Quantitative Approach to Fragmented QRS in Arrhythmogenic Cardiomyopathy: From Disease towards Asymptomatic Carriers of Pathogenic Variants
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Data Collection
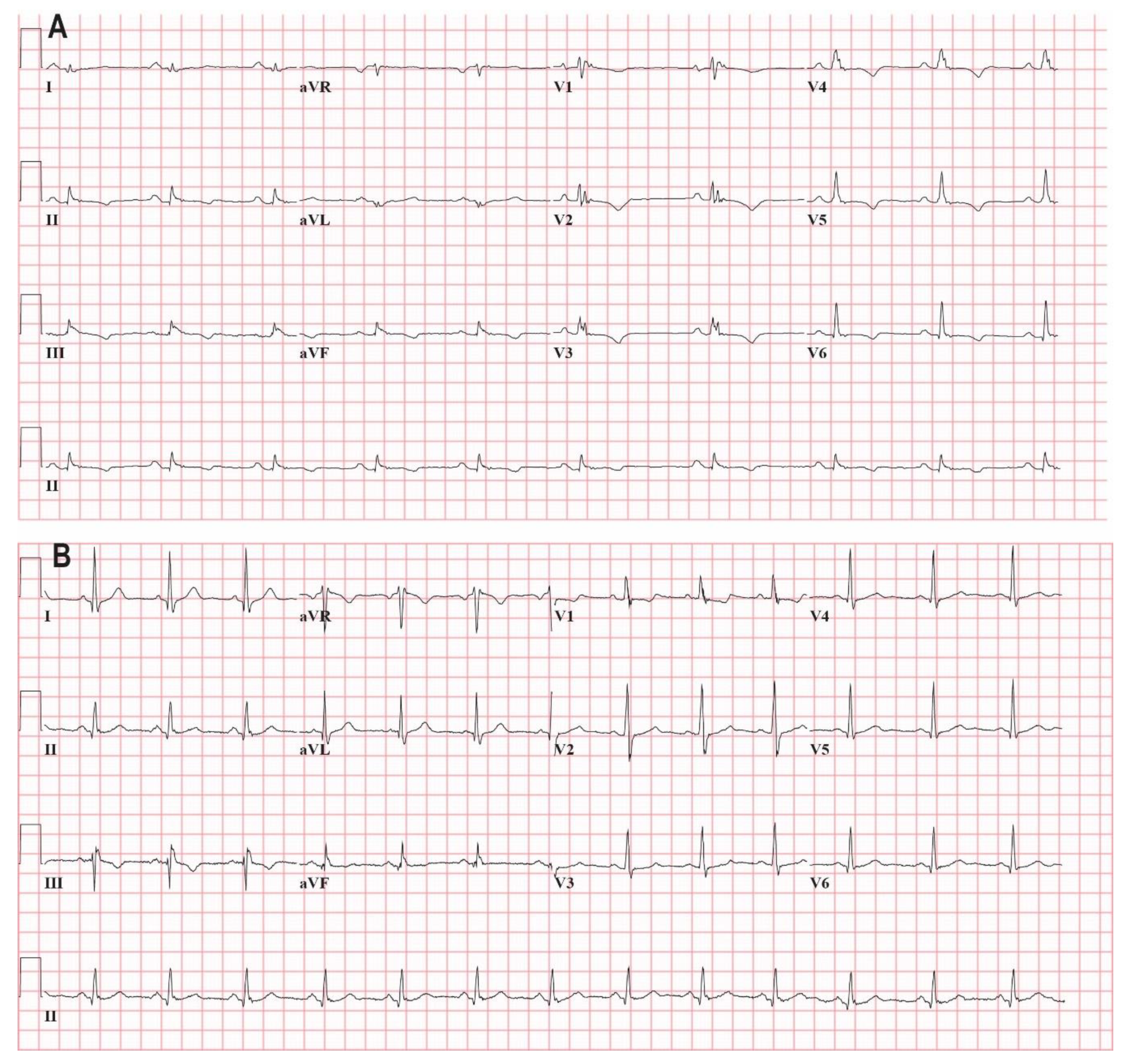
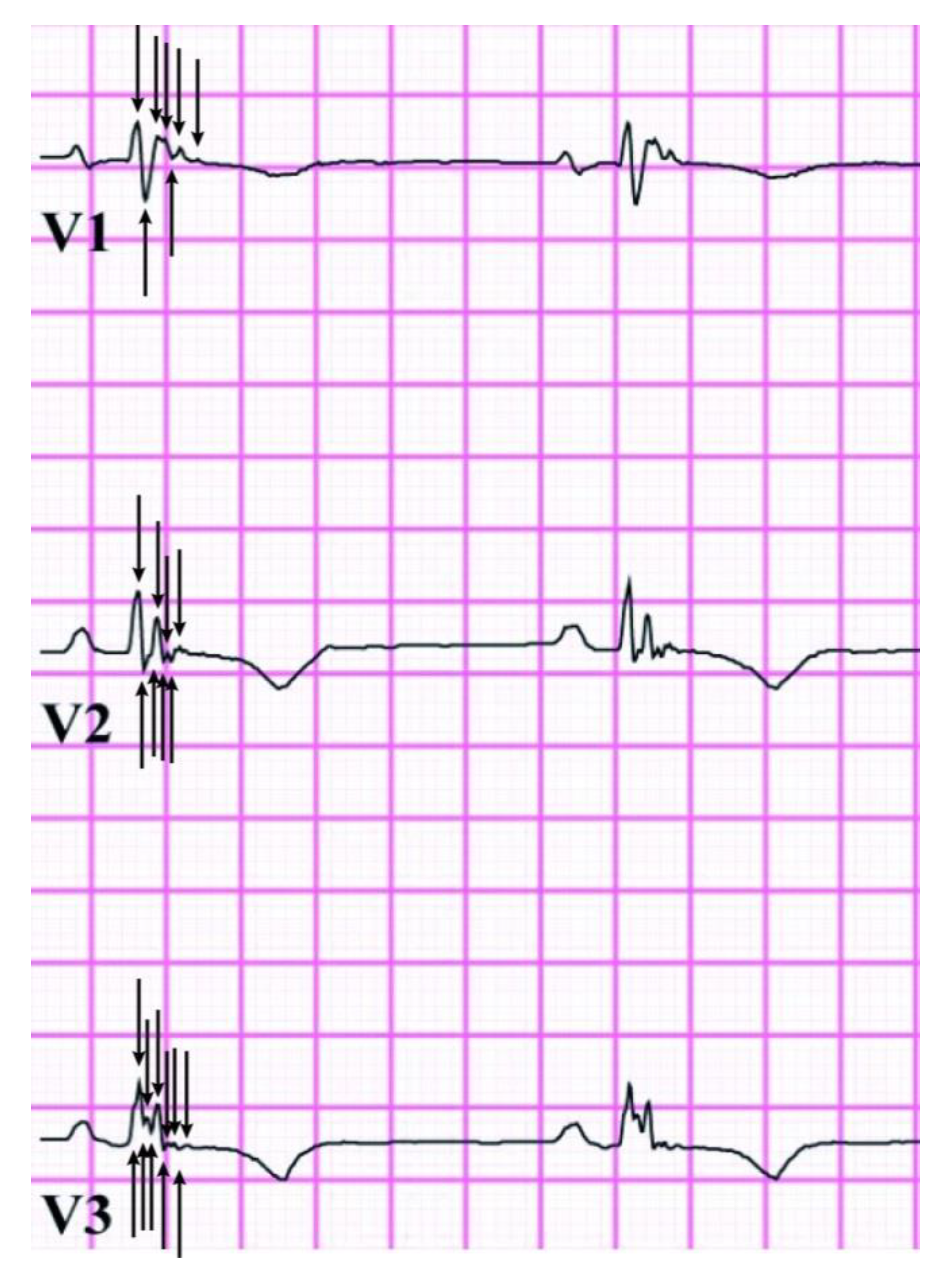
2.3. Total Q-fQRS
2.4. Follow-up
2.5. Statistical Methods
3. Results
3.1. Study Population
3.2. Feasibility and Reproducibility
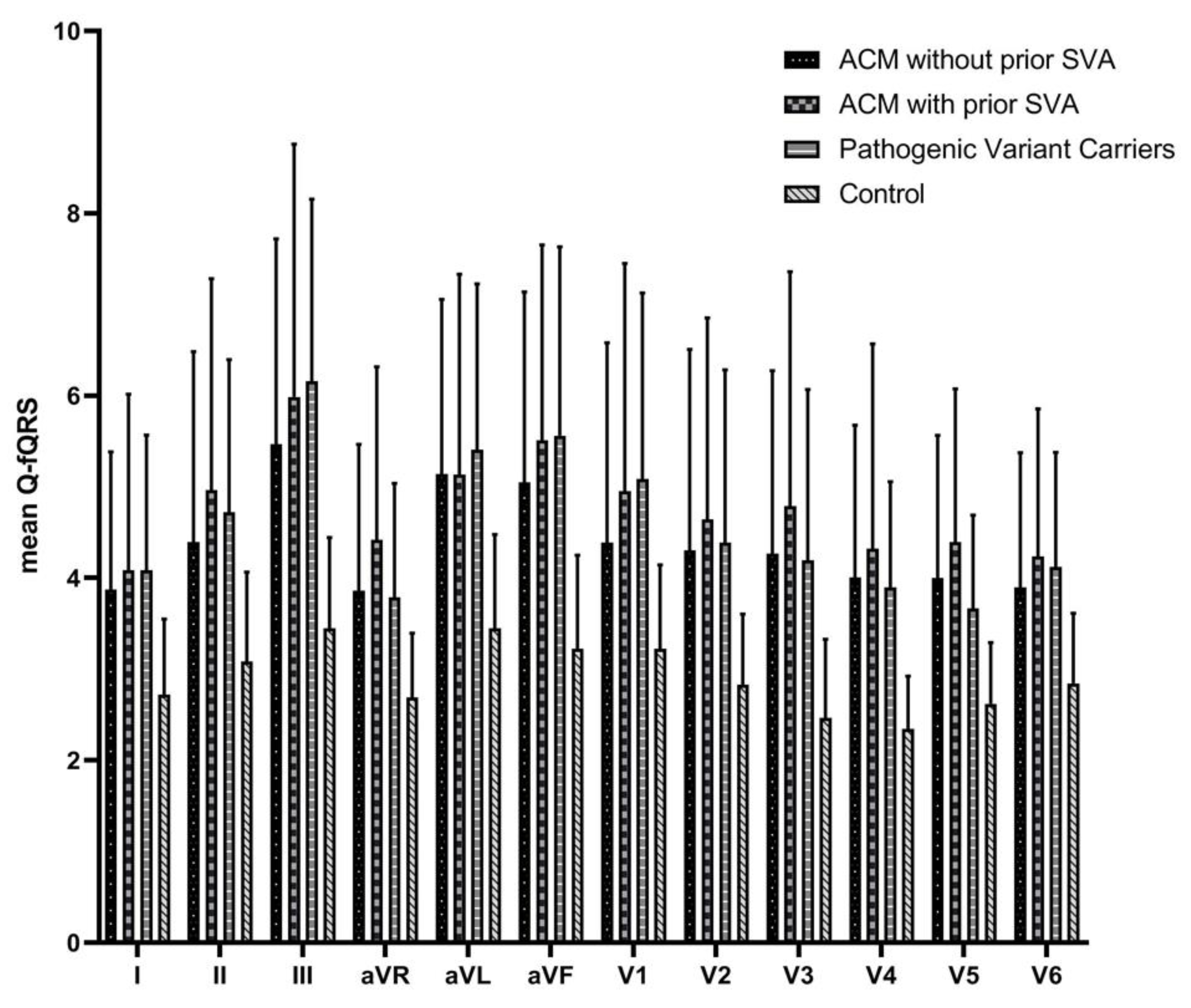
3.3. Relation of Q-fQRS with Disease Status
3.4. Relation of Q-fQRS with Outcome
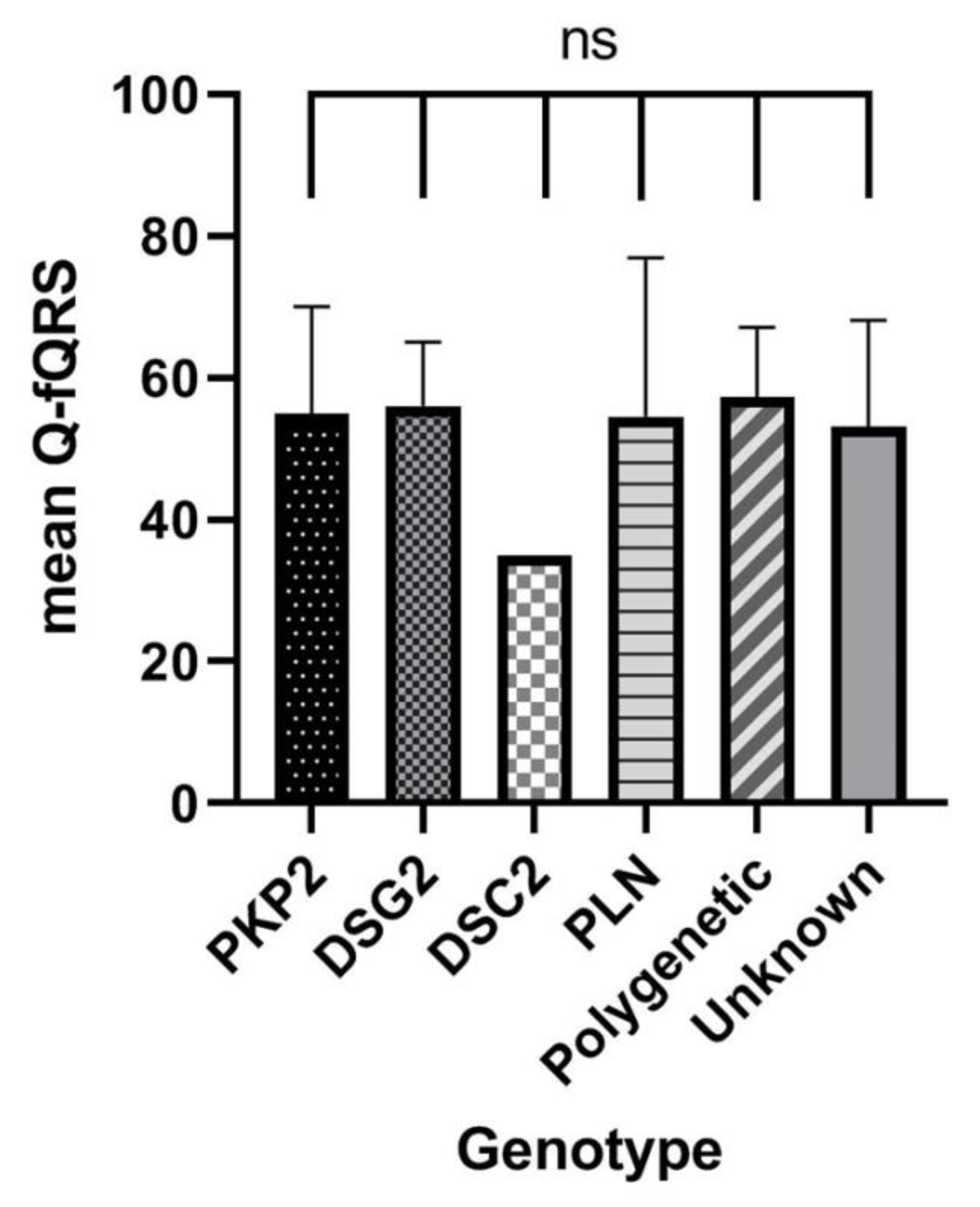
3.5. Relation of Q-fQRS with Genotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017, 376, 1489–1490. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.P.J.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation 2010, 121, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Tschabrunn, C.M.; Haqqani, H.M.; Santangeli, P.; Zado, E.S.; Marchlinski, F.E. 12-Lead Electrocardiogram to Localize Region of Abnormal Electroanatomic Substrate in Arrhythmogenic Right Ventricular Cardiomyopathy. JACC Clin. Electrophysiol. 2017, 3, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; El Masry, H. Fragmented QRS and other depolarization abnormalities as a predictor of mortality and sudden cardiac death. Curr. Opin. Cardiol. 2010, 25, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Khan, B.; Jacob, S.; Kumar, A.; Mahenthiran, P. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006, 113, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- De Bakker, J.M.; van Capelle, F.J.; Janse, M.J.; Tasseron, S.; Vermeulen, J.T.; de Jonge, N.; Lahpor, J.R. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation 1993, 88, 915–926. [Google Scholar] [CrossRef]
