Front. Pediatr. 2017, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.M.; Rehbinder, E.M.; Lødrup Carlsen, K.C.; Gudbrandsgard, M.; Carlsen, K.H.; Haugen, G.; Hedlin, G.; Jonassen, C.M.; Sjøborg, K.D.; Landrø, L.; et al. Food and nutrient intake and adherence to dietary recommendations during pregnancy: A nordic mother–child population-based cohort. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Hillesund, E.R.; Bere, E.; Sagedal, L.R.; Vistad, I.; Seiler, H.L.; Torstveit, M.K.; Øverby, N.C. Pre-pregnancy and early pregnancy dietary behavior in relation to maternal and newborn health in the norwegian fit for delivery study—A post hoc observational analysis. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Koletzko, B.; Brands, B.; Chourdakis, M.; Cramer, S.; Grote, V.; Hellmuth, C.; Kirchberg, F.; Prell, C.; Rzehak, P.; Uhl, O.; et al. The Power of Programming and the EarlyNutrition project: Opportunities for health promotion by nutrition during the first thousand days of life and beyond. Ann. Nutr. Metab. 2014, 64, 187–196. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, N.; Schneider, E.; Lehnen, H.; Haaf, T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 2014, 148, R111–R120. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.L.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, J.M.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O.; et al. Hyperglycemia and adverse Pregnancy Outcome follow-up study (HAPO FUS): Maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar] [CrossRef]
- Brown, F.M.; Isganaitis, E.; James-Todd, T. Much to HAPO FUS About: Increasing Maternal Glycemia in Pregnancy Is Associated With Worsening Childhood Glucose Metabolism. Diabetes Care 2019, 42, 393–395. [Google Scholar] [CrossRef]
- Moses, R.G.; Cefalu, W.T. Considerations in theManagement of Gestational Diabetes Mellitus: “You Are What Your Mother Ate!”. Diabetes Care 2016, 39, 13–15. [Google Scholar] [CrossRef][Green Version]
- Langer, O. Obesity or diabetes: Which is more hazardous to the health of the offspring? J. Matern. Neonatal Med. 2016, 29, 186–190. [Google Scholar] [CrossRef]
