Psychosocial Impact of Predictive Genetic Testing in Hereditary Heart Diseases: The PREDICT Study
Abstract
1. Introduction
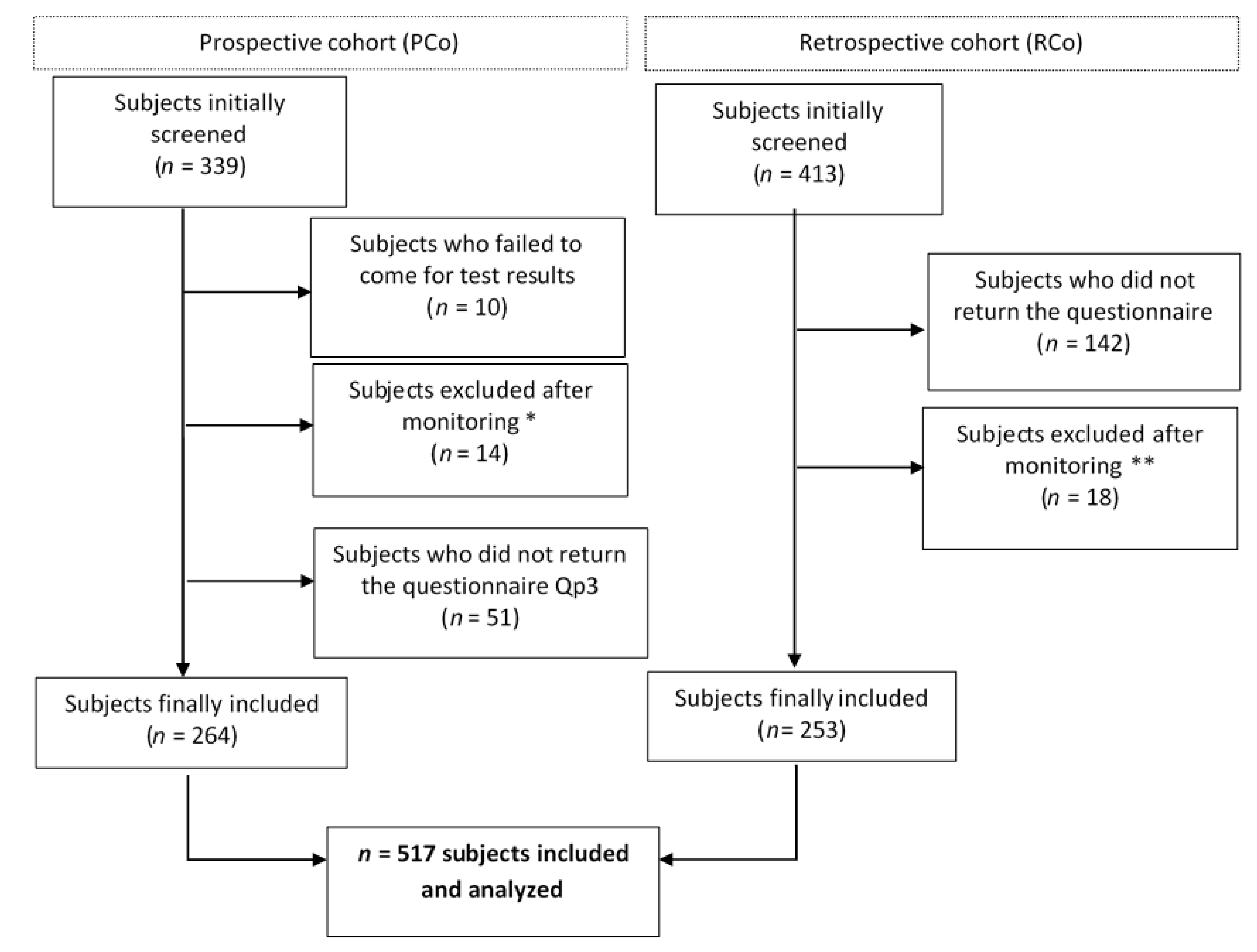
2. Methods
2.1. Population
2.2. Questionnaires
2.3. Statistical Analysis
3. Results
3.1. Expectations From the First Provision of Information
3.2. Reasons for Undergoing Predictive Genetic Testing
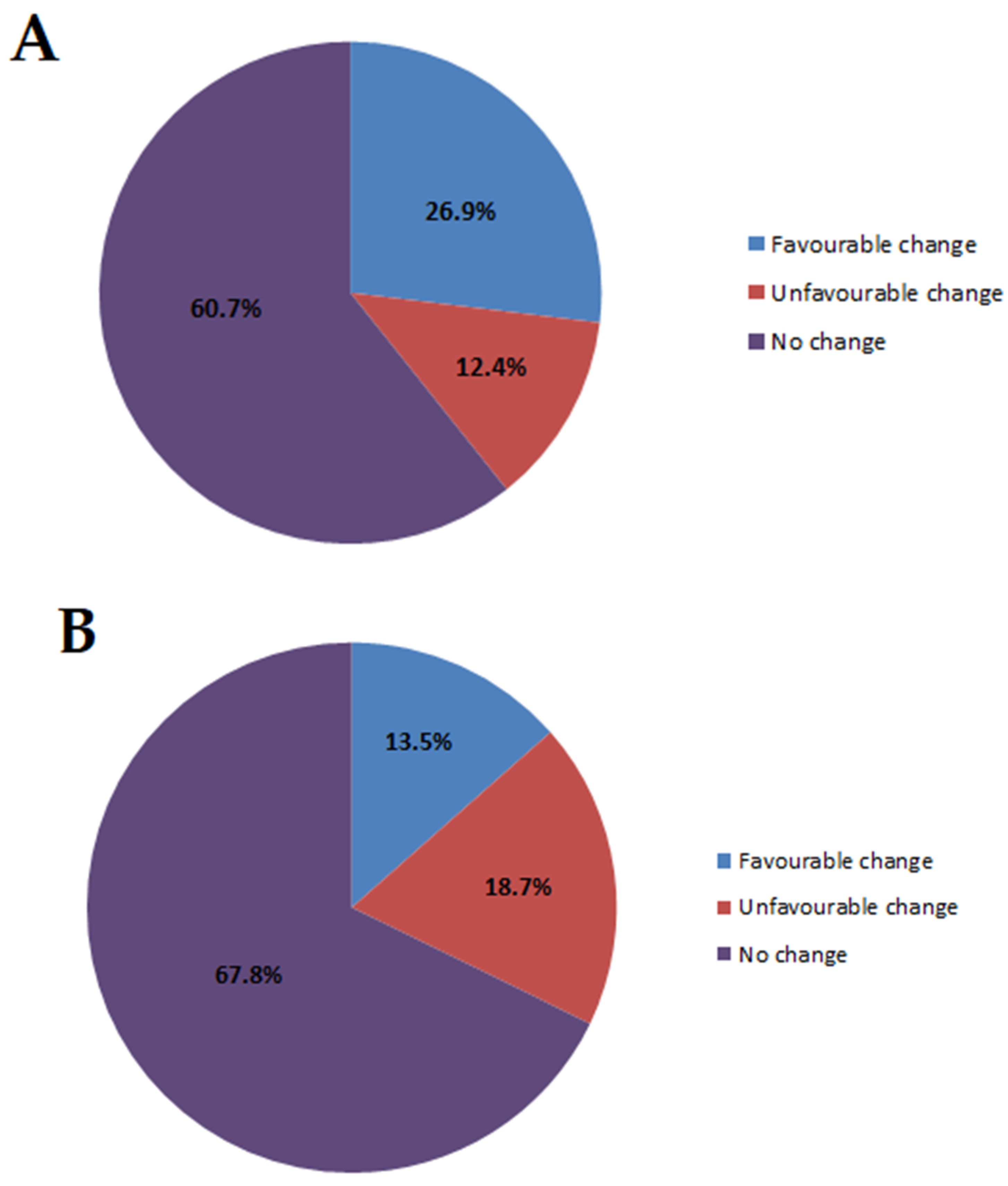
3.3. Global Life Changes After Predictive Genetic Testing
3.4. Changes in Social, Professional or Family Relationships After Predictive Genetic Testing
3.5. Psychological Impact of Disclosure of Genetic Status
3.6. Regret at Having Undergone Predictive Genetic Testing
4. Discussion
5. Limitations and Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Broadstock, M.; Michie, S.; Marteau, T.M. Psychological consequences of predictive genetic testing: A systematic review. Eur. J. Hum. Genet. 2000, 8, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Skirton, H.; Goldsmith, L.; Jackson, L.; Tibben, A. Quality in genetic counselling for presymptomatic testing—Clinical guidelines for practice across the range of genetic conditions. Eur. J. Hum. Genet. 2012, 21, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Mularczyk, E.; Decruyenaere, M.; Denayer, L.; Evers-Kiebooms, G. A theoretical psychological perspective on predictive testing for late onset hereditary diseases. Genet. Couns. (Geneva Switz.) 2007, 18, 367–378. [Google Scholar]
- Almqvist, E.; Brinkman, R.R.; Wiggins, S.; Hayden, M.R. Psychological consequences and predictors of adverse events in the first 5 years after predictive testing for Huntington’s disease. Clin. Genet. 2003, 64, 300–309. [Google Scholar] [CrossRef]
- Wiggins, S.; Whyte, P.; Huggins, M.; Adam, S.; Theilmann, J.; Bloch, M.; Sheps, S.B.; Schechter, M.T.; Hayden, M.R. The Canadian Collaborative Study of Predictive Testing* The Psychological Consequences of Predictive Testing for Huntington’s Disease. N. Engl. J. Med. 1992, 327, 1401–1405. [Google Scholar] [CrossRef]
- Decruyenaere, M.; Evers-Kiebooms, G.; Cloostermans, T.; Boogaerts, A.; Demyttenaere, K.; Dom, R.; Fryns, J.P. Psychological distress in the 5-year period after predictive testing for Huntington’s disease. Eur. J. Hum. Genet. 2003, 11, 30–38. [Google Scholar] [CrossRef]