- De Bakker, J.M.; van Capelle, F.J.; Janse, M.J.; Tasseron, S.; Vermeulen, J.T.; de Jonge, N.; Lahpor, J.R. Fractionated electrograms in dilated cardiomyopathy: Origin and relation to abnormal conduction. J. Am. Coll. Cardiol. 1996, 27, 1071–1078. [Google Scholar] [CrossRef]
- Canpolat, U.; Kabakçi, G.; Aytemir, K.; Dural, M.; Sahiner, L.; Yorgun, H.; Summan, H.; Kaya, E.B.; Tokgözoğlu, L.; Oto, A. Fragmented QRS Complex Predicts the Arrhythmic Events in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia. J. Cardiovasc. Electrophysiol. 2013, 24, 1260–1266. [Google Scholar] [CrossRef]
- Peters, S.; Trummel, M.; Koehler, B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy. Heart Rhythm 2008, 5, 1417–1421. [Google Scholar] [CrossRef]
- Malik, M. Electrocardiographic smoke signals of fragmented QRS complex. J. Cardiovasc. Electrophysiol. 2013, 24, 1267–1270. [Google Scholar] [CrossRef]
- Bosman, L.P.; Sammani, A.; James, C.A.; Cadrin-Tourigny, J.; Calkins, H.; van Tintelen, J.P.; Hauer, R.N.W.; Hauer, F.W.; te Riele, A.J.S.M. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: A systematic review and meta-analysis. Heart Rhythm 2018, 15, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Haukilahti, M.A.; Eranti, A.; Kentta, T.; Huikuri, H.V. QRS Fragmentation Patterns Representing Myocardial Scar Need to Be Separated from Benign Normal Variants: Hypotheses and Proposal for Morphology based Classification. Front. Physiol. 