Clinical Utility of Extracorporeal Shock Wave Therapy on Hypertrophic Scars of the Hand Caused by Burn Injury: A Prospective, Randomized, Double-Blinded Study
Abstract
1. Introduction
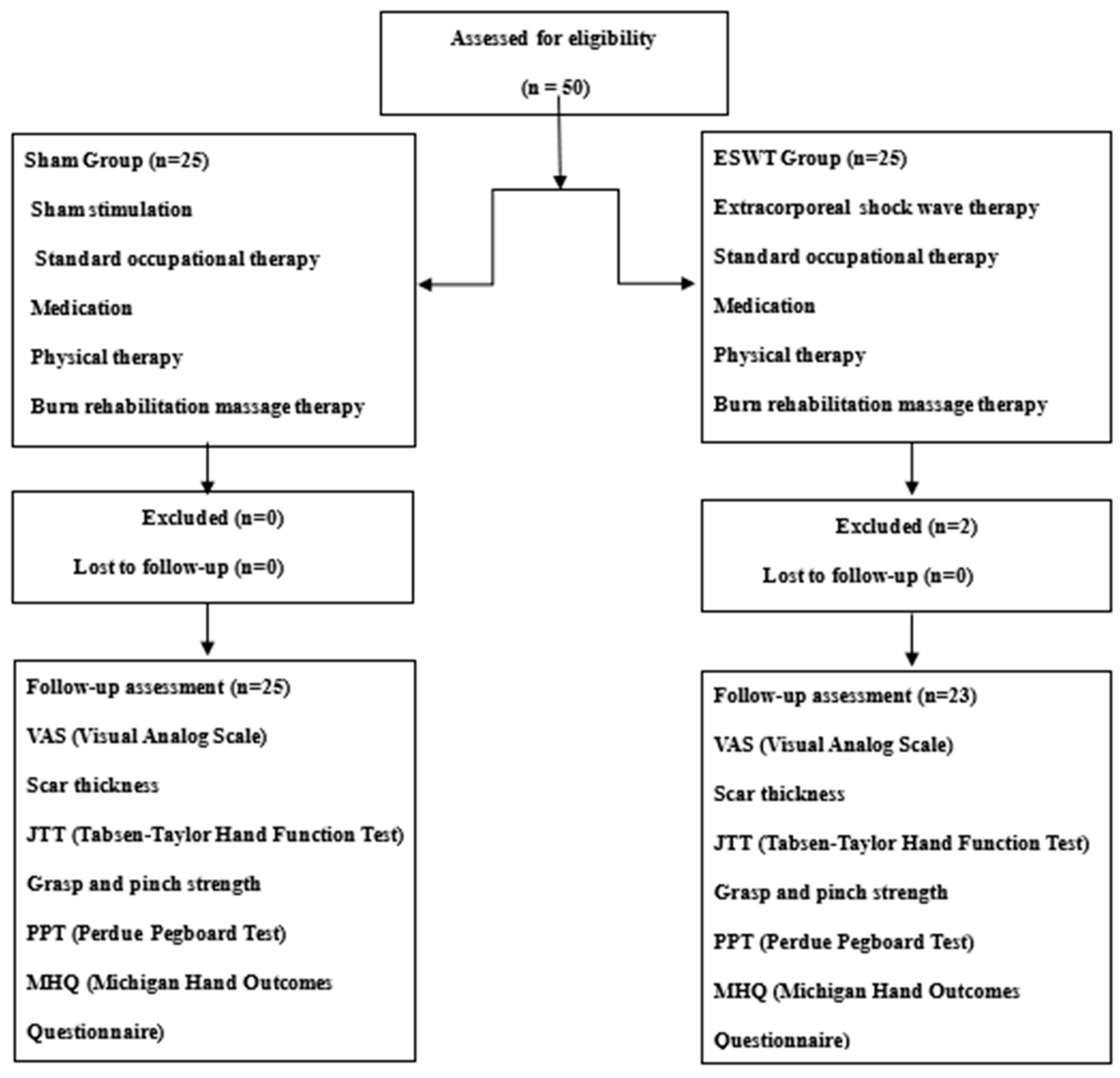
2. Experimental Section
2.1. Methods
Study Design and Statement of Ethics
2.2. Study Group
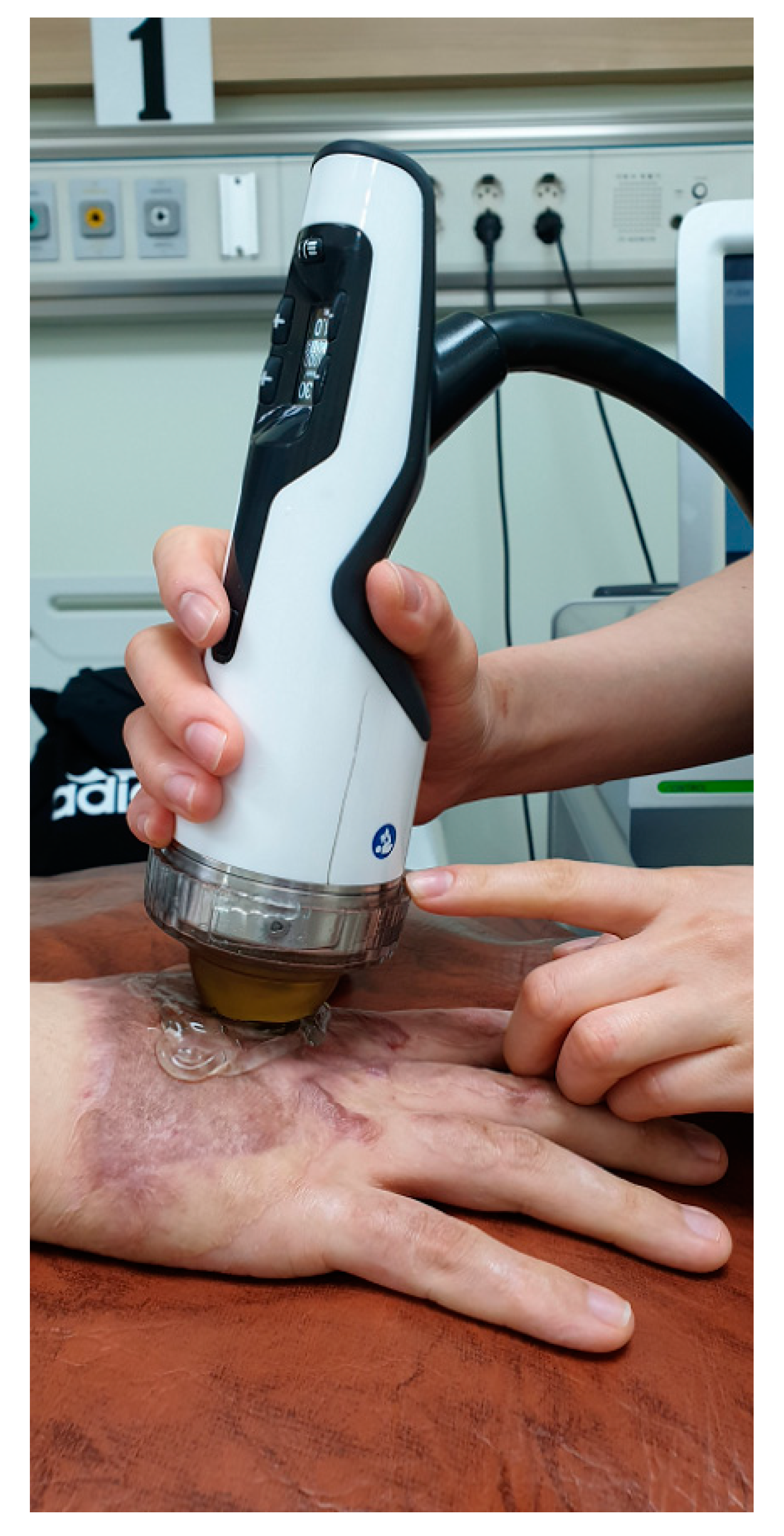
2.3. Intervention
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cui, H.S.; Hong, A.R.; Kim, J.B.; Yu, J.H.; Cho, Y.S.; Joo, S.Y.; Seo, C.H. Extracorporeal Shock Wave Therapy Alters the Expression of Fibrosis-Related Molecules in Fibroblast Derived from Human Hypertrophic Scar. Int. J. Mol. Sci. 2018, 19, 124. [Google Scholar] [CrossRef]
- Arno, A.; Garcia, O.; Hernan, I.; Sancho, J.; Acosta, A.; Barret, J.P. Extracorporeal shock waves, a new non-surgical method to treat severe burns. Burns J. Int. Soc. 2010, 36, 844–849. [Google Scholar] [CrossRef]
- Van der Veer, W.M.; Bloemen, M.C.; Ulrich, M.M.; Molema, G.; van Zuijlen, P.P.; Middelkoop, E.; Niessen, F.B. Potential cellular and molecular causes of hypertrophic scar formation. Burns J. Int. Soc. 2009, 35, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Saggini, A.; Spagnoli, A.M.; Dodaj, I.; Cigna, E.; Maruccia, M.; Soda, G.; Bellomo, R.G.; Scuderi, N. Extracorporeal Shock Wave Therapy: An Emerging Treatment Modality for Retracting Scars of the Hands. Ultrasound Med. Biol. 2016, 42, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Nuchtern, J.G.; Engrav, L.H.; Nakamura, D.Y.; Dutcher, K.A.; Heimbach, D.M.; Vedder, N.B. Treatment of fourth-degree hand burns. J. Burn Care Rehabil. 1995, 16, 36–42. [Google Scholar] [CrossRef] [PubMed]
- De Lima Morais, T.M.; Meyer, P.F.; de Vasconcellos, L.S.; e Silva, J.C.; e Andrade, I.F.; de Farias, V.A.F.; da Silva, I.C.; Araújo, R.M.F.G.; da Silva, R.M.V.; Pacheco, E.F.; et al. Effects of the extracorporeal shock wave therapy on the skin: An experimental study. Lasers Med. Sci. 2019, 34, 389–396. [Google Scholar] [CrossRef]
- Kuo, Y.R.; Wu, W.S.; Hsieh, Y.L.; Wang, F.S.; Wang, C.T.; Chiang, Y.C.; Wang, C.J. Extracorporeal shock wave enhanced extended skin flap tissue survival via increase of topical blood perfusion and associated with suppression of tissue pro-inflammation. J. Surg. Res. 2007, 143, 385–392. [Google Scholar] [CrossRef]
