Adenosine and the Cardiovascular System: The Good and the Bad
Abstract
1. Introduction
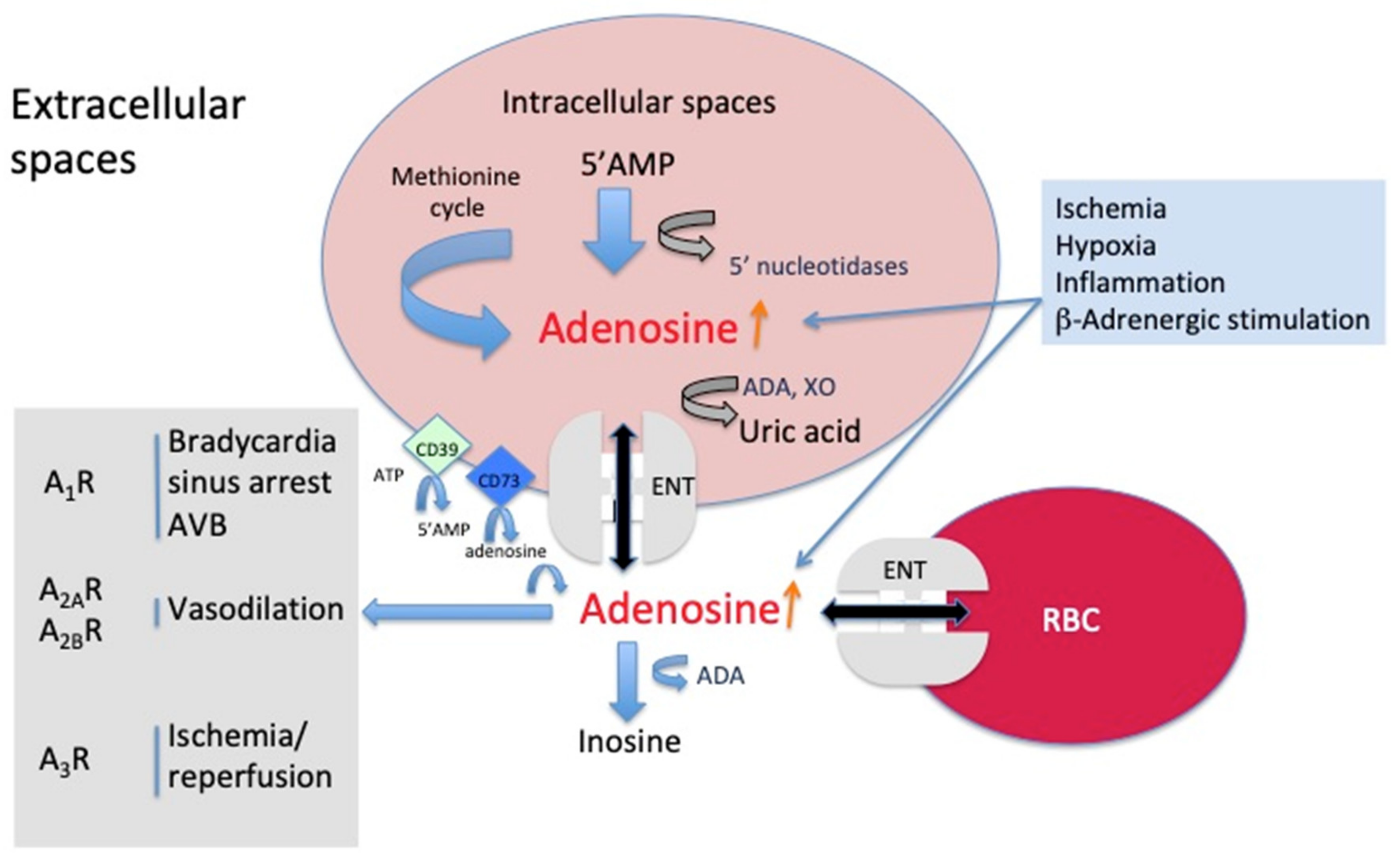
2. Source and Mechanism of Action of Adenosine
3. Adenosine Receptors
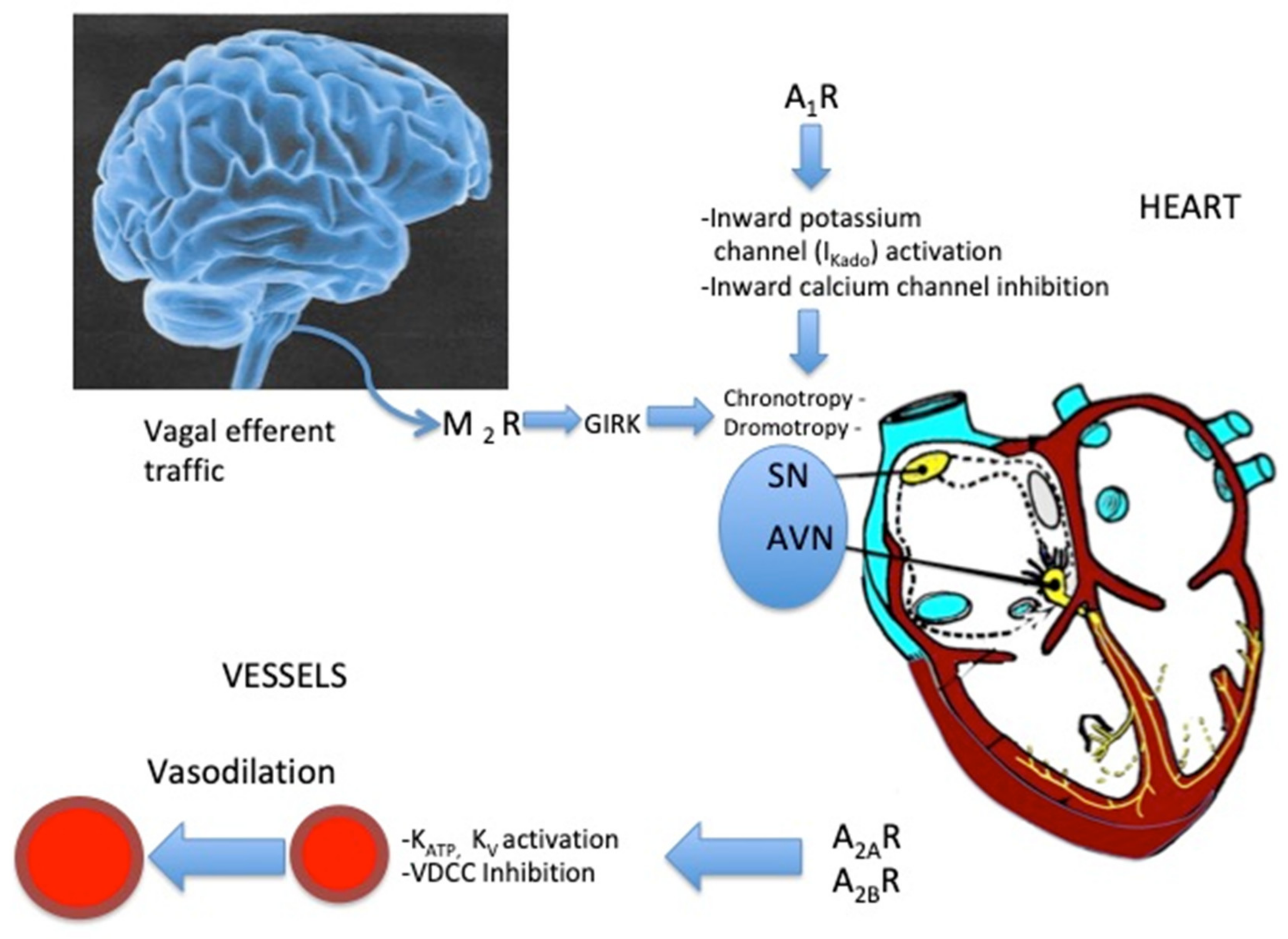
4. Effects of Adenosine on Vessels
5. Effects of Adenosine on the Sinus Node and Atrio–Ventricular Junction (Figure 2)
6. Adenosine and Ventricular Myocytes
7. Clinical Aspects
7.1. Adenosine and Atrial Fibrillation
7.2. Effects of Exogenous Adenosine
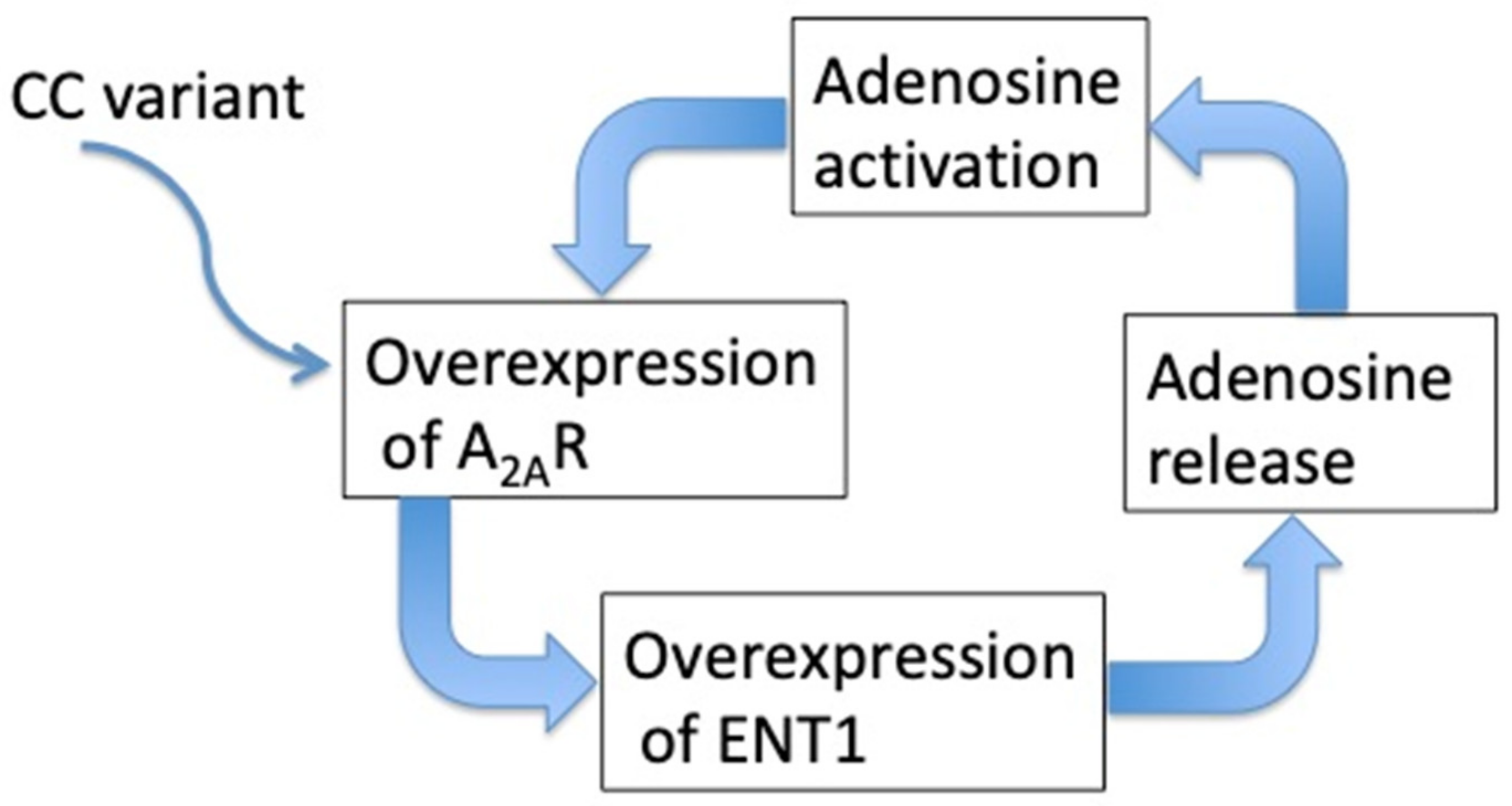
7.3. Adenosine and Reflex (Neurohumoral) Syncope
7.4. Adenosine and Hypoxia
7.4.1. Syncope in Hypoxic Conditions
7.4.2. Obstructive Sleep Apnea Syndrome
7.4.3. Altitude Hypoxia
7.5. Myocardial Ischemia Reperfusion Protection
7.6. Adenosine and Vascular Injury and Repair
7.7. Adenosine and Systemic Hypertension
7.8. Adenosine and Pulmonary Hypertension
7.9. Adenosine and Heart Failure
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Eltzschig, H.K.; Faigle, M.; Knapp, S.; Karhausen, J.; Ibla, J.; Rosenberger, P.; Odegard, K.C.; Laussen, P.C.; Thompson, L.F.; Colgan, S.P. Endothelial catabolism of extracellular adenosine during hypoxia: The role of surface adenosine deaminase and CD26. Blood 2006, 108, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Grenz, A.; Homann, D.; Eltzschig, H.K. Extracellular Adenosine: A Safety Signal That Dampens Hypoxia-Induced Inflammation During Ischemia. Antioxidants Redox Signal. 2011, 15, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Le, G.; Essackjee, H.; Ballard, H. Intracellular adenosine formation and release by freshly-isolated vascular endothelial cells from rat skeletal muscle: Effects of hypoxia and/or acidosis. Biochem. Biophys. Res. Commun. 2014, 450, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef]
- Sumi, Y.; Woehrle, T.; Chen, Y.; Yao, Y.; Li, A.; Junger, W.G. Adrenergic receptor activation involves ATP release and feedback through purinergic receptors. Am. J. Physiol. Physiol. 2010, 299, C1118–C1126. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Ijzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Onyedibe, K.I.; Wang, M.; Sintim, H.O. ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors-A STING in the Tale of ENPP1. Molecules 2019, 24, 4192. [Google Scholar] [CrossRef]
- Plagemann, P.G.W.; Wohlhueter, R.M.; Kraupp, M. Adenosine uptake, transport, and metabolism in human erythrocytes. J. Cell. Physiol. 1985, 125, 330–336. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Who Is Who in Adenosine Transport. Front. Pharmacol. 2018, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Riegel, A.-K.; Eltzschig, H.K. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 2014, 124, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Shryock, J.C.; Belardinelli, L. Adenosine and Adenosine Receptors in the Cardiovascular System: Biochemistry, Physiology, and Pharmacology. Am. J. Cardiol. 1997, 79, 2–10. [Google Scholar] [CrossRef]
- Headrick, J.P.; Ashton, K.J.; Rose’Meyer, R.B.; Peart, J.N. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef]
- Musser, B.; E Morgan, M.; Leid, M.; Murray, T.F.; Linden, J.; E Vestal, R. Species comparison of adenosine and beta-adrenoceptors in mammalian atrial and ventricular myocardium. Eur. J. Pharmacol. 1993, 246, 105–111. [Google Scholar] [CrossRef]
- Hussain, T.; Mustafa, S.J. Binding of A1Adenosine Receptor Ligand [3H]8-Cyclopentyl-1,3-Dipropylxanthine in Coronary Smooth Muscle. Circ. Res. 1995, 77, 194–198. [Google Scholar] [CrossRef]
- Iwamoto, T.; Umemura, S.; Toya, Y.; Uchibori, T.; Kogi, K.; Takagi, N.; Ishii, M. Identification of Adenosine A2 Receptor-cAMP System in Human Aortic Endothelial Cells. Biochem. Biophys. Res. Commun. 1994, 199, 905–910. [Google Scholar] [CrossRef]
- Marala, R.B.; Mustafa, S.J. Immunological characterization of adenosine A2A receptors in human and porcine cardiovascular tissues. J. Pharmacol. Exp. Ther. 1998, 286, 1051–1057. [Google Scholar]
- Monahan, T.S.; Sawmiller, D.R.; Fenton, R.A.; Dobson, J.G. Adenosine A(2a)-receptor activation increases contractility in isolated perfused hearts. Am. J. Physiol. Circ. Physiol. 2000, 279, H1472–H1481. [Google Scholar] [CrossRef]