- Goizet, C.; Lesca, G.; Durr, A. Presymptomatic testing in Huntington’s disease and autosomal dominant cerebellar ataxias. Neurology 2002, 59, 1330–1336. [Google Scholar] [CrossRef]
- Timman, R.; Roos, R.; Maat-Kievit, A.; Tibben, A. Adverse Effects of Predictive Testing for Huntington Disease Underestimated: Long-Term Effects 7-10 Years After the Test. Health Psychol. 2004, 23, 189–197. [Google Scholar] [CrossRef]
- International Huntington Association and the World Federation of Neurology Research Group on Huntington’s Chorea. Guidelines for the molecular genetics predictive test in Huntington’s disease. J. Med. Genet. 1994, 31, 555–559. [Google Scholar] [CrossRef]
- Sturm, A.C.; Hershberger, R.E. Genetic testing in cardiovascular medicine. Curr. Opin. Cardiol. 2013, 28, 317–325. [Google Scholar] [CrossRef]
- Cirino, A.L.; Harris, S.; Lakdawala, N.K.; Michels, M.; Olivotto, I.; Day, S.M.; Abrams, M.J.; Charron, P.; Caleshu, C.; Semsarian, C.; et al. Role of Genetic Testing in Inherited Cardiovascular Disease. JAMA Cardiol. 2017, 2, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Charron, P.; Arad, M.; Arbustini, E.; Basso, C.; Bilinska, Z.T.; Elliott, P.; Helio, T.; Keren, A.; McKenna, W.J.; Monserrat, L.; et al. Genetic counselling and testing in cardiomyopathies: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2010, 31, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, A.J.; Ellinor, P.T.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA Expert Consensus Statement on the State of Genetic Testing for the Channelopathies and Cardiomyopathies: This document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). EP Eur. 2011, 13, 1077–1109. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, S.; Ferrari, F.; Manfrinati, A.; Pravettoni, G. A Systematic Review of the Psychological Implications of Genetic Testing: A Comparative Analysis Among Cardiovascular, Neurodegenerative and Cancer Diseases. Front. Genet. 2018, 9, 9. [Google Scholar] [CrossRef]
