Recommendations for Standards of Network Care for Patients with Parkinson’s Disease in Germany
Abstract
1. Introduction
2. Roundtable Participants and Format
3. The Patient Journey—Where to Go When PD Is Suspected?
4. What Symptoms and Complaints Should Prompt a GP to Consider a Diagnosis of PD, and When Should a Neurologist Be Consulted?
5. What Diagnostic Tests Should Be Performed by the Neurologist at the First Visit?
6. Which Patients Should Be Referred to a Specialized Movement Disorder Center?
- Doubts about correct diagnosis;
- Young onset and side effects under prescribed medication;
- Troublesome motor fluctuations;
- ≥1 h of troublesome dyskinesia/day;
- ≥2 h ‘off’ symptoms/day;
- ≥5 oral levodopa doses/day;
- Freezing of gait;
- Acute or subacute camptocormia;
- High burden of NMS;
- Nontransitory troublesome hallucinations;
- Repeated falls despite assumed treatment.
7. Which Patients Should Be Admitted for PD Multimodal Complex Treatment (MCT)?
8. Which Patients Should Be Admitted to a PD Day Clinic?
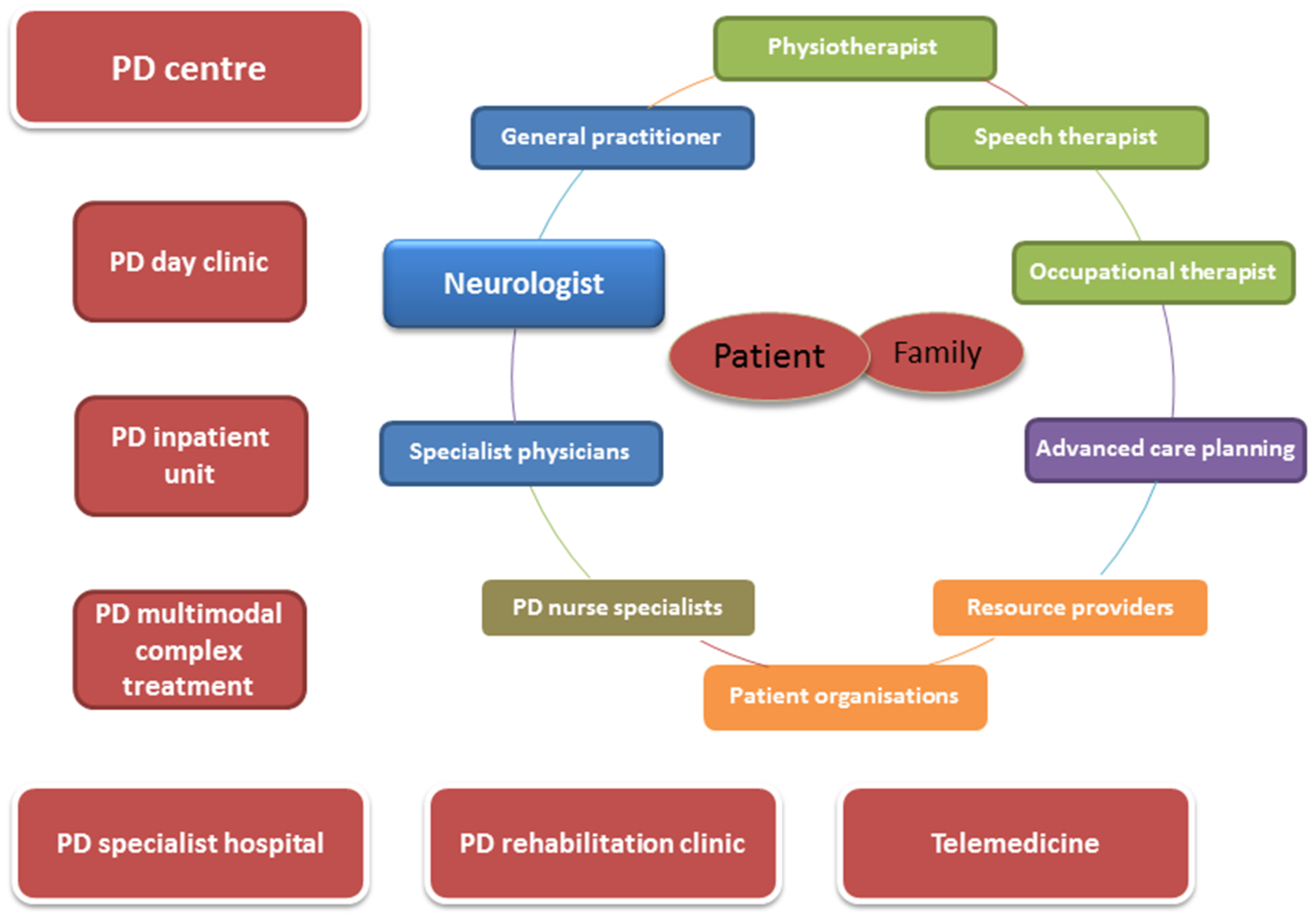
9. Who Else Should Be Involved in the Treatment of Patients with PD?
10. What Is the Relevance of Self-Management in Patients with PD?
11. What Is the Role of Telemedicine in the Network Care of Patients with PD?
12. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Nonmotor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22 (Suppl. 1), S119–S122. [Google Scholar] [CrossRef] [PubMed]
- Ri Richter, D.; Bartig, D.; Muhlack, S.; Hartelt, E.; Scherbaum, R.; Katsanos, A.H.; Müller, T.; Jost, W.H.; Ebersbach, G.; Gold, R.; et al. Dynamics of Parkinson’s Disease Multimodal Complex Treatment in Germany from 2010–2016: Patient Characteristics, Access to Treatment, and Formation of Regional Centers. Cells 2019, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Tonges, L.; Bartig, D.; Muhlack, S.; Jost, W.; Gold, R.; Krogias, C. Characteristics and dynamics of inpatient treatment of patients with Parkinson’s disease in Germany: Analysis of 1.5 million patient cases from 2010 to 2015. Der Nervenarzt 2019, 90, 167–174. [Google Scholar]