- Dobson, J.G., Jr.; Fenton, R.A. Adenosine A2 receptor function in rat ventricularmyocytes. Cardiovasc. Res. 1997, 34, 337–347. [Google Scholar] [CrossRef]
- Morrison, R.R.; Talukder, M.A.H.; Ledent, C.; Mustafa, S.J. Cardiac effects of adenosine in A2A receptor knockout hearts: Uncovering A2B receptors. Am. J. Physiol. Circ. Physiol. 2002, 282, H437–H444. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.K.; Park, W.S.; Ko, J.-H.; Han, J.; Kim, N.; E Earm, Y. Protein kinase A-dependent activation of inward rectifier potassium channels by adenosine in rabbit coronary smooth muscle cells. Biochem. Biophys. Res. Commun. 2005, 337, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Francis, C.E.; Ravid, K. An A3-Subtype Adenosine Receptor Is Highly Expressed in Rat Vascular Smooth Muscle Cells: Its Role in Attenuating Adenosine-Induced Increase in cAMP. Microvasc. Res. 1997, 54, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Makaritsis, K.; E Francis, C.; Gavras, H.; Ravid, K. A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: Studies in knock-out mice. Biochim. Biophys. Acta (BBA) Bioenerg. 2000, 1500, 280–290. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef]
- Sanjani, M.S.; Teng, B.; Krahn, T.; Tilley, S.; Ledent, C.; Mustafa, J.S. Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double-knockout mice. Am. J. Physiol. Circ. Physiol. 2011, 301, H2322–H2333. [Google Scholar] [CrossRef]
- Ledent, C.; Vaugeois, J.-M.; Schiffmann, S.N.; Pedrazzini, T.; El Yacoubi, M.; Vanderhaeghen, J.-J.; Costentin, J.; Heath, J.; Vassart, G.; Parmentier, M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 1997, 388, 674–678. [Google Scholar] [CrossRef]
- Wolska, N.; Rozalski, M. Blood Platelet Adenosine Receptors as Potential Targets for Anti-Platelet Therapy. Int. J. Mol. Sci. 2019, 20, 5475. [Google Scholar] [CrossRef]
- Fenouillet, E.; Mottola, G.; Kipson, N.; Paganelli, F.; Guieu, R.; Ruf, J. Adenosine Receptor Profiling Reveals an Association between the Presence of Spare Receptors and Cardiovascular Disorders. Int. J. Mol. Sci. 2019, 20, 5964. [Google Scholar] [CrossRef]
- Shryock, J.C.; Snowdy, S.; Baraldi, P.G.; Cacciari, B.; Spalluto, G.; Monopoli, A.; Ongini, E.; Baker, S.P.; Belardinelli, L. A2A-adenosine receptor reserve for coronary vasodilation. Circulation 1998, 98, 711–718. [Google Scholar] [CrossRef]
- Berne, R.M. Cardiac nucleotides in hypoxia: Possible role in regulation ofcoronary blood flow. Am. J. Physiol. 1963, 204, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Mubagwa, K.; Mullane, K.; Flameng, W. Role of adenosine in the heart and circulation. Cardiovasc. Res. 1996, 32, 797–813. [Google Scholar] [CrossRef]
- Ponnoth, D.S.; Sanjani, M.S.; Ledent, C.; Roush, K.; Krahn, T.; Mustafa, S.J. Absence of adenosine-mediated aortic relaxation in A(2A) adenosine receptor knockout mice. Am. J. Physiol. Circ. Physiol. 2009, 297, H1655–H1660. [Google Scholar] [CrossRef] [PubMed]
- Kusano, Y.; Echeverry, G.; Miękisiak, G.; Kulik, T.B.; Aronhime, S.N.; Chen, J.F.; Winn, H.R. Role of Adenosine A2 Receptors in Regulation of Cerebral Blood Flow during Induced Hypotension. Br. J. Pharmacol. 2009, 30, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Arsyad, A.; Dobson, G.P. Adenosine relaxation in isolated rat aortic rings and possible roles of smooth muscle Kv channels, KATP channels and A2a receptors. BMC Pharmacol. Toxicol. 2016, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Kleppisch, T.; Nelson, M.T. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1995, 92, 12441–12445. [Google Scholar] [CrossRef]
- Berwick, Z.C.; A Payne, G.; Lynch, B.; Dick, G.; Sturek, M.; Tune, J.D. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: Role of K(V) and K(ATP) channels. Microcirculation 2010, 17, 600–607. [Google Scholar] [CrossRef]
- Pelleg, A.; Hurt, C.; Miyagawa, A.; Michelson, E.L.; Dreifus, L.S. Differential sensitivity of cardiac pacemakers to exogenous adenosine in vivo. Am. J. Physiol. Circ. Physiol. 1990, 258, H1815–H1822. [Google Scholar] [CrossRef]