- Djedovic, G.; Kamelger, F.S.; Jeschke, J.; Piza-Katzer, H. Effect of extracorporeal shock wave treatment on deep partial-thickness burn injury in rats: A pilot study. Plast. Surg. Int. 2014, 2014, 495967. [Google Scholar] [CrossRef]
- Haupt, G.; Chvapil, M. Effect of shock waves on the healing of partial-thickness wounds in piglets. J. Surg. Res. 1990, 49, 45–48. [Google Scholar] [CrossRef]
- Aguilera-Saez, J.; Munoz, P.; Serracanta, J.; Monte, A.; Barret, J.P. Extracorporeal shock wave therapy role in the treatment of burn patients. A systematic literature review. Burns J. Int. Soc. 2019. [Google Scholar] [CrossRef]
- Fioramonti, P.; Cigna, E.; Onesti, M.G.; Fino, P.; Fallico, N.; Scuderi, N. Extracorporeal shock wave therapy for the management of burn scars. Dermatol. Surg. 2012, 38, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Joo, S.Y.; Cui, H.; Cho, S.R.; Yim, H.; Seo, C.H. Effect of extracorporeal shock wave therapy on scar pain in burn patients: A prospective, randomized, single-blind, placebo-controlled study. Medicine 2016, 95, e4575. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.Y.; Cho, Y.S.; Seo, C.H. The clinical utility of extracorporeal shock wave therapy for burn pruritus: A prospective, randomized, single-blind study. Burns J. Int. Soc. 2018, 44, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Yang, X.; Wu, G.; Liu, M.; Han, J.; Tao, K.; Hu, D. The use of autologous platelet-rich plasma gel increases wound healing and reduces scar development in split-thickness skin graft donor sites. J. Plast. Surg. Hand Surg. 2019, 53, 356–360. [Google Scholar] [CrossRef]
- Cowan, A.C.; Stegink-Jansen, C.W. Rehabilitation of hand burn injuries: Current updates. Injury 2013, 44, 391–396. [Google Scholar] [CrossRef]
- Joo, S.Y.; Cho, Y.S.; Lee, S.Y.; Seok, H.; Seo, C.H. Effects of Virtual Reality-Based Rehabilitation on Burned Hands: A Prospective, Randomized, Single-Blind Study. J. Clin. Med. 2020, 9, 731. [Google Scholar] [CrossRef]
- Schoneveld, K.; Wittink, H.; Takken, T. Clinimetric evaluation of measurement tools used in hand therapy to assess activity and participation. J. Hand 2009, 22, 221–235. [Google Scholar] [CrossRef]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef]