- Binder, S.G.S.; Woitalla, D.; Müller, T.; Wellach, I.; Klucken, J.; Eggers, C.; Liersch, S.; Amelung, V. Patients’ Perspective on Provided Health Services in Parkinson’s Disease in Germany–a Cross-Sectional Survey. Akt Neurol. 2018, 45, 703–713. [Google Scholar] [CrossRef]
- Tonges, L.; Ehret, R.; Lorrain, M.; Riederer, P.; Mungersdorf, M. Epidemiology of Parkinson’s Disease and Current Concepts of Outpatient Care in Germany. Fortschr. Neurol. Psychiatr. 2017, 85, 329–335. [Google Scholar]
- Eggers, C.; Wolz, M.; Warnecke, T.; Prell, T.; Tonges, L. Parkinson Networks in Germany: Future or Utopia? Fortschr. Neurol. Psychiatr. 2020. [Google Scholar] [CrossRef]
- Bloem, B.R.; Rompen, L.; Vries, N.M.; Klink, A.; Munneke, M.; Jeurissen, P. ParkinsonNet: A Low-Cost Health Care Innovation With A Systems Approach From The Netherlands. Health Aff. 2017, 36, 1987–1996. [Google Scholar] [CrossRef]
- Bloem, B.R.; Munneke, M. Revolutionising management of chronic disease: The ParkinsonNet approach. BMJ 2014, 348, g1838. [Google Scholar] [CrossRef]
- Espay, A.J.; Hausdorff, J.M.; Sánchez-Ferro, Á.; Klucken, J.; Merola, A.; Bonato, P.; Paul, S.S.; Horak, F.B.; Vizcarra, J.A.; Mestre, T.A.; et al. A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov. Disord. 2019, 34, 657–663. [Google Scholar] [CrossRef]
- Klucken, J.; Kruger, R.; Schmidt, P.; Bloem, B.R. Management of Parkinson’s Disease 20 Years from Now: Towards Digital Health Pathways. J. Park. Dis. 2018, 8, S85–S94. [Google Scholar] [CrossRef] [PubMed]
- Ypinga, J.H.; de Vries, N.M.; Boonen, L.H.; Koolman, X.; Munneke, M.; Zwinderman, A.H.; Bloem, B.R. Effectiveness and costs of specialized physiotherapy given via ParkinsonNet: A retrospective analysis of medical claims data. Lancet Neurol. 2018, 17, 153–161. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; E Lang, A.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.J.; Lang, A.E.; Halliday, G.; Goetz, C.G.; Chan, P.; Slow, E.; Seppi, K.; et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2018, 33, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- S3 Leitlinie Idiopathisches Parkinsonsyndrom. Available online: https://www.dgn.org/leitlinien/3219-030-010-idiopathisches-parkinson-syndrom (accessed on 1 January 2016).