- Mustafa, S.J.; Morrison, R.R.; Teng, B.; Pelleg, A. Adenosine Receptors and the Heart: Role in Regulation of Coronary Blood Flow and Cardiac Electrophysiology. Handb. Exp. Pharmacol. 2009, 193, 161–188. [Google Scholar] [CrossRef]
- Belardinelli, L.; Giles, W.R.; West, A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J. Physiol. 1988, 405, 615–633. [Google Scholar] [CrossRef]
- DiFrancesco, D.; Borer, J.S. The funny current: Cellular basis for the control of heart rate. Drugs 2007, 67, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, L. Modulation of atrioventricular transmission by adenosine. Prog. Clin. Boil. Res. 1987, 230, 109–118. [Google Scholar]
- Pelleg, A.; Hurt, C.M.; Hewlett, E.L. ATP shortens atrial action potential duration in the dog: Role of adenosine, the vagus nerve, and G protein. Can. J. Physiol. Pharmacol. 1996, 74, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, N.S.; Headrick, J.P.; Matherne, G.P. Myocardial function in the working mouse heart overexpressing cardiac A1 adenosine receptors. J. Mol. Cell. Cardiol. 1998, 30, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Boknik, P.; Begrow, F.; Hanske, G.; Justus, I.; Mat’Us, M.; Reinke, U.; Matherne, G.P.; Schmitz, W. Altered signal transduction in cardiac ventricle overexpressing A(1)-adenosine receptors. Cardiovasc. Res. 2003, 60, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Hove-Madsen, L.; Prat-Vidal, C.; Llach, A.; Ciruela, F.; Casadó, V.; Lluís, C.; Bayés-Genís, A.; Cinca, J.; Franco, R. Adenosine A2A receptors are expressed in human atrial myocytes and modulate spontaneous sarcoplasmic reticulum calcium release. Cardiovasc. Res. 2006, 72, 292–302. [Google Scholar] [CrossRef]
- Belardinelli, L.; Isenberg, G. Actions of adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ. Res. 1983, 53, 287–297. [Google Scholar] [CrossRef]
- Song, Y.; Thedford, S.; Lerman, B.B.; Belardinelli, L. Adenosine-sensitive afterdepolarizations and triggered activity in guinea pig ventricular myocytes. Circ. Res. 1992, 70, 743–753. [Google Scholar] [CrossRef]
- Xu, J.; Hurt, C.M.; Pelleg, A. Digoxin-induced ventricular arrhythmias in the guinea pig heart in vivo: Evidence for a role of endogenous catecholamines in the genesis of delayed. Heart Vessel. 1995, 10, 119–127. [Google Scholar] [CrossRef]
- Song, Y.; Shryock, J.C.; Knot, H.J.; Belardinelli, L. Selective attenuation by adenosine of arrhythmogenic action of isoproterenol on ventricular myocytes. Am J Physiol. Heart. Circ. Physiol. 2001, 280, H2789–H2795. [Google Scholar] [CrossRef]
- Zulkifly, H.; Lip, G.Y.H.; A Lane, D. Epidemiology of atrial fibrillation. Int. J. Clin. Pr. 2018, 72, e13070. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.A.; Allessie, M. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Carnagarin, R.; Kiuchi, M.G.; Ho, J.K.; Matthews, V.B.; Schlaich, M.P. Sympathetic Nervous System Activation and Its Modulation: Role in Atrial Fibrillation. Front. Mol. Neurosci. 2019, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Zipes, U.P. Role of the Autonomic Nervous System in Modulating Cardiac Arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-A.; Hsieh, M.H.; Tai, C.-T.; Tsai, C.F.; Prakash, V.S.; Yu, W.C.; Hsu, T.L.; A Ding, Y.; Chang, M.S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999, 100, 1879–1886. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Metayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Gencel, L.; Pradeau, V.; Garrigues, S.; Chouairi, S.; Hocini, M.; Métayer, P.; Roudaut, R.; et al. Right and Left Atrial Radiofrequency Catheter Therapy of Paroxysmal Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 1996, 7, 1132–1144. [Google Scholar] [CrossRef]
- Lustgarten, D.L.; Keane, D.; Ruskin, J. Cryothermal ablation: Mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Prog. Cardiovasc. Dis. 1999, 41, 481–498. [Google Scholar] [CrossRef]
- Ücer, E.; Fredersdorf, S.; Seegers, J.; Poschenrieder, F.; Hauck, C.; Maier, L.; Jungbauer, C. High Predictive Value of Adenosine Provocation in Predicting Atrial Fibrillation Recurrence After Pulmonary Vein Isolation With Visually Guided Laser Balloon Compared With Radiofrequency Ablation. Circ. J. 2020, 84, 404–410. [Google Scholar] [CrossRef]