- Ormondroyd, E.; Oates, S.; Parker, M.; Blair, E.; Watkins, H. Pre-symptomatic genetic testing for inherited cardiac conditions: A qualitative exploration of psychosocial and ethical implications. Eur. J. Hum. Genet. 2013, 22, 88–93. [Google Scholar] [CrossRef]
- Hendriks, K.S.; Hendriks, M.M.; Birnie, E.; Grosfeld, F.J.; Wilde, A.A.M.; Bout, J.V.D.; Smets, E.M.; Van Tintelen, J.P.; Kroode, H.F.T.; Van Langen, I.M. Familial disease with a risk of sudden death: A longitudinal study of the psychological consequences of predictive testing for long QT syndrome. Heart Rhythm. 2008, 5, 719–724. [Google Scholar] [CrossRef]
- Charron, P.; Heron, D.; Gargiulo, M.; Richard, P.; Dubourg, O.; Desnos, M.; Bouhour, J.-B.; Feingold, J.; Carrier, L.; Hainque, B.; et al. Genetic testing and genetic counselling in hypertrophic cardiomyopathy: The French experience. J. Med. Genet. 2002, 39, 741–746. [Google Scholar] [CrossRef]
- MacLeod, R.; Beach, A.; Henriques, S.; Knopp, J.; Nelson, K.; Kerzin-Storrar, L. Experiences of predictive testing in young people at risk of Huntington’s disease, familial cardiomyopathy or hereditary breast and ovarian cancer. Eur. J. Hum. Genet. 2013, 22, 396–401. [Google Scholar] [CrossRef][Green Version]
- Hodges, W.F.; Spielberger, C.D. Digit span: An indicant of trait or state anxiety? J. Consult. Clin. Psychol. 1969, 33, 430–434. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Bruchon-Schweitzer, M.; Paulhan, I. STAI-Y: Inventaire d’anxiété état-trait Forme Y.; Éditions du centre de psychologie appliquée: Paris, France, 1993. [Google Scholar]
- Horowitz, M.; Wilner, N.; Alvarez, W. Impact of Event Scale: A Measure of Subjective Stress. Psychosom. Med. 1979, 41, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.; Holland, D.T.; Duong, J.; Ahimaz, P.; Chung, W.K. Examining the Psychosocial Impact of Genetic Testing for Cardiomyopathies. J. Genet. Couns. 2017, 27, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Christiaans, I.; Van Langen, I.M.; Birnie, E.; Bonsel, G.J.; Wilde, A.A.; Smets, E.M. Genetic counseling and cardiac care in predictively tested hypertrophic cardiomyopathy mutation carriers: The patients’ perspective. Am. J. Med. Genet. A 2009, 149, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Ingles, J.; Yeates, L.; O’Brien, L.; McGaughran, J.; Scuffham, P.; Atherton, J.; Semsarian, C. Genetic testing for inherited heart diseases: Longitudinal impact on health-related quality of life. Genet. Med. 2012, 14, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Ingles, J. Psychological Issues in Managing Families with Inherited Cardiovascular Diseases. Cold Spring Harb. Perspect. Med. 2019, a036558. [Google Scholar] [CrossRef] [PubMed]
- Hickey, K.T.; Sciacca, R.R.; Biviano, A.B.; Whang, W.; Dizon, J.M.; Garan, H.; Chung, W.K. The effect of cardiac genetic testing on psychological well-being and illness perceptions. Heart Lung 2014, 43, 127–132. [Google Scholar] [CrossRef]
- Baig, S.S.; Strong, M.; Rosser, E.; Taverner, N.V.; Glew, R.; Miedzybrodzka, Z.; Clarke, A.; Craufurd, D.; Quarrell, O. 22 Years of predictive testing for Huntington’s disease: The experience of the UK Huntington’s Prediction Consortium. Eur. J. Hum. Genet. 2016, 24, 1396–1402. [Google Scholar] [CrossRef]
- Erskine, K.E.; Hidayatallah, N.Z.; Walsh, C.A.; McDonald, T.V.; Cohen, L.; Marion, R.W.; Dolan, S. Motivation to pursue genetic testing in individuals with a personal or family history of cardiac events or sudden cardiac death. J. Genet. Couns. 2014, 23, 849–859. [Google Scholar] [CrossRef]
- Leray, E.; Camara, A.; Drapier, D.; Riou, F.; Bougeant, N.; Pélissolo, A.; Lloyd, K.; Bellamy, V.; Roelandt, J.; Millet, B. Prevalence, characteristics and comorbidities of anxiety disorders in France: Results from the “Mental Health in General Population” Survey (MHGP). Eur. Psychiatry 2011, 26, 339–345. [Google Scholar] [CrossRef]
- Aspinwall, L.G.; Taber, J.M.; Leaf, S.L.; Kohlmann, W.; Leachman, S.A. Melanoma genetic counseling and test reporting improve screening adherence among unaffected carriers 2 years later. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1687–1697. [Google Scholar] [CrossRef]