2016, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Bosman, L.P.; Verstraelen, T.E.; van Lint, F.H.M.; Cox, M.G.P.J.; Groeneweg, J.A.; Mast, T.P.; van der Zwaag, P.A.; Volders, P.G.A.; Evertz, R.; Wong, L.; et al. The Netherlands Arrhythmogenic Cardiomyopathy Registry: Design and status update. Neth. Heart J. 2019, 27, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Prakken, N.H.; Velthuis, B.K.; Teske, A.J.; Mosterd, A.; Mail, W.P.; Cremar, M.J. Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 17, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Vaughan Williams, E.M. A classification of antiarrhythmic actions reassessed after a decade of new drugs. J. Clin. Pharmacol. 1984, 24, 129–147. [Google Scholar] [CrossRef]
- Cox, M.G.; Nelen, M.R.; Wilde, A.A.W.; Wiesfeld, A.C.; Smagt, J.J.; Loh, P.; Cramer, M.J.; Doevendans, P.A.; Tintelen, J.P.V.; de Bakker, J.M.P.; et al. Activation delay and VT parameters in arrhythmogenic right ventricular dysplasia/cardiomyopathy: Toward improvement of diagnostic ECG criteria. J. Cardiovasc. Electrophysiol. 2008, 19, 775–781. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Rubin, D.B.; Schenker, N. Multiple imputation in health-care databases: An overview and some applications. Stat. Med. 1991, 10, 585–598. [Google Scholar] [CrossRef]
- Van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 2007, 16, 219–242. [Google Scholar] [CrossRef]