- Pelleg, A.; Pennock, R.S.; Kutalek, S.P. Proarrhythmic effects of adenosine: One decade of clinical data. Am. J. Ther. 2002, 9, 141–147. [Google Scholar] [CrossRef]
- Ip, J.E.; Cheung, J.W.; Chung, J.H.; Liu, C.F.; Thomas, G.; Markowitz, S.M.; Lerman, B.B. Adenosine-Induced Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2013, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Strickberger, S.A.; Man, K.C.; Daoud, E.G.; Goyal, R.; Brinkman, K.; Knight, B.P.; Weiss, R.; Bahu, M.; Morady, F. Adenosine-induced atrial arrhythmia: A prospective analysis. Ann. Intern. Med. 1997, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Isa-Param, R.; Pérez-Castellano, N.; Villacastín, J.; Moreno, J.; Salinas, J.; Alonso, R.; Ruiz, E.; Doblado, M.; Morales, R.; Macaya, C. [Inducibility of atrial arrhythmias after adenosine and isoproterenol infusion in patients referred for atrial fibrillation ablation]. Revista Española de Cardiología 2006, 59, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Maille, B.; Marlinge, M.; Vairo, D.; Mottola, G.; Koutbi, L.; Deharo, P.; Gastaldi, M.; Gaudry, M.; Guiol, C.; Bottone, S.; et al. Adenosine plasma level in patients with paroxysmal or persistent atrial fibrillation and normal heart during ablation procedure and/or cardioversion. Purinergic Signal. 2018, 15, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-D.; Deng, H.; Guo, P.; Liu, F.-Z.; Chen, R.-Y.; Fang, X.-H.; Zhan, X.-Z.; Liao, H.-T.; Huang, W.-X.; Liu, Y.; et al. High prevalence of hyperuricaemia and its impact on non-valvular atrial fibrillation: The cross-sectional Guangzhou (China) Heart Study. BMJ Open 2019, 9, e028007. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Guo, P.; Zheng, M.; Huang, J.; Xue, Y.; Zhan, X.; Wang, F.; Liu, Y.; Fang, X.; Liao, H.; et al. Epidemiological Characteristics of Atrial Fibrillation in Southern China: Results from the Guangzhou Heart Study. Sci. Rep. 2018, 8, 17829. [Google Scholar] [CrossRef]
- Kawasoe, S.; Kubozono, T.; Yoshifuku, S.; Ojima, S.; Miyata, M.; Miyahara, H.; Maenohara, S.; Ohishi, M. Uric Acid Level and New-Onset Atrial Fibrillation in the Japanese General Population―Longitudinal Study. Circ. J. 2018, 83, 156–163. [Google Scholar] [CrossRef]
- Llach, A.; E Molina, C.; Prat-Vidal, C.; Fernandes, J.; Casadó, V.; Ciruela, F.; Lluís, C.; Franco, R.; Cinca, J.; Hove-Madsen, L. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur. Heart J. 2010, 32, 721–729. [Google Scholar] [CrossRef]
- Li, N.; Hansen, B.J.; Fedorov, V.V. Response by Li et al to Letter Regarding Article, “Adenosine-Induced Atrial Fibrillation: Localized Reentrant Drivers in Lateral Right Atria Due to Heterogeneous Expression of Adenosine A1 Receptors and GIRK4 Subunits in the Human Heart”. Circulation 2016, 134, e648–e649. [Google Scholar] [CrossRef]
- Saadjian, A.Y.; Lévy, S.; Franceschi, F.; Zouher, I.; Paganelli, F.; Guieu, R.P. Role of Endogenous Adenosine as a Modulator of Syncope Induced During Tilt Testing. Circulation 2002, 106, 569–574. [Google Scholar] [CrossRef]
- Deharo, J.-C.; Mechulan, A.; Giorgi, R.; Franceschi, F.; Prevot, S.; Peyrouse, E.; Condo, J.; By, Y.; Ruf, J.; Brignole, M.; et al. Adenosine plasma level and A2A adenosine receptor expression: Correlation with laboratory tests in patients with neurally mediated syncope. Heart 2012, 98, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Saadjian, A.Y.; Gerolami, V.; Giorgi, R.; Mercier, L.; Berge-Lefranc, J.-L.; Paganelli, F.; Ibrahim, Z.; By, Y.; Guéant, J.L.; Lévy, S.; et al. Head-up tilt induced syncope and adenosine A2A receptor gene polymorphism. Eur. Heart J. 2009, 30, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Guieu, R.; Deharo, J.-C.; Ruf, J.; Mottola, G.; Kipson, N.; Bruzzese, L.; Gérolami, V.; Franceschi, F.; Ungar, A.; Tomaino, M.; et al. Adenosine and Clinical Forms of Neurally-Mediated Syncope. J. Am. Coll. Cardiol. 2015, 66, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Guieu, R.; Tomaino, M.; Iori, M.; Ungar, A.; Bertolone, C.; Unterhuber, M.; Bottoni, N.; Tesi, F.; Deharo, J.C. Mechanism of syncope without prodromes with normal heart and normal electrocardiogram. Heart Rhythm. 2017, 14, 234–239. [Google Scholar] [CrossRef]