- Ertmański, S.; Metcalfe, K.; Trempała, J.; Głowacka, M.D.; Lubinski, J.; Narod, S.A.; Gronwald, J. Identification of Patients at High Risk of Psychological Distress After BRCA1 Genetic Testing. Genet. Test. Mol. Biomark. 2009, 13, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, M.; Lejeune, S.; Tanguy, M.-L.; Lahlou-Laforêt, K.; Faudet, A.; Cohen, D.; Feingold, J.; Dürr, A. Long-term outcome of presymptomatic testing in Huntington disease. Eur. J. Hum. Genet. 2008, 17, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Caleshu, C.; Kasparian, N.A.; Edwards, K.S.; Yeates, L.; Semsarian, C.; Perez, M.V.; Ashley, E.A.; Turner, C.J.; Knowles, J.W.; Ingles, J. Interdisciplinary psychosocial care for families with inherited cardiovascular diseases. Trends Cardiovasc. Med. 2016, 26, 647–653. [Google Scholar] [CrossRef] [PubMed]
| Prospective Cohort (n = 264) | Retrospective Cohort (n = 253) | |
|---|---|---|
| Age | 42.3 ± 16.7 years | 43.0 ± 15.2 years |
| Gender: | ||
| Female | 160 (60.6%) | 154 (60.9%) |
| Male | 104 (39.4%) | 99 (39.1%) |
| Familial disease: | ||
| Hypertrophic cardiomyopathy | 139 (52.9%) | 119 (47.0%) |
| Dilated cardiomyopathy | 39 (14.8%) | 36 (14.2%) |
| Long QT syndrome | 28 (10.6%) | 48 (19.0%) |
| Brugada syndrome | 0 (0.0%) | 4 (1.6%) |
| ARVC | 42 (16.0%) | 40 (15.8%) |
| Other | 15 (5.7%) | 6 (2.4%) |
| Genetic test result: | ||
| Carrier of the variant | 104 (39.4%) | 125 (49.4%) |
| Non-carrier | 160 (60.6%) | 128 (50.6%) |
| Sporting activity: | ||
| Yes | 132 (54.8%) | 144 (56.9%) |
| No | 109 (45.2%) | 109 (43.1%) |
| Marital status: | ||
| Single | 65 (28.5%) | 47 (24.0%) |
| In relationship | 163 (71.5%) | 149 (76.0%) |
| Prospective Cohort n = 264 | Retrospective Cohort n = 253 | |
|---|---|---|
| For children (to know if they are at risk) | 167 (64.0%) | 143 (56.5%) |
| To remove doubt | 171 (65.3%) | 129 (51.0%) |
| To benefit from medical monitoring | 91 (34.9%) | 93 (36.8%) |
| To prepare for the future | 64 (24.4%) | 39 (15.4%) |
| At the request of a close relative | 62 (23.8%) | 59 (23.3%) |
| Because of a planned pregnancy | 14 (5.3%) | 29 (11.5%) |
| To participate in a research protocol | 49 (18.8%) | 43 (17.0%) |
| I don’t know | 5 (1.9%) | 2 (0.8%) |
| Other | 48 (18.4%) | 19 (7.5%) |
| State-Trait Anxiety Inventory state score | ||||||
|---|---|---|---|---|---|---|
| All Subjects * | Non-Carriers | Mutation Carriers | ||||
| Mean ± SD | >35 (n) | Mean ± SD | >35 & (n) | Mean ± SD | >35 & (n) | |