- Forno, E.; Young, O.M.; Kumar, R.; Simhan, H.; Celedón, J.C. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 2014, 134, e5354–e5356. [Google Scholar] [CrossRef] [PubMed]
- Polinski, K.J.; Liu, J.; Boghossian, N.S.; McLain, A.C. Maternal obesity, gestational weight gain, and asthma in offspring. Prev. Chronic Dis. 2017, 14, e109. [Google Scholar] [CrossRef] [PubMed]
- Netting, M.J.; Middleton, P.F.; Makrides, M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systematic review of food-based approaches. Nutrition 2014, 30, 1225–1241. [Google Scholar] [CrossRef] [PubMed]
- Dumas, O.; Varraso, R.; Gillman, M.W.; Field, A.E.; Camargo, C.A. Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Brown, K.R.; Maslin, K.; Palmer, D.J. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr. Allergy Immunol. 2017, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Baïz, N.; Just, J.; Chastang, J.; Forhan, A.; De Lauzon-Guillain, B.; Magnier, A.M.; Annesi-Maesano, I. Maternal diet before and during pregnancy and risk of asthma and allergic rhinitis in children. Allergy Asthma Clin. Immunol. 2019, 15, 40. [Google Scholar] [CrossRef]
- Tuokkola, J.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Vaarala, O.; Kenward, M.G.; Virta, L.J.; Veijola, R.; Simell, O.; Ilonen, J.; et al. Maternal diet during pregnancy and lactation and cow’s milk allergy in offspring. Eur. J. Clin. Nutr. 2016, 70, 554–559. [Google Scholar] [CrossRef]
- Rice, J.L.; Romero, K.M.; Galvez Davila, R.M.; Meza, C.T.; Bilderback, A.; Williams, D.L.; Breysse, P.N.; Bose, S.; Checkley, W.; Hansel, N.N.; et al. Association Between Adherence to the Mediterranean Diet and Asthma in Peruvian Children. Lung 2015, 193, 893–899. [Google Scholar] [CrossRef]
- Griffiths, P.S.; Walton, C.; Samsell, L.; Perez, M.K.; Piedimonte, G. Maternal high-fat hypercaloric diet during pregnancy results in persistent metabolic and respiratory abnormalities in offspring. Pediatr. Res. 2016, 79, 278–286. [Google Scholar] [CrossRef]