- Das, M.; Suszko, A.M.; Nayyar, S.; Viswanathan, K.; Spears, D.A.; Tomlinson, G.; Pinter, A.; Crystal, E.; Dalvi, R.; Krishnan, S.; et al. Automated Quantification of Low-Amplitude Abnormal QRS Peaks from High-Resolution ECG Recordings Predicts Arrhythmic Events in Patients with Cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2017, 10, e004874. [Google Scholar] [CrossRef]
- Te Riele, A.S.; James, C.A.; Rastegar, N.; Bhonsale, A.; Murray, B.; Tichnell, C.; Judge, D.P.; Bluemke, D.A.; Zimmerman, S.L.; Kamel, I.R.; et al. Yield of serial evaluation in at-risk family members of patients with ARVD/C. J. Am. Coll. Cardiol. 2014, 64, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Mast, T.P.; Teske, A.J.; Walmsley, J.; van der Heijden, J.F.; van Es, R.; Prinzen, F.W.; Delhaas, T.; van Veen, T.A.; Loh, P.; Doevendans, P.A.; et al. Right Ventricular Imaging and Computer Simulation for Electromechanical Substrate Characterization in Arrhythmogenic Right Ventricular Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2185–2197. [Google Scholar] [CrossRef]
- Assis, F.R.; Krishnan, A.; Zhou, X.; James, C.A.; Murray, B.; Tichnell, C.; Berger, R.; Calkins, H.; Tandri, H.; Mandal, K. Cardiac sympathectomy for refractory ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2019, 16, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; Arora, R.; Buckley, U.; Shivkumar, K. Autonomic Nervous System Dysfunction: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1189–1206. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 336) | Definite ACM (n = 221) | Carriers (n = 57) | Control (n = 58) | p Value # | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years.) | 39 ± 15 | 42 ± 15 | 35 ± 16 * | 27 ± 6 | <0.001 |