- Brignole, M.; Solari, D.; Iori, M.; Bottoni, N.; Guieu, R.; Deharo, J.C.; Information, P.E.K.F.C. Efficacy of theophylline in patients affected by low adenosine syncope. Heart Rhythm. 2016, 13, 1151–1154. [Google Scholar] [CrossRef]
- Joulia, F.; Coulange, M.; Desplantes, A.; Barberon, B.; Kipson, N.; Gérolami, V.; Jammes, Y.; Kerbaul, F.; Nee, L.; Fromonot, J.; et al. Purinergic profile of fainting divers is different from patients with vasovagal syncope. Int. J. Cardiol. 2014, 174, 741–743. [Google Scholar] [CrossRef]
- Hira, H.S.; Samal, P.; Kaur, A.; Kapoor, S. Plasma level of hypoxanthine/xanthine as markers of oxidative stress with different stages of obstructive sleep apnea syndrome. Ann. Saudi Med. 2014, 34, 308–313. [Google Scholar] [CrossRef]
- Saito, H.; Nishimura, M.; Shibuya, E.; Makita, H.; Tsujino, I.; Miyamoto, K.; Kawakami, Y. Tissue hypoxia in sleep apnea syndrome assessed by uric acid and adenosine. Chest 2002, 122, 1686–1694. [Google Scholar] [CrossRef]
- Hira, H.S.; Shukla, A.; Kaur, A.; Kapoor, S. Serum uric acid and lactate levels among patients with obstructive sleep apnea syndrome: Which is a better marker of hypoxemia? Ann. Saudi Med. 2012, 32, 37–42. [Google Scholar] [CrossRef]
- Song, A.; Zhang, Y.; Han, L.; Yegutkin, G.; Liu, H.; Sun, K.; D’Alessandro, A.; Li, J.; Karmouty-Quintana, H.; Iriyama, T.; et al. Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nat. Commun. 2017, 8, 14108. [Google Scholar] [CrossRef]
- Tang, L.; Parker, M.; Fei, Q.; Loutzenhiser, R. Afferent arteriolar adenosine A2a receptors are coupled to KATP in in vitro perfused hydronephrotic rat kidney. Am. J. Physiol. Content 1999, 277, F926–F933. [Google Scholar] [CrossRef] [PubMed]
- Kost, C.K.; Herzer, W.A.; Rominski, B.R.; Mi, Z.; Jackson, E.K. Diuretic response to adenosine A(1) receptor blockade in normotensive and spontaneously hypertensive rats: Role of pertussis toxin-sensitive G-proteins. J. Pharmacol. Exp. Ther. 2000, 292, 752–760. [Google Scholar] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev. Esp. Cardiol. (Engl. Ed.) 2016, 69, 177. [Google Scholar] [PubMed]
- Modesti, P.A.; Vanni, S.; Morabito, M.; Modesti, A.; Marchetta, M.; Gamberi, T.; Sofi, F.; Savia, G.; Mancia, G.; Gensini, G.F.; et al. Role of Endothelin-1 in Exposure to High Altitude. Circulation 2006, 114, 1410–1416. [Google Scholar] [CrossRef]
- Man, F.H.-D.; Tu, L.; Handoko, L.; Rain, S.; Ruiter, G.; François, C.; Schalij, I.; Dorfmüller, P.; Simonneau, G.; Fadel, E.; et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 780–789. [Google Scholar] [CrossRef]
- Xu, M.; Gong, Y.; Su, M.; Dai, Z.; Dai, S.; Bao, S.; Li, N.; Zheng, R.; He, J.; Chen, J.; et al. Absence of the adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice. J. Vasc. Res. 2010, 48, 171–183. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev. Esp. Cardiol. (Engl. Ed.) 2016, 69, 1167. [Google Scholar]
- Morgan, J.M.; McCormack, D.G.; Griffiths, M.J.; Morgan, C.J.; Barnes, P.J.; Evans, T.W. Adenosine as a vasodilator in primary pulmonary hypertension. Circ. 1991, 84, 1145–1149. [Google Scholar] [CrossRef]
- Rankin, A.C.; Brooks, R.; Ruskin, J.N.; McGovern, B.A. Adenosine and the treatment of supraventricular tachycardia. Am. J. Med. 1992, 92, 655–664. [Google Scholar] [CrossRef]
- Coli, S.; Mantovani, F.; Ferro, J.; Gonzi, G.; Zardini, M.; Ardissino, D. Adenosine-induced severe bronchospasm in a patient without pulmonary disease. Am. J. Emerg. Med. 2012, 30, 2082.e3–2082.e5. [Google Scholar] [CrossRef]
- Crea, F.; El-Tamimi, H.; Vejar, M.; Kaski, J.C.; Davies, G.; Maseri, A. Adenosine-induced chest pain in patients with silent and painful myocardial ischaemia: Another clue to the importance of generalized defective perception of painful stimuli as a cause of silent ischaemia. Eur. Heart J. 1988, 9, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.A.; Hwang, C.; Hunter, D.; Chen, P.-S.; Smith, D.; Ariani, M.; Johnston, W.D.; Allen, B.J.; Chun, J.G.; Gold, C.R. Life-threatening alterations in heart rate after the use of adenosine in atrial flutter. Am. Heart J. 1995, 130, 564–571. [Google Scholar] [CrossRef]