| Qp1 | 30.5 ± 9.6 | 68 (28.6%) | 30.5 ± 9.3 | 41 (28.5%) | 30.6 ± 10.1 | 27 (28.7%) |
| Qp2 | 34.7 ± 12.1 | 90 (39.5%) | 34.9 ± 12.7 | 55 (40.1%) | 34.4 ± 11.2 | 35 (38.5%) |
| Qp3 | 30.0 ± 10.4 | 58 (23.3%) | 28.9 ± 9.9 | 29 (19.3%) | 31.7 ± 11.0 | 29 (29.3%) |
| Qr | 35.2 ± 11.7 | 92 (40.4%) | 34.8 ± 11.8 | 43 (36.8%) | 35.7 ± 11.7 | 49 (44.1%) |
| Impact of Event Scale Score | |||
|---|---|---|---|
| All Subjects * | Non-Carriers & | Mutation Carriers & | |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Qp1 | 6.9 ± 9.8 | 6.8 ± 10.7 | 7.0 ± 8.1 |
| Qp2 | 8.7 ± 10.5 | 9.1 ± 11.1 | 8.0 ± 9.5 |
| Qp3 | 6.5 ± 10.0 | 5.8 ± 9.7 | 7.6 ± 10.4 |
| Qr | 12.9 ± 14.0 | 10.0 ± 12.4 | 15.6 ± 15.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordet, C.; Brice, S.; Maupain, C.; Gandjbakhch, E.; Isidor, B.; Palmyre, A.; Moerman, A.; Toutain, A.; Akloul, L.; Brehin, A.-C.; et al. Psychosocial Impact of Predictive Genetic Testing in Hereditary Heart Diseases: The PREDICT Study. J. Clin. Med. 2020, 9, 1365. https://doi.org/10.3390/jcm9051365
Bordet C, Brice S, Maupain C, Gandjbakhch E, Isidor B, Palmyre A, Moerman A, Toutain A, Akloul L, Brehin A-C, et al. Psychosocial Impact of Predictive Genetic Testing in Hereditary Heart Diseases: The PREDICT Study. Journal of Clinical Medicine. 2020; 9(5):1365. https://doi.org/10.3390/jcm9051365
Chicago/Turabian StyleBordet, Céline, Sandrine Brice, Carole Maupain, Estelle Gandjbakhch, Bertrand Isidor, Aurélien Palmyre, Alexandre Moerman, Annick Toutain, Linda Akloul, Anne-Claire Brehin, and et al. 2020. "Psychosocial Impact of Predictive Genetic Testing in Hereditary Heart Diseases: The PREDICT Study" Journal of Clinical Medicine 9, no. 5: 1365. https://doi.org/10.3390/jcm9051365
APA StyleBordet, C., Brice, S., Maupain, C., Gandjbakhch, E., Isidor, B., Palmyre, A., Moerman, A., Toutain, A., Akloul, L., Brehin, A.-C., Sawka, C., Rooryck, C., Schaefer, E., Nguyen, K., Dupin Deguine, D., Rouzier, C., Billy, G., Séné, K., Denjoy, I., ... Charron, P. (2020). Psychosocial Impact of Predictive Genetic Testing in Hereditary Heart Diseases: The PREDICT Study. Journal of Clinical Medicine, 9(5), 1365. https://doi.org/10.3390/jcm9051365