| Sex (male) | 181 (54) | 119 (54) | 29 (51) | 33 (57) | 0.811 |
| Symptoms | 182 (54) | 171 (77) | 11 (19) * | 0 (0) | <0.001 |
| Cardiac syncope | 52 (16) | 52 (24) | 0 (0) | 0 (0) | <0.001 |
| Genetics | |||||
| Pathogenic variant | 226 (67) | 169 (77) | 57 (100) | NA | <0.001 |
| PKP2 | 163 (49) | 125 (57) | 38 (67) | NA | <0.001 |
| DSG2 | 9 (3) | 3 (1) | 6 (11) | NA | <0.001 |
| PLN | 49 (15) | 36 (16) | 13 (23) | NA | <0.001 |
| ECG | |||||
| PR interval (ms) | 157 ± 27 | 161 ± 28 | 145 ± 20 | 154 ± 24 | <0.001 |
| QRS duration (ms) | 96 ± 15 | 97 ± 17 | 92 ± 12 | 97 ± 10 | 0.066 |
| QTc interval (ms) | 419 ± 26 | 425 ± 28 | 413 ± 24 | 405 ± 14 | <0.001 |
| TWI V1–2 | 136 (41) | 127 (58) | 8 (14) * | 1 (2) | <0.001 |
| TWI V1–3 | 102 (30) | 102 (46) | 0 (0) | 0 (0) | <0.001 |
| TWI V4–6 | 18 (5) | 18 (8) | 0 (0) | 0 (0) | 0.007 |
| TAD (ms) | 56 ± 10 | 56 ± 16 | 52 ± 14 | 46 ± 9 | <0.001 |
| Arrhythmia | |||||
| PVC count/24 h on Holter | 851 (113–2623) | 1076 (534–3403) | 20 (2–492) | NA | <0.001 |
| NSVT | 111 (33) | 101 (46) | 10 (18) | NA | <0.001 |
| SVA at baseline | 81 (24) | 81 (37) | 0 (0) | NA | <0.001 |
| Imaging | |||||
| RVEF (%) | 48 (45–53) | 48 (41–48) | 53 (49–59) | 53 (49–56) | <0.001 |
| LVEF (%) | 59 (53–62) | 62 (52–62) | 58 (53–60) | 58 (54–63) | 0.255 |
| RV Volume (mL/m2) | 100 (90–118) | 100 (90–118) | 93 (83–107) | 102 (93–120) | 0.001 |
| Parameters | ACM with Prior SVA (n = 81) | ACM without Prior SVA (n = 140) | Pathogenic Variant Carriers (n = 57) | Control (n = 58) | p Value |