- Webster, D.P.; Daar, A.A. Prolonged bradyasystole and seizures following intravenous adenosine for supraventricular tachycardia. Am. J. Emerg. Med. 1993, 11, 192–194. [Google Scholar] [CrossRef]
- Christopher, M.; Key, C.B.; E Persse, D. Refractory asystole and death following the prehospital administration of adenosine. Prehospital. Emerg. Care 2000, 4, 196–198. [Google Scholar] [CrossRef]
- Dunn, J.S.; Brost, B. Fetal bradycardia after IV adenosine for maternal PSVT. Am. J. Emerg. Med. 2000, 18, 234–235. [Google Scholar] [CrossRef]
- Tan, H.L.; Spekhorst, H.H.; Peters, R.J.; Wilde, A.A. Adenosine induced ventricular arrhythmias in the emergency room. Pacing Clin. Electrophysiol. 2001, 24, 450–455. [Google Scholar] [CrossRef]
- Mallet, M. Proarrhythmic effects of adenosine: A review of the literature. Emerg. Med. J. 2004, 21, 408–410. [Google Scholar] [CrossRef]
- Harrington, M.G.R.; Froelich, C.E.G.; Harrington, G.R.; Froelich, E.G. Adenosine-induced Torsades de Pointes. Chest 1993, 103, 1299–1301. [Google Scholar] [CrossRef]
- Viskin, S.; Rosso, R.; Rogowski, O.; Belhassen, B.; Levitas, A.; Wagshal, A.; Katz, A.; Fourey, D.; Zeltser, D.; Oliva, A.; et al. Provocation of sudden heart rate oscillation with adenosine exposes abnormal QT responses in patients with long QT syndrome: A bedside test for diagnosing long QT syndrome. Eur. Heart J. 2005, 27, 469–475. [Google Scholar] [CrossRef]
- Wesley, R.C.; Turnquest, P. Torsades de pointe after intravenous adenosine in the presence of prolonged QT syndrome. Am. Heart J. 1992, 123, 794–796. [Google Scholar] [CrossRef]
- Celiker, A.; Tokel, K.; Cil, E.; Özkutlu, S.; Özme, Ç. Adenosine Induced Torsades de Pointes in a Child with Congenital Long QT Syndrome. Pacing Clin. Electrophysiol. 1994, 17, 1814–1817. [Google Scholar] [CrossRef] [PubMed]
- Teodorovich, N.; Margolin, E.; Kogan, Y.; Paz, O.; Swissa, M. Torsades de pointes after adenosine administration. J. Electrocardiol. 2016, 49, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Deharo, J.-C.; Guieu, R. Syncope and Idiopathic (Paroxysmal) AV Block. Cardiol. Clin. 2015, 33, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Day, S.C.; Cook, E.; Funkenstein, H.; Goldman, L. Evaluation and outcome of emergency room patients with transient loss of consciousness. Am. J. Med. 1982, 73, 15–23. [Google Scholar] [CrossRef]
- Silverstein, M.D.; Singer, D.E.; Mulley, A.G.; Thibault, G.E.; Barnett, G.O. Patients with syncope admitted to medical intensive care units. JAMA 1982, 248, 1185–1189. [Google Scholar] [CrossRef]
- Del Rosso, A.; Bartoletti, A.; Brignole, M. The clinical utility and diagnostic value of the head-up tilt testing (HUT) protocol. J. Cardiovasc. Electrophysiol. 2004, 15, author reply 615–616. [Google Scholar]
- Brignole, M.; Gaggioli, G.; Menozzi, C.; Gianfranchi, L.; Bartoletti, A.; Bottoni, N.; Lolli, G.; Oddone, D.; Del Rosso, A.; Pellinghelli, G. Adenosine-induced atrioventricular block in patients with unexplained syncope: The diagnostic value of ATP testing. Circulation 1997, 96, 3921–3927. [Google Scholar] [CrossRef]
- Fromonot, J.; Chaumet, G.; Gavarry, O.; Rostain, J.-C.; Lucciano, M.; Joulia, F.; Brignole, M.; Deharo, J.-C.; Guieu, R.; Boussuges, A. Hyperoxia Improves Hemodynamic Status During Head-up Tilt Testing in Healthy Volunteers. Medicine 2016, 95, e2876. [Google Scholar] [CrossRef]
- Pinto-Duarte, A.; Coelho, J.E.; Cunha, R.A.; Ribeiro, J.; Sebastião, A.M. Adenosine A2A receptors control the extracellular levels of adenosine through modulation of nucleoside transporters activity in the rat hippocampus. J. Neurochem. 2005, 93, 595–604. [Google Scholar] [CrossRef]
- Deharo, J.-C.; Brignole, M.; Guieu, R. Adenosine hypersensitivity and atrioventricular block. Herzschrittmachertherapie und Elektrophysiologie 2018, 29, 166–170. [Google Scholar] [CrossRef]