- Hellwig, S.; Amtage, F.; Kreft, A.; Buchert, R.; Winz, O.H.; Vach, W.; Spehl, T.S.; Rijntjes, M.; Hellwig, B.; Weiller, C.; et al. [18F] FDG-PET is superior to [123I] IBZM-SPECT for the differential diagnosis of parkinsonism. Neurology 2012, 79, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Walker, Z.; Gandolfo, F.; Orini, S.; Garibotto, V.; Agosta, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Nestor, P.J.; Boccardi, M.; et al. Clinical utility of FDG PET in Parkinson’s disease and atypical parkinsonism associated with dementia. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1534–1545. [Google Scholar] [CrossRef]
- Eggers, C.; On behalf of the CPN study group; Dano, R.; Schill, J.; Fink, G.R.; Hellmich, M.; Timmermann, L. Patient-centered integrated healthcare improves quality of life in Parkinson’s disease patients: A randomized controlled trial. J. Neurol. 2018, 265, 764–773. [Google Scholar] [CrossRef]
- Van der Marck, M.A.; Bloem, B.R.; Borm, G.F.; Overeem, S.; Munneke, M.; Guttman, M. Effectiveness of multidisciplinary care for Parkinson’s disease: A randomized, controlled trial. Mov. Disord. 2013, 28, 605–611. [Google Scholar] [CrossRef]
- Antonini, A.; Stoessl, A.J.; Kleinman, L.S.; Skalicky, A.M.; Marshall, T.S.; Sail, K.R.; Onuk, K.; Odin, P.L.A. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multicountry Delphi-panel approach. Curr. Med Res. Opin. 2018, 34, 2063–2073. [Google Scholar] [CrossRef]
- Odin, P.; Chaudhuri, K.R.; Slevin, J.; Volkmann, J.; Dietrichs, E.; Martinez-Martin, P.; Krauss, J.; Henriksen, T.; Katzenschlager, R.; Antonini, A.; et al. Collective physician perspectives on nonoral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Park. Relat. Disord. 2015, 21, 1133–1144. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Chaudhuri, K.R.; Ruiz, P.J.G.; LeWitt, P.; Katzenschlager, R.; Sixel-Döring, F.; Henriksen, T.; Sesar, Á.; Poewe, W.; Ceballos-Baumann, A. Expert Consensus Group report on the use of apomorphine in the treatment of Parkinson’s disease–Clinical practice recommendations. Park. Relat. Disord. 2015, 21, 1023–1030. [Google Scholar] [CrossRef]
- Müller, T.; Öhm, G.; Eilert, K.; Möhr, K.; Rotter, S.; Haas, T.; Küchler, M.; Lütge, S.; Marg, M.; Rothe, H. Benefit on motor and nonmotor behavior in a specialized unit for Parkinson’s disease. J. Neural Transm. 2017, 124, 715–720. [Google Scholar] [CrossRef]
- Scherbaum, R.; Hartelt, E.; Kinkel, M.; Gold, R.; Muhlack, S.; Tönges, L. Parkinson’s Disease Multimodal Complex Treatment improves motor symptoms, depression and quality of life. J. Neurol. 2019, 267, 954–965. [Google Scholar] [CrossRef]
- Fründt, O.; Mainka, T.; Schönwald, B.; Müller, B.; Dicusar, P.; Gerloff, C.; Buhmann, C. The Hamburg Parkinson day-clinic: A new treatment concept at the border of in- and outpatient care. J. Neural Transm. 2018, 125, 1461–1472. [Google Scholar] [CrossRef]
- Buhmann, C.; Bass, H.; Hahne, M.; Jost, W.; Redecker, C.; Schwarz, M.; Woitalla, D. Parkinson’s Disease at the Border Between Inpatient and Outpatient Care. Fortschr. Neurol. Psychiatr. 2016, 84 (Suppl. 1), S36–S40. [Google Scholar]
- Ellis, T.; Katz, D.I.; White, D.K.; DePiero, T.J.; Hohler, A.D.; Saint-Hilaire, M. Effectiveness of an inpatient multidisciplinary rehabilitation program for people with Parkinson disease. Phys. Ther. 2008, 88, 812–819. [Google Scholar] [CrossRef]
- Oguh, O.; Videnovic, A. Inpatient management of Parkinson disease: Current challenges and future directions. Neurohospitalist 2012, 2, 28–35. [Google Scholar] [CrossRef]
- Zertifizierungskriterien. Available online: https://www.parkinson-vereinigung.de/diverse-inhalte/fachkliniken/zertifizieungskriterien.html (accessed on 20 April 2020).