|---|---|---|---|---|---|
| Q-fQRS anterior leads (V1–4) | 19 ± 8 | 17 ± 6 | 18 ± 4 | 11 ± 2 | <0.001 |
| Q-fQRS inferior leads (II, III, aVF) | 16 ± 7 | 15 ± 6 | 16 ± 5 | 10 ± 3 | <0.001 |
| Q–fQRS lateral leads (I, aVL, V5–6) | 18 ± 6 | 17 ± 5 | 17 ± 3 | 12 ± 2 | <0.001 |
| Q-fQRS lead aVR | 4 ± 2 | 4 ± 2 | 4 ± 1 | 3 ± 1 | <0.001 |
| Total Q-fQRS | 57 ± 20 | 53 ± 16 | 55 ± 10 | 35 ± 5 | <0.001 |
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | ||
| Age | 0.98 (0.96–1.00) | 0.071 | Age | 0.96 (0.93–0.99) | 0.008 |
| Sex | 2.19 (1.08–4.44) | 0.030 | Sex | 2.01 (0.92–4.41) | 0.080 |
| Symptoms | 3.05 (1.17–7.93) | 0.022 | Symptoms | 4.91 (1.43–16.84) | 0.011 |
| PR interval | 0.99 (0.98–1.01) | 0.206 | |||
| QRS duration | 0.99 (0.97–1.02) | 0.529 | |||
| QTc interval | 1.00 (0.99–1.01) | 0.856 | |||
| RVEF | 0.96 (0.92–0.99) | 0.013 | RVEF | 0.97 (0.93–1.00) | 0.064 |
| LVEF | 0.99 (0.94–1.03) | 0.536 | |||
| Total Q-fQRS | 0.99 (0.96–1.02) | 0.335 | |||
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | ||
| Age | 0.99 (0.96–1.02) | 0.591 | Age | 0.96 (0.92–0.99) | 0.044 |
| Sex | 2.32 (1.01–5.30) | 0.046 | Sex | 3.71 (1.25–11.03) | 0.018 |
| Symptoms | 3.03 (1.03–8.93) | 0.044 | Symptoms | 4.93 (1.05–23.05) | 0.042 |
| PR interval | 0.99 (0.98–1.01) | 0.476 | |||
| QRS duration | 0.98 (0.95–1.01) | 0.312 | |||
| QTc interval | 1.00 (0.99–1.01) | 0.897 | |||
| RVEF | 0.93 (0.89–0.97) | 0.002 | RVEF | 0.93 (0.88–0.98) | 0.010 |
| LVEF | 0.98 (0.93–1.03) | 0.372 | |||