- Bédard, A.; Northstone, K.; Henderson, A.J.; Shaheen, S.O. Maternal intake of sugar during pregnancy and childhood respiratory and atopic outcomes. Eur. Respir. J. 2017, 50, 1700073. [Google Scholar] [CrossRef]
- Azad, M.B.; Moyce, B.L.; Guillemette, L.; Pascoe, C.D.; Wicklow, B.; McGavock, J.M.; Halayko, A.J.; Dolinsky, V.W. Diabetes in pregnancy and lung health in offspring: Developmental origins of respiratory disease. Paediatr. Respir. Rev. 2017, 21, 19–26. [Google Scholar] [CrossRef] [PubMed]
- H Al Wattar, B.; Dodds, J.; Placzek, A.; Beresford, L.; Spyreli, E.; Moore, A.; Gonzalez Carreras, F.J.; Austin, F.; Murugesu, N.; Roseboom, T.J.; et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019, 16, e1002857. [Google Scholar] [CrossRef] [PubMed]
- Assaf-Balut, C.; García De La Torre, N.; Durán, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Ortolá, A.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, N.G.; Assaf-Balut, C.; Varas, I.J.; Del Valle, L.; Durán, A.; Fuentes, M.; Del Prado, N.; Bordiú, E.; Valerio, J.J.; Herraiz, M.A.; et al. Effectiveness of following mediterranean diet recommendations in the real world in the incidence of gestational diabetes mellitus (GDM) and adverse maternal-foetal outcomes: A prospective, universal, interventional study with a single group. the st carlos study. Nutrients 2019, 11, 1210. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; Garcia de la Torre, N.; Durán, A.; Bordiu, E.; del Valle, L.; Familiar, C.; Valerio, J.; Jimenez, I.; Herraiz, M.A.; Izquierdo, N.; et al. An Early, Universal Mediterranean Diet-Based Intervention in Pregnancy Reduces Cardiovascular Risk Factors in the “Fourth Trimester”. J. Clin. Med. 2019, 8, 1499. [Google Scholar] [CrossRef]
- Myrskylä, M.; Fenelon, A. Maternal Age and Offspring Adult Health: Evidence from the Health and Retirement Study. Demography 2012, 49, 1231–1257. [Google Scholar] [CrossRef]