- Weerkamp, N.J.; Tissingh, G.; Poels, P.J.; Zuidema, S.U.; Munneke, M.; Koopmans, R.T.; Bloem, B.R. Parkinson disease in long term care facilities: A review of the literature. J. Am. Med. Dir. Assoc. 2014, 15, 90–94. [Google Scholar] [CrossRef]
- Tosserams, A.; de Vries, N.M.; Bloem, B.R.; Nonnekes, J. Multidisciplinary Care to Optimize Functional Mobility in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 159–172. [Google Scholar] [CrossRef]
- Domingos, J.; Keus, S.H.J.; Dean, J.; de Vries, N.M.; Ferreira, J.J.; Bloem, B.R. The European Physiotherapy Guideline for Parkinson’s Disease: Implications for Neurologists. J. Park. Dis. 2018, 8, 499–502. [Google Scholar]
- Munneke, M.; Nijkrake, M.J.; Keus, S.H.J.; Kwakkel, G.; Berendse, H.W.; Roos, R.A.C.; Borm, G.F.; Adang, E.M.; Overeem, S.; Bloem, B.R.; et al. Efficacy of community-based physiotherapy networks for patients with Parkinson’s disease: A cluster-randomized trial. Lancet Neurol. 2010, 9, 46–54. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.-Y.; Barton, B.; De Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C.; on behalf of the Movement Disorder Society Evidence-Based Medicine Committee; Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef] [PubMed]
- Radder, D.L.M.; Sturkenboom, I.H.; van Nimwegen, M.; Keus, S.H.; Bloem, B.R.; de Vries, N.M. Physical therapy and occupational therapy in Parkinson’s disease. Int. J. Neurosci. 2017, 127, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Sturkenboom, I.H.W.M.; Graff, M.J.L.; Hendriks, J.C.M.; Veenhuizen, Y.; Munneke, M.; Bloem, B.R.; van der Sander, M.W.N.; OTiP Study Group. Efficacy of occupational therapy for patients with Parkinson’s disease: A randomized controlled trial. Lancet Neurol. 2014, 13, 557–566. [Google Scholar] [CrossRef]
- Suttrup, I.; Warnecke, T. Dysphagia in Parkinson’s Disease. Dysphagia 2016, 31, 24–32. [Google Scholar] [CrossRef]
- Lennaerts, H.; Groot, M.; Rood, B.; Gilissen, K.; Tulp, H.; Van Wensen, E.; Munneke, M.; Van Laar, T.; Bloem, B.R. A Guideline for Parkinson’s Disease Nurse Specialists, with Recommendations for Clinical Practice. J. Park. Dis. 2017, 7, 749–754. [Google Scholar] [CrossRef]
- Radder, D.L.; Lennaerts, H.H.; Vermeulen, H.; Van Asseldonk, T.; Delnooz, C.C.S.; Hagen, R.H.; Munneke, M.; Bloem, B.R.; De Vries, N.M. The cost-effectiveness of specialized nursing interventions for people with Parkinson’s disease: The NICE-PD study protocol for a randomized controlled clinical trial. Trials 2020, 21, 88. [Google Scholar] [CrossRef]
- Oliver, D.; Veronese, S. Specialist palliative care for Parkinson’s disease. Ann. Palliat. Med. 2020, 9, S52–S62. [Google Scholar] [CrossRef]
- Ng, J. Palliative care for Parkinson’s disease. Ann. Palliat. Med. 2018, 7, 296–303. [Google Scholar] [CrossRef]
- Lau, F.; Downing, M.; Lesperance, M.; Karlson, N.; Kuziemsky, C.; Yang, J. Using the Palliative Performance Scale to Provide Meaningful Survival Estimates. J. Pain. Symptom Manag. 2009, 38, 134–144. [Google Scholar] [CrossRef]