| Total Q-fQRS | 0.99 (0.96–1.03) | 0.693 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roudijk, R.W.; Bosman, L.P.; van der Heijden, J.F.; de Bakker, J.M.T.; Hauer, R.N.W.; van Tintelen, J.P.; Asselbergs, F.W.; te Riele, A.S.J.M.; Loh, P. Quantitative Approach to Fragmented QRS in Arrhythmogenic Cardiomyopathy: From Disease towards Asymptomatic Carriers of Pathogenic Variants. J. Clin. Med. 2020, 9, 545. https://doi.org/10.3390/jcm9020545
Roudijk RW, Bosman LP, van der Heijden JF, de Bakker JMT, Hauer RNW, van Tintelen JP, Asselbergs FW, te Riele ASJM, Loh P. Quantitative Approach to Fragmented QRS in Arrhythmogenic Cardiomyopathy: From Disease towards Asymptomatic Carriers of Pathogenic Variants. Journal of Clinical Medicine. 2020; 9(2):545. https://doi.org/10.3390/jcm9020545
Chicago/Turabian StyleRoudijk, Rob W., Laurens P. Bosman, Jeroen F. van der Heijden, Jacques M. T. de Bakker, Richard N. W. Hauer, J. Peter van Tintelen, Folkert W. Asselbergs, Anneline S. J. M. te Riele, and Peter Loh. 2020. "Quantitative Approach to Fragmented QRS in Arrhythmogenic Cardiomyopathy: From Disease towards Asymptomatic Carriers of Pathogenic Variants" Journal of Clinical Medicine 9, no. 2: 545. https://doi.org/10.3390/jcm9020545
APA StyleRoudijk, R. W., Bosman, L. P., van der Heijden, J. F., de Bakker, J. M. T., Hauer, R. N. W., van Tintelen, J. P., Asselbergs, F. W., te Riele, A. S. J. M., & Loh, P. (2020). Quantitative Approach to Fragmented QRS in Arrhythmogenic Cardiomyopathy: From Disease towards Asymptomatic Carriers of Pathogenic Variants. Journal of Clinical Medicine, 9(2), 545. https://doi.org/10.3390/jcm9020545