- Zhao, J.C.; Zhang, B.R.; Hong, L.; Shi, K.; Wu, W.W.; Yu, J.A. Extracorporeal shock wave therapy with low-energy flux density inhibits hypertrophic scar formation in an animal model. Int. J. Mol. Med. 2018, 41, 1931–1938. [Google Scholar] [CrossRef]
- Zhao, J.C.; Zhang, B.R.; Shi, K.; Wang, J.; Yu, Q.H.; Yu, J.A. Lower energy radial shock wave therapy improves characteristics of hypertrophic scar in a rabbit ear model. Exp. Ther. Med. 2018, 15, 933–939. [Google Scholar] [CrossRef]
- Meirer, R.; Kamelger, F.S.; Piza-Katzer, H. Shock wave therapy: An innovative treatment method for partial thickness burns. Burns J. Int. Soc. 2005, 31, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Ottomann, C.; Hartmann, B.; Tyler, J.; Maier, H.; Thiele, R.; Schaden, W.; Stojadinovic, A. Prospective randomized trial of accelerated re-epithelization of skin graft donor sites using extracorporeal shock wave therapy. J. Am. Coll. Surg. 2010, 211, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Ottomann, C.; Stojadinovic, A.; Lavin, P.T.; Gannon, F.H.; Heggeness, M.H.; Thiele, R.; Schaden, W.; Hartmann, B. Prospective randomized phase II Trial of accelerated reepithelialization of superficial second-degree burn wounds using extracorporeal shock wave therapy. Ann. Surg. 2012, 255, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Meirer, R.; Kamelger, F.S.; Huemer, G.M.; Wanner, S.; Piza-Katzer, H. Extracorporal shock wave may enhance skin flap survival in an animal model. Br. J. Plast. Surg. 2005, 58, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Kisch, T.; Sorg, H.; Forstmeier, V.; Knobloch, K.; Liodaki, E.; Stang, F.; Mailänder, P.; Krämer, R. Remote effects of extracorporeal shock wave therapy on cutaneous microcirculation. J. Tissue Viability 2015, 24, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Stojadinovic, A.; Anam, K.; Amare, M.; Naik, S.; Peoples, G.E.; Tadaki, D.; Elster, E.A. Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int. Wound J. 2009, 6, 11–21. [Google Scholar] [CrossRef]
- Pinitkwamdee, S.; Laohajaroensombat, S.; Orapin, J.; Woratanarat, P. Effectiveness of Extracorporeal Shockwave Therapy in the Treatment of Chronic Insertional Achilles Tendinopathy. Foot Ankle Int. 2020. [Google Scholar] [CrossRef]