- Lorig, K.R.; Holman, H. Self-management education: History, definition, outcomes, and mechanisms. Ann. Behav. Med. 2003, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.; Liddy, C. Self-management support programs for persons with Parkinson’s disease: An integrative review. Patient Educ. Couns. 2017, 100, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Groppa, S.; Woitalla, D.; Müller, T.; Wellach, I.; Klucken, J.; Eggers, C.; Liersch, S.; Amelung, V.E. Patientenperspektive auf die Versorgungssituation im Krankheitsbild Morbus Parkinson in Deutschland–eine Querschnittserhebung. Aktuelle Neurol. 2018, 45, 703–713. [Google Scholar] [CrossRef]
- Schneider, R.B.; Biglan, K.M. The promise of telemedicine for chronic neurological disorders: The example of Parkinson’s disease. Lancet Neurol. 2017, 16, 541–551. [Google Scholar] [CrossRef]
- Ben-Pazi, H.; Browne, P.; Chan, P.; Cubo, E.; Guttman, M.; Hassan, A.; Hatcher-Martin, J.; Mari, Z.; Moukheiber, E.; the International Parkinson and Movement Disorder Society Telemedicine Task Force; et al. The Promise of Telemedicine for Movement Disorders: An Interdisciplinary Approach. Curr. Neurol. Neurosci. Rep. 2018, 18, 26. [Google Scholar] [CrossRef]
- Spear, K.L.; Auinger, P.; Simone, R.; Dorsey, E.R.; Francis, J. Patient Views on Telemedicine for Parkinson Disease. J. Park. Dis. 2019, 9, 401–404. [Google Scholar] [CrossRef]
- Weck, C.E.; Lex, K.M.; Lorenzl, S. Telemedicine in Palliative Care: Implementation of New Technologies to Overcome Structural Challenges in the Care of Neurological Patients. Front. Neurol. 2019. [Google Scholar] [CrossRef]
- Marxreiter, F.; Buttler, U.; Gassner, H.; Gandor, F.; Gladow, T.; Eskofier, B.; Winkler, J.; Ebersbach, G.; Klucken, J. The Use of Digital Technology and Media in German Parkinson’s Disease Patients. J. Park. Dis. 2019, 10, 717–727. [Google Scholar] [CrossRef]
- Jarman, B.; Hurwitz, B.; Cook, A.; Bajekal, M.; Lee, A. Effects of community based nurses specialising in Parkinson’s disease on health outcome and costs: Randomized controlled trial. BMJ 2002, 324, 1072–1075. [Google Scholar] [CrossRef][Green Version]
- Strandberg-Larsen, M. Measuring integrated care. Dan. Med. Bull. 2011, 58, B4245. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prell, T.; Siebecker, F.; Lorrain, M.; Eggers, C.; Lorenzl, S.; Klucken, J.; Warnecke, T.; Buhmann, C.; Tönges, L.; Ehret, R.; et al. Recommendations for Standards of Network Care for Patients with Parkinson’s Disease in Germany. J. Clin. Med. 2020, 9, 1455. https://doi.org/10.3390/jcm9051455
Prell T, Siebecker F, Lorrain M, Eggers C, Lorenzl S, Klucken J, Warnecke T, Buhmann C, Tönges L, Ehret R, et al. Recommendations for Standards of Network Care for Patients with Parkinson’s Disease in Germany. Journal of Clinical Medicine. 2020; 9(5):1455. https://doi.org/10.3390/jcm9051455
Chicago/Turabian StylePrell, Tino, Frank Siebecker, Michael Lorrain, Carsten Eggers, Stefan Lorenzl, Jochen Klucken, Tobias Warnecke, Carsten Buhmann, Lars Tönges, Reinhard Ehret, and et al. 2020. "Recommendations for Standards of Network Care for Patients with Parkinson’s Disease in Germany" Journal of Clinical Medicine 9, no. 5: 1455. https://doi.org/10.3390/jcm9051455
APA StylePrell, T., Siebecker, F., Lorrain, M., Eggers, C., Lorenzl, S., Klucken, J., Warnecke, T., Buhmann, C., Tönges, L., Ehret, R., Wellach, I., & Wolz, M. (2020). Recommendations for Standards of Network Care for Patients with Parkinson’s Disease in Germany. Journal of Clinical Medicine, 9(5), 1455. https://doi.org/10.3390/jcm9051455