- Hinkle, S.N.; Albert, P.S.; Mendola, P.; Sjaarda, L.A.; Yeung, E.; Boghossian, N.S.; Laughon, S.K. The association between parity and birthweight in a longitudinal consecutive pregnancy cohort. Paediatr. Perinat. Epidemiol. 2014, 28, 106–115. [Google Scholar] [CrossRef]
- Sonneveldt, E.; Decormier Plosky, W.; Stover, J. Linking high parity and maternal and child mortality: What is the impact of lower health services coverage among higher order births? BMC Public Health 2013, 13, S7. [Google Scholar] [CrossRef]
- Moore Simas, T.A.; Waring, M.E.; Callaghan, K.; Leung, K.; Ward Harvey, M.; Buabbud, A.; Chasan-Taber, L. Weight gain in early pregnancy and risk of gestational diabetes mellitus among Latinas. Diabetes Metab. 2019, 45, 26–31. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; Familiar, C.; García de la Torre, N.; Rubio, M.A.; Bordiú, E.; Del Valle, L.; Lara, M.; Ruiz, T.; Ortolá, A.; Crespo, I.; et al. Gestational diabetes mellitus treatment reduces obesity-induced adverse pregnancy and neonatal outcomes: The St. Carlos gestational study. BMJ Open Diabetes Res. Care 2016, 4, e000314. [Google Scholar] [CrossRef]
- Assaf-Balut, C.; García de la Torre, N.; Duran, A.; Fuentes, M.; Bordiú, E.; Del Valle, L.; Familiar, C.; Valerio, J.; Jiménez, I.; Herraiz, M.A.; et al. A Mediterranean Diet with an Enhanced Consumption of Extra Virgin Olive Oil and Pistachios Improves Pregnancy Outcomes in Women Without Gestational Diabetes Mellitus: A Sub-Analysis of the St. Carlos Gestational Diabetes Mellitus Prevention Study. Ann. Nutr. Metab. 2019, 74, 69–79. [Google Scholar] [CrossRef]
- Amati, F.; Hassounah, S.; Swaka, A. The impact of mediterranean dietary patterns during pregnancy on maternal and offspring health. Nutrients 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhang, W.; Yang, W.; Liu, H. Systematic review and meta-analysis of whether cesarean section contributes to the incidence of allergic diseases in children: A protocol for systematic review and meta analysis. Medicine 2019, 98, e18394. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, E.; Bozzola, M.; Calcaterra, V.; Barberi, S.; Villani, A. Infectious diseases and vaccination strategies: How to protect the “unprotectable”? ISRN Prev. Med. 2013, 2013, e765354. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Sicherer, S.H.; Wesley Burks, A.; Abrams, S.A.; Fuchs, G.J.; Kim, J.H.; Wesley Lindsey, C.; Magge, S.N.; Rome, E.S.; Schwarzenberg, S.J. The effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Greenspan, L.C.; Faith, M.S.; Hurston, S.R.; Quesenberry, C.P. Breastfeeding and growth during infancy among offspring of mothers with gestational diabetes mellitus: A prospective cohort study. Pediatr. Obes. 2018, 13, 492–504. [Google Scholar] [CrossRef]
- Fernández-Barrés, S.; Romaguera, D.; Valvi, D.; Martínez, D.; Vioque, J.; Navarrete-Muñoz, E.M.; Amiano, P.; Gonzalez-Palacios, S.; Guxens, M.; Pereda, E.; et al. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: The INMA birth cohort study. Pediatr. Obes. 2016, 11, 491–499. [Google Scholar] [CrossRef]
- Lahti-Pulkkinen, M.; Bhattacharya, S.; Wild, S.H.; Lindsay, R.S.; Räikkönen, K.; Norman, J.E.; Bhattacharya, S.; Reynolds, R.M. Consequences of being overweight or obese during pregnancy on diabetes in the offspring: A record linkage study in Aberdeen, Scotland. Diabetologia 2019, 62, 1412–1419. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Cañueto, D.; Giardina, S.; Salas-Salvadó, J.; Cañellas, N.; Correig, X.; Bulló, M. Effect of pistachio consumption on the modulation of urinary gut microbiota-related metabolites in prediabetic subjects. J. Nutr. Biochem. 2017, 45, 48–53. [Google Scholar] [CrossRef]
- Edwards, S.M.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The Maternal Gut Microbiome during Pregnancy. Mcn Am. J. Matern. Nurs. 2017, 42, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A.L.; Mulle, J.G.; Ferranti, E.P.; Edwards, S.; Dunn, A.B.; Corwin, E.J. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv. Neonatal Care 2015, 15, 377–385. [Google Scholar] [CrossRef] [PubMed]
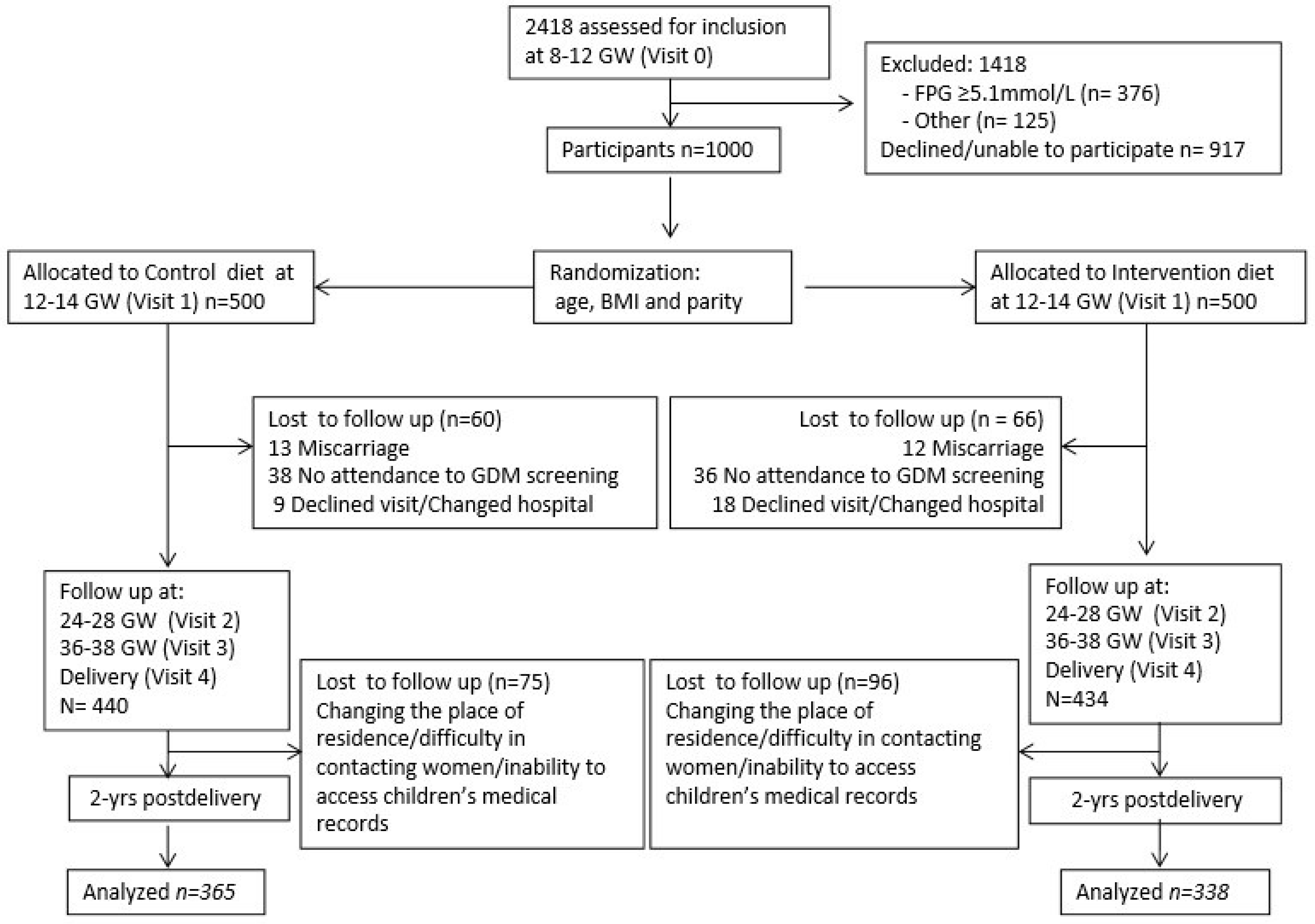
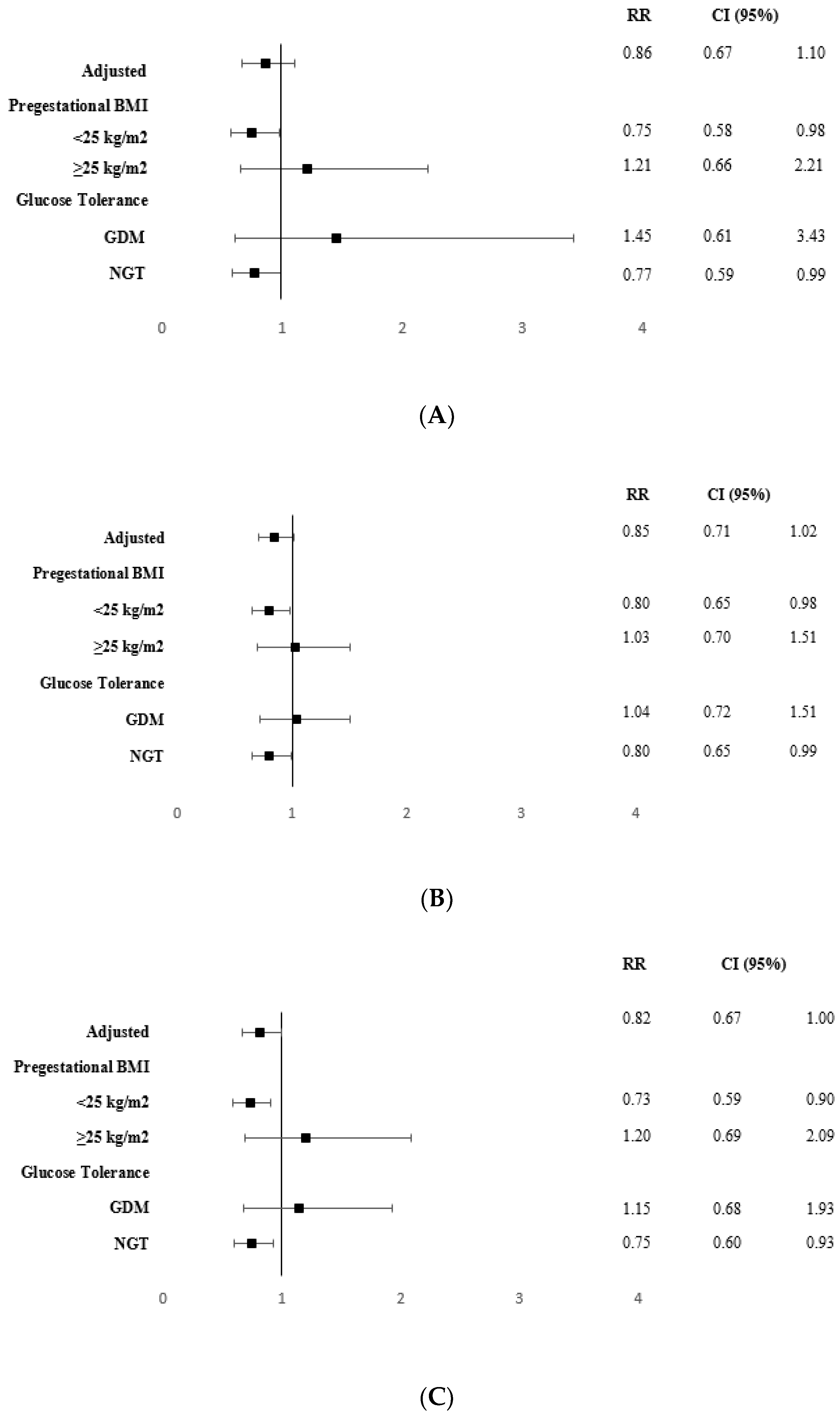
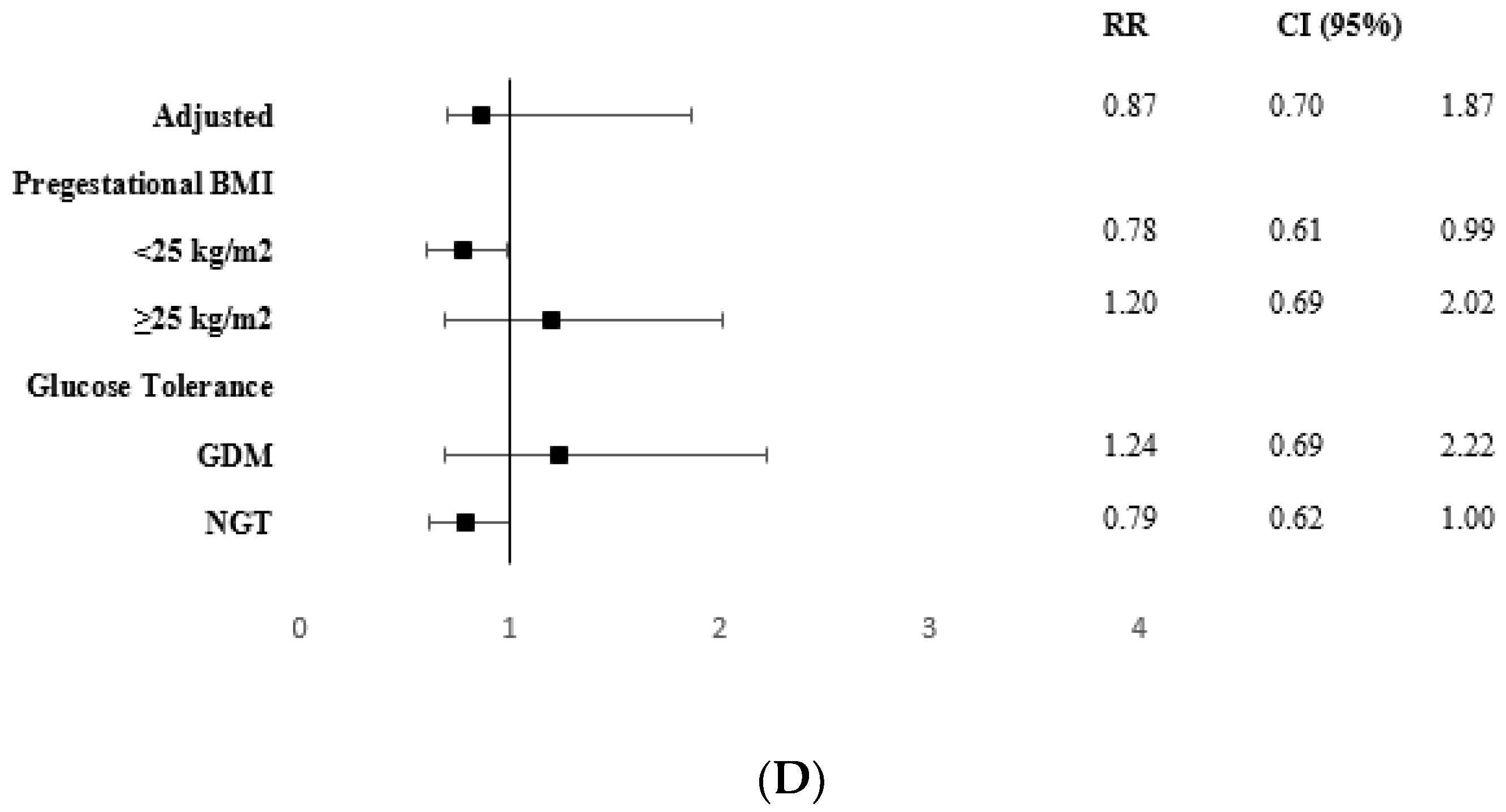