- Cohen, F.R.; Lazareno, S.; Birdsall, N.J. The affinity of adenosine for the high- and low-affinity states of the human adenosine A1 receptor. Eur. J. Pharmacol. 1996, 309, 111–114. [Google Scholar] [CrossRef]
- Brignole, M.; Iori, M.; Solari, D.; Bottoni, N.; Rivasi, G.; Ungar, A.; Deharo, J.C.; Guieu, R. Efficacy of theophylline in patients with syncope without prodromes with normal heart and normal ECG. Int. J. Cardiol. 2019, 289, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Joulia, F.; Coulange, M.; Lemaitre, F.; Costalat, G.; Franceschi, F.; Gariboldi, V.; Nee, L.; Fromonot, J.; Bruzzese, L.; Gravier, G.; et al. Plasma adenosine release is associated with bradycardia and transient loss of consciousness during experimental breath-hold diving. Int. J. Cardiol. 2013, 168, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, J.F.; Melo, B.; Conde, S.V. Adenosine Mediates Hypercapnic Response in the Rat Carotid Body via A2A and A2B Receptors. Results Probl. Cell Differ. 2018, 107, 89–93. [Google Scholar] [CrossRef]
- American Sleep Apnea Assocication. Available online: https://www.sleepapnea.org/learn/sleep-apnea-information-clinicians/ (accessed on 5 April 2020).
- Harding, S.M. Complications and consequences of obstructive sleep apnea. Curr. Opin. Pulm. Med. 2000, 6, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Morote-Garcia, J.C.; Rosenberger, P.; Kuhlicke, J.; Eltzschig, H.K. HIF-1–dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 2008, 111, 5571–5580. [Google Scholar] [CrossRef]
- Jensen, M.L.F.; Vestergaard, M.B.; Tønnesen, P.; Larsson, H.B.W.; Jennum, P. Cerebral blood flow, oxygen metabolism, and lactate during hypoxia in patients with obstructive sleep apnea. Sleep 2018, 41. [Google Scholar] [CrossRef]
- Bjorness, T.E.; Greene, R.W. Adenosine and Sleep. Curr. Neuropharmacol. 2009, 7, 238–245. [Google Scholar] [CrossRef]
- Carley, D.W.; Radulovacki, M. Role of Peripheral Adenosine A1 Receptors in the Regulation of Sleep Apneas in Rats. Exp. Neurol. 1999, 159, 545–550. [Google Scholar] [CrossRef]
- Görlach, A. Control of adenosine transport by hypoxia. Circ. Res. 2005, 97, 1–3. [Google Scholar] [CrossRef]
- Coney, A.; Marshall, J.M. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J. Physiol. 1998, 509, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Feoktistov, I.; Ryzhov, S.; Zhong, H.; Goldstein, A.E.; Matafonov, A.; Zeng, D.; Biaggioni, I. Hypoxia Modulates Adenosine Receptors in Human Endothelial and Smooth Muscle Cells Toward an A2B Angiogenic Phenotype. Hypertension 2004, 44, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, L.; Fromonot, J.; By, Y.; Durand-Gorde, J.M.; Condo, J.; Kipson, N.; Guieu, R.; Fenouillet, E.; Ruf, J. NF-kappaB enhances hypoxia-driven T-cell immunosuppression via upregulation of adenosine A(2A) receptors. Cell Signal. 2014, 26, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Abdulla, P.; Hoffman, E.; Hamilton, K.E.; Daniels, D.; Schönfeld, C.; Löffler, M.; Reyes, G.; Duszenko, M.; Karhausen, J.; et al. HIF-1–dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 2005, 202, 1493–1505. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Wu, H.; D’Alessandro, A.; Yegutkin, G.; Song, A.; Sun, K.; Li, J.; Cheng, N.-Y.; Huang, A.; et al. Beneficial Role of Erythrocyte Adenosine A2B Receptor-Mediated AMP-Activated Protein Kinase Activation in High-Altitude Hypoxia. Circulatyion 2016, 134, 405–421. [Google Scholar] [CrossRef]
- Sun, K.; Liu, H.; Song, A.; Manalo, J.M.; D’Alessandro, A.; Hansen, K.C.; Kellems, R.E.; Eltzschig, H.K.; Blackburn, M.R.; Roach, R.; et al. Erythrocyte purinergic signaling components underlie hypoxia adaptation. J. Appl. Physiol. 2017, 123, 951–956. [Google Scholar] [CrossRef]
- Paterson, G.G.; Young, J.M.; Willson, J.A.; Graham, C.J.; Dru, R.C.; Lee, E.W.; Torpey, G.S.; Walmsley, S.R.; Chan, M.V.; Warner, T.D.; et al. Hypoxia Modulates Platelet Purinergic Signalling Pathways. Thromb. Haemost. 2019, 120, 253–261. [Google Scholar] [CrossRef]