- Ochiai, N.; Ohtori, S.; Sasho, T.; Nakagawa, K.; Takahashi, K.; Takahashi, N.; Murata, R.; Moriya, H.; Wada, Y.; Saisu, T. Extracorporeal shock wave therapy improves motor dysfunction and pain originating from knee osteoarthritis in rats. Osteoarthr. Cartil. 2007, 15, 1093–1096. [Google Scholar] [CrossRef]
- Henderson, J.; Terenghi, G.; McGrouther, D.A.; Ferguson, M.W. The reinnervation pattern of wounds and scars may explain their sensory symptoms. J. Plast. Reconstr. Aesthetic Surg. 2006, 59, 942–950. [Google Scholar] [CrossRef]
- Dedes, V.; Stergioulas, A.; Kipreos, G.; Dede, A.M.; Mitseas, A.; Panoutsopoulos, G.I. Effectiveness and Safety of Shockwave Therapy in Tendinopathies. Mater. Sociomed. 2018, 30, 131–146. [Google Scholar] [CrossRef]
- Dedes, V.; Tzirogiannis, K.; Polikandrioti, M.; Dede, A.M.; Mitseas, A.; Panoutsopoulos, G.I. Comparison of radial extracorporeal shockwave therapy with ultrasound therapy in patients with lateral epicondylitis. J. Med. Ultrason. 2020, 47, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Padua, R.; Bondi, R.; Ceccarelli, E.; Ripanti, S.; Bondi, L.; Campi, A. Extracorporeal shock wave therapy for chronic calcifying tendinitis of the shoulder. J. Orthop. Traumatol. 2002, 2, 147–150. [Google Scholar] [CrossRef]
- Seok, H.; Kim, S.H. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2013, 92, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Zyluk, A.; Mosiejczuk, H. Outcomes of the treatment of trigger digits by extracorporal shock wave therapy (ESWT). Handchir. Mikrochir. Plast. Chir. 2020, 52, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Samhan, A.F.; Abdelhalim, N.M. Impacts of low-energy extracorporeal shockwave therapy on pain, pruritus, and health-related quality of life in patients with burn: A randomized placebo-controlled study. Burns J. Int. Soc. Burn Inj. 2019, 45, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
| ESWT Group (n = 23) | Sham Group (n = 25) | p-Value | |
|---|---|---|---|
| Male:Female | 22:1 | 23:2 | 0.53 |
| Age (years) | 47.09 ± 11.09 | 48.56 ± 11.18 | 0.94 |
| Cause of burn | 0.43 | ||
| Flame burn | 15 | 18 | |
| Electrical burn | 1 | 3 | |
| Contact burn | 0 | 1 | |