| CONTROL GROUP n = 365 | INTERVENTION GROUP n = 338 | p | |
|---|---|---|---|
| Age (years) | 32.8 ± 5.3 | 33.2 ± 5.0 | 0.316 |
| Race/Ethnicity | |||
| Caucasian | 237 (64.9) | 222 (65.6) | |
| Hispanic | 114 (31.2) | 109 (32.2) | |
| Others | 14 (3.9) | 7 (2.2) | 0.804 |
| Family history of | |||
| Type 2 Diabetes | 82 (23.5) | 92 (27.7) | |
| MetS (>2 components) | 65 (17.8) | 76 (22.5) | 0.154 |
| Previous history of | |||
| GDM | 11 (3.0) | 11 (3.3) | |
| Miscarriages | 117 (32.0) | 117 (34.6) | 0.688 |
| Educational status | |||
| Elementary education | 41 (11.2) | 21 (6.2) | |
| Secondary School | 145 (39.7) | 146 (43.2) | |
| University Degree | 174 (47.7) | 168 (49.7) | |
| UNK | 5 (1.4) | 3 (0.9) | 0.467 |
| Employment | 276 (75.6) | 266 (78.7) | 0.950 |
| Number of pregnancies | |||
| Primiparous | 144 (39.5) | 142 (42.1) | |
| Second pregnancy | 119 (32.6) | 115 (34.1) | |
| >2 pregnancies | 102 (27.9) | 81 (22.8) | 0.622 |
| Smoker | |||
| Never | 202 (55.3) | 184 (54.1) | |
| Current | 26 (7.1) | 26 (7.7) | 0.994 |
| Gestational Age (weeks) at baseline | 12.1 ± 0.6 | 12.0 ± 0.3 | 0.899 |
| Pre-pregnancy Body Weight (kg) | 61.5 ± 11.1 | 60.3 ± 9.9 | 0.131 |
| Weight gain at: | |||
| 24–28 GW | 7.73 ± 4.22 | 7.04 ± 3.71 | 0.022 |
| 36–38 GW | 11.02 ± 6.71 | 11.49 ± 6.87 | 0.452 |
| Pre-pregnancy BMI (kg/m2) | 23.3 ± 3.9 | 23.1 ± 3.5 | 0.354 |
| BMI ≥ 25 kg/m2 | 107 (29.3) | 86 (25.4) | 0.486 |
| Systolic BP/Diastolic BP (mm Hg) | |||
| 12 GW | 107 ± 11/64 ± 9 | 107 ± 10/66 ± 9 | 0.957/0.061 |
| 24 GW | 105 ± 11/63 ± 9 | 105 ± 11/63 ± 8 | 0.370/0.747 |
| 36 GW | 113 ± 13/72 ± 9 | 113 ± 13/73 ± 9 | 0.319/0.303 |
| Fasting Blood Glucose (mg/dl) | |||
| 12 GW | 81.4 ± 6.1 | 81.2 ± 6.0 | 0.687 |
| 24 GW | 85.8 ± 6.7 | 84.0 ± 6.5 | 0.001 |
| 36 GW | 77.1 ± 7.7 | 75.0 ± 7.7 | 0.007 |
| GDM at 24–28 GW n (%) | 91 (24.9) | 58 (17.2) | 0.036 |
| Caesarean Section n (%) | 56 (15.3) | 54 (16.0) | 0.111 |
| TSH mcUI/mL | |||
| 12 GW | 1.9 ± 1.2 | 2.1 ± 1.4 | 0.223 |
| 24 GW | 2.0 ± 1.2 | 2.1 ± 1.1 | 0.464 |