| Scalding burn | 3 | 1 | |
| Spark burn | 4 | 2 | |
| Time to treatment (days) | 64.04 ± 36.62 | 63.48 ± 14.43 | 0.12 |
| TBSA (%) | 28.39 ± 17.86 | 27.80 ± 19.46 | 0.91 |
| ESWT Group (n = 23) | Sham Group (n = 25) | p-Value | |
|---|---|---|---|
| VAS | 7.00 ± 1.24 | 6.96 ± 1.21 | 0.84 |
| Thickness (cm) | 0.21 ± 0.11 | 0.19 ± 0.06 | 0.36 |
| Vancouver Scar Scale | |||
| Pigmentation | 3.00 ± 0.00 | 2.92 ± 0.28 | 0.17 |
| Pliability | 1.87 ± 0.76 | 2.20 ± 0.76 | 0.14 |
| Height | 1.43 ± 0.51 | 1.56 ± 0.58 | 0.33 |
| Vascularity | 2.17 ± 0.72 | 2.32 ± 0.75 | 0.45 |
| Total | 8.48 ± 1.70 | 9.00 ± 1.85 | 0.32 |
| Grasp and Pinch Power Test | |||
| Grasp (kg) | 7.50 ± 7.40 | 5.81 ± 7.72 | 0.38 |
| Lateral Pinch (kg) | 3.21 ± 1.67 | 3.58 ± 5.21 | 0.43 |
| Tip Pinch (kg) | 1.40 ± 0.91 | 1.32 ± 1.33 | 0.56 |
| Jebsen−Taylor Hand Function Test | |||
| Writing | 11.04 ± 4.80 | 10.64 ± 3.49 | 0.10 |
| Cards | 3.57 ± 2.78 | 3.04 ± 2.42 | 0.53 |
| Small | 5.57 ± 3.86 | 5.04 ± 4.23 | 0.66 |
| Checkers | 9.26 ± 4.11 | 8.00 ± 5.38 | 0.72 |
| Feeding | 10.09 ± 4.07 | 8.88 ± 5.23 | 0.73 |
| Light | 8.78 ± 4.52 | 9.68 ± 3.69 | 0.60 |
| Heavy | 8.52 ± 4.33 | 8.08 ± 4.47 | 0.76 |
| Perdue Pegboard Test | |||
| Affected hand | 7.78 ± 5.51 | 8.52 ± 3.37 | 0.58 |
| Both hands | 5.78 ± 4.49 | 6.36 ± 2.61 | 0.59 |
| Assembly | 12.13 ± 12.33 | 14.00 ± 8.45 | 0.26 |
| Michigan Hand Outcomes Questionnaire | |||
| Function | 21.09 ± 12.96 | 23.80 ± 10.54 | 0.30 |
| ADL | 21.52 ± 12.38 | 23.20 ± 12.74 | 0.49 |
| Work | 15.57 ± 14.24 | 15.20 ± 18.06 | 0.63 |
| Pain | 34.35 ± 28.13 | 24.92 ± 21.27 | 0.22 |
| Esthetics | 12.36 ± 16.36 | 10.08 ± 13.78 | 0.74 |
| Satisfaction | 24.96 ± 12.35 | 25.08 ± 14.10 | 0.75 |
| ESWT Group (n = 23) | Sham Group (n = 25) | p-Value | |
|---|---|---|---|
| VAS | −1.48 ± 1.04 | −0.52 ± 0.77 | 0.001 ** |
| Scar thickness (cm) | 0.01 ± 0.07 | 0.07 ± 0.07 | 0.018 ** |
| Vancouver Scar Scale | |||
| Pigmentation | −0.04 ± 0.21 | −0.16 ± 0.37 | 0.19 |
| Pliability | 0.13 ± 0.81 | 0.08 ± 1.08 | 0.78 |
| Height | 0.09 ± 0.60 | 0.44 ± 0.58 | 0.06 |
| Vascularity | −0.52 ± 0.73 | −0.04 ± 0.61 | 0.015 ** |
| Total | −0.35 ± 1.61 | 0.32 ± 1.75 | 0.19 |
| Grasp and Pinch Power Test | |||
| Grasp (kg) | 4.71 ± 4.90 | 4.71 ± 3.77 | 0.99 |