| 36 GW | 1.7 ± 1.3 | 1.6 ± 0.9 | 0.890 |
| MEDAS Score | |||
| 12 GW | 4.1 ± 1.7 | 4.4 ± 1.6 | 0.090 |
| 24 GW | 4.5 ± 1.7 | 6.3 ± 1.7 | 0.001 |
| 36 GW | 5.5 ± 1.9 | 6.6 ± 2.1 | 0.001 |
| Nutrition Score | |||
| 12 GW | 0.6 ± 3.3 | 0.2 ± 3.1 | 0.078 |
| 24 GW | 1.2 ± 3.4 | 4.2 ± 3.2 | 0.001 |
| 36 GW | 3.6 ± 3.7 | 5.3 ± 3.6 | 0.001 |
| Physical Activity Score | |||
| 12 GW | −1.7 ± 1.0 | −1.9 ± 1.0 | 0.059 |
| 24 GW | −1.8 ± 0.9 | −1.8 ± 0.9 | 0.482 |
| 36 GW | −1.8 ± 0.7 | −1.6 ± 0.9 | 0.055 |
| CG | IG | p | |
|---|---|---|---|
| Number (n) | 365 | 338 | |
| Born, n (%) | |||
| Preterm (<37 GW) | 14 (3.8) | 5 (1.5) | 0.477 |
| Small for gestational age (SGA) | 23 (6.3) | 5 (1.5) | 0.002 |
| Large for gestational age (LGA) | 10 (2.7) | 4 (1.2) | 0.049 |
| Age (months) | 23.13 ± 2.55 | 23.29 ± 2.51 | 0.433 |
| Body Weight (kg) | 12.11 ± 1.48 | 12.17 ± 1.54 | 0.555 |
| Percentile | 47.7 ± 27.0 | 49.2 ± 27.4 | 0.483 |
| Height (cm) | 86.26 ±3.96 | 86.16 ± 4.01 | 0.759 |
| Percentile | 39.3 ± 27.0 | 39.7 ± 28.6 | 0.867 |
| Breastfeeding n (%) | 340 (94.4%) | 314 (93.5%) | 0.703 |
| Exclusive (months) | 5.20 ± 1.50 | 5.36 ± 1.47 | 0.194 |
| Mixed (months) | 10.34 ± 7.74 | 10.60 ± 7.55 | 0.705 |
| Cereal Introduction (months) | |||
| Gluten-free cereal | 4.77 ± 0.81 | 4.74 ± 0.81 | 0.702 |
| Gluten cereal | 6.58 ± 1.28 | 6.62 ± 2.02 | 0.787 |
| Nursery | |||
| n (%) | 247 (67.7) | 237 (70.7) | 0.213 |
| Age (months) | 15.8 ± 6.0 | 14.8 ± 6.8 | 0.139 |
| Vaccinations n (%) | |||
| Compulsory | 359 (99.4%) | 338 (100%) | 0.270 |
| Recommended n (%) | |||
| Meningitis | 210 (58.2) | 214 (64.3) | 0.059 |
| Rotavirus | 251 (69.5) | 240 (72.1) | 0.257 |
| Outpatient diseases n (%) | |||
| Treatment with antibiotics | 251 (68.8%) | 234 (69.4%) | 0.456 |
| Treatment with corticosteroids | 187 (51.2%) | 166 (49.3%) | 0.327 |
| Diagnoses n (%) | |||
| Food allergies | 29 (8.0%) | 21 (6.2%) | 0.225 |
| Asthma | 7 (1.9%) | 11 (3.3%) | 0.189 |
| Bronchiolitis/respiratory problems | 74 (20.3%) | 75 (22.3%) | 0.298 |
| Atopic dermatitis | 101 (27.7%) | 106 (31.5%) | 0.161 |