| Lateral Pinch (kg) | 1.10 ± 1.69 | 0.74 ± 1.61 | 0.46 |
| Tip Pinch (kg) | 0.80 ± 1.13 | 0.48 ± 0.78 | 0.26 |
| Jebsen−Taylor Hand Function Test | |||
| Writing | 1.57 ± 4.11 | 0.88 ± 3.43 | 0.12 |
| Cards | 2.48 ± 3.54 | 0.40 ± 2.22 | 0.02 * |
| Small | 3.70 ± 4.30 | 0.16 ± 2.87 | 0.004 ** |
| Checkers | 1.30 ± 4.48 | 3.32 ± 4.40 | 0.15 |
| Feeding | 1.70 ± 3.97 | 1.48 ± 2.38 | 0.99 |
| Light | 1.78 ± 4.77 | 0.04 ± 2.17 | 0.16 |
| Heavy | 1.39 ± 5.06 | 0.60 ± 1.68 | 0.90 |
| Perdue Pegboard Test | |||
| Affected hand | 2.26 ± 3.51 | 0.00 ± 3.65 | 0.12 |
| Both hands | 1.61 ± 2.97 | 1.44 ± 4.23 | 0.97 |
| Assembly | 4.04 ± 10.55 | 1.72 ± 10.15 | 0.44 |
| Michigan Hand Outcomes Questionnaire | |||
| Function | 13.78 ± 18.12 | 8.60 ± 15.65 | 0.17 |
| ADL | 11.74 ± 20.43 | 12.20 ± 20.77 | 0.51 |
| Work | 19.22 ± 19.47 | 13.72 ± 22.91 | 0.12 |
| Pain | −9.39 ± 24.78 | −6.48 ± 18.45 | 0.45 |
| Esthetics | 10.55 ± 16.46 | 9.60 ± 21.33 | 0.15 |
| Satisfaction | 22.86 ± 21.59 | 10.30 ± 19.37 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, S.Y.; Lee, S.Y.; Cho, Y.S.; Seo, C.H. Clinical Utility of Extracorporeal Shock Wave Therapy on Hypertrophic Scars of the Hand Caused by Burn Injury: A Prospective, Randomized, Double-Blinded Study. J. Clin. Med. 2020, 9, 1376. https://doi.org/10.3390/jcm9051376
Joo SY, Lee SY, Cho YS, Seo CH. Clinical Utility of Extracorporeal Shock Wave Therapy on Hypertrophic Scars of the Hand Caused by Burn Injury: A Prospective, Randomized, Double-Blinded Study. Journal of Clinical Medicine. 2020; 9(5):1376. https://doi.org/10.3390/jcm9051376
Chicago/Turabian StyleJoo, So Young, Seung Yeol Lee, Yoon Soo Cho, and Cheong Hoon Seo. 2020. "Clinical Utility of Extracorporeal Shock Wave Therapy on Hypertrophic Scars of the Hand Caused by Burn Injury: A Prospective, Randomized, Double-Blinded Study" Journal of Clinical Medicine 9, no. 5: 1376. https://doi.org/10.3390/jcm9051376
APA StyleJoo, S. Y., Lee, S. Y., Cho, Y. S., & Seo, C. H. (2020). Clinical Utility of Extracorporeal Shock Wave Therapy on Hypertrophic Scars of the Hand Caused by Burn Injury: A Prospective, Randomized, Double-Blinded Study. Journal of Clinical Medicine, 9(5), 1376. https://doi.org/10.3390/jcm9051376