| Severe Diseases inpatients treatment | |||
| All-cause hospital stays n (%) | 65 (17.8%) | 51 (15.1%) | 0.193 |
| Children n (%) | 54 (14.9%) | 46 (13.6%) | 0.079 |
| Duration (days) | 11.9 ± 25.2 | 6.8 ± 9.1 | 0.020 |
| Asthma/bronchiolitis disease | 27 (7.4%) | 18 (5.3%) | 0.167 |
| Treatment with antibiotics | 59 (16.2%) | 40 (11.9%) | 0.063 |
| Treatment with corticosteroids | 41 (11.2%) | 25 (7.4%) | 0.054 |
| Treatment with antibiotics and corticosteroids | 36 (9.9%) | 25 (7.4%) | 0.155 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melero, V.; Assaf-Balut, C.; Torre, N.G.d.l.; Jiménez, I.; Bordiú, E.; Valle, L.d.; Valerio, J.; Familiar, C.; Durán, A.; Runkle, I.; et al. Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study. J. Clin. Med. 2020, 9, 1454. https://doi.org/10.3390/jcm9051454
Melero V, Assaf-Balut C, Torre NGdl, Jiménez I, Bordiú E, Valle Ld, Valerio J, Familiar C, Durán A, Runkle I, et al. Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study. Journal of Clinical Medicine. 2020; 9(5):1454. https://doi.org/10.3390/jcm9051454
Chicago/Turabian StyleMelero, Verónica, Carla Assaf-Balut, Nuria García de la Torre, Inés Jiménez, Elena Bordiú, Laura del Valle, Johanna Valerio, Cristina Familiar, Alejandra Durán, Isabelle Runkle, and et al. 2020. "Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study." Journal of Clinical Medicine 9, no. 5: 1454. https://doi.org/10.3390/jcm9051454
APA StyleMelero, V., Assaf-Balut, C., Torre, N. G. d. l., Jiménez, I., Bordiú, E., Valle, L. d., Valerio, J., Familiar, C., Durán, A., Runkle, I., Miguel, M. P. d., Montañez, C., Barabash, A., Cuesta, M., Herraiz, M. A., Izquierdo, N., Rubio, M. A., & Calle-Pascual, A. L. (2020). Benefits of Adhering to a Mediterranean Diet Supplemented with Extra Virgin Olive Oil and Pistachios in Pregnancy on the Health of Offspring at 2 Years of Age. Results of the San Carlos Gestational Diabetes Mellitus Prevention Study. Journal of Clinical Medicine, 9(5), 1454. https://doi.org/10.3390/jcm9051454