- Laxson, D.D.; Homans, D.C.; Bache, R.J. Inhibition of adenosine-mediated coronary vasodilation exacerbates myocardial ischemia during exercise. Am. J. Physiol. Circ. Physiol. 1993, 265, H1471–H1477. [Google Scholar] [CrossRef]
- Duncker, D.J.; Stubenitsky, R.; Verdouw, P.D. Role of adenosine in the regulation of coronary blood flow in swine at rest and during treadmill exercise. Am. J. Physiol. Content 1998, 275, H1663–H1672. [Google Scholar] [CrossRef]
- Bardenheuer, H.; Schrader, J. Supply-to-demand ratio for oxygen determines formation of adenosine by the heart. Am. J. Physiol. Circ. Physiol. 1986, 250, H173–H180. [Google Scholar] [CrossRef]
- Martin, B.J.; McClanahan, T.B.; Van Wylen, D.G.; Gallagher, K.P. Effects of ischemia, preconditioning, and adenosine deaminase inhibition on interstitial adenosine levels and infarct size. Basic Res. Cardiol 1997, 92, 240–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harrison, G.; Willis, R.J.; Headrick, J.P. Extracellular adenosine levels and cellular energy metabolism in ischemically preconditioned rat heart. Cardiovasc. Res. 1998, 40, 74–87. [Google Scholar] [CrossRef]
- Toombs, C.F.; McGee, S.; Johnston, W.E.; Vinten-Johansen, J. Myocardial protective effects of adenosine. Infarct size reduction with pretreatment and continued receptor stimulation during ischemia. Circulation 1992, 86, 986–994. [Google Scholar] [CrossRef]
- Cohen, M.V.; Downey, J.M. Adenosine: Trigger and mediator of cardioprotection. Basic Res. Cardiol. 2007, 103, 203–215. [Google Scholar] [CrossRef]
- Headrick, J.P.; Lasley, R.D. Adenosine Receptors and Reperfusion Injury of the Heart. Handb. Exp. Pharmacol. 2009, 193, 189–214. [Google Scholar] [CrossRef]
- Reichelt, M.; Shanu, A.; Willems, L.; Witting, P.K.; Ellis, N.A.; Blackburn, M.; Headrick, J.P. Endogenous Adenosine Selectively Modulates Oxidant Stress via the A1 Receptor in Ischemic Hearts. Antioxid. Redox Signal. 2009, 11, 2641–2650. [Google Scholar] [CrossRef]
- Rothermel, B.A.; Hill, J. Adenosine A3 receptor and cardioprotection: Enticing, enigmatic, elusive. Circulation 2008, 118, 1691–1693. [Google Scholar] [CrossRef]
- Mubagwa, K.; Flameng, W. Adenosine, adenosine receptors and myocardial protection: An updated overview. Cardiovasc. Res. 2001, 52, 25–39. [Google Scholar] [CrossRef]
- Paganelli, F.; Saadjian, A.; Sampol, J.J.; Maixent, J.; Levy, S.; Guieu, R. Effects of percutaneous transluminal coronary angioplasty on coronary adenosineconcentrations in humans. Eur. J. Clin. Investig. 2000, 30, 105–110. [Google Scholar] [CrossRef]
- Tommasi, S.; Carluccio, E.; Bentivoglio, M.; Corea, L.; Picano, E. Low-dose dipyridamole infusion acutely increases exercise capacity in angina pectoris: A double-blind, placebo controlled crossover stress echocardiographic study. J. Am. Coll. Cardiol. 2000, 35, 83–88. [Google Scholar] [CrossRef][Green Version]
- Heidland, U.E.; Heintzen, M.P.; Michel, C.J.; Strauer, B.E. Intracoronary administration of dipyridamole prior to percutaneous transluminal coronary angioplasty provides a protective effect exceeding that of ischemic preconditioning. Coron. Artery Dis. 2000, 11, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Sirker, A.; Loke, Y.K.; Garcia-Dorado, D.; Hausenloy, D.J. Clinical benefit of adenosine as an adjunct to reperfusion in ST-elevation myocardial infarction patients: An updated meta-analysis of randomized controlled trials. Int. J. Cardiol. 2015, 202, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Skyschally, A.; Caster, P.; Iliodromitis, E.K.; Schulz, R.; Kremastinos, D.T.; Heusch, G. Ischemic postconditioning: Experimental models and protocol algorithms. Basic Res. Cardiol. 2009, 104, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Johnston-Cox, H.A.; Koupenova, M.; Ravid, K. A2 adenosine receptors and vascular pathologies. Arter. Thromb. Vasc. Boil. 2012, 32, 870–878. [Google Scholar] [CrossRef]
- Feoktistov, I.; Biaggioni, I.; Cronstein, B.N. Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb. Exp. Pharmacol. 2009, 193, 383–397. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Gadangi, P.; Longaker, M.; Sung, J.; Levine, J.; Nilsen, D.; Reibman, J.; Li, M.; Jiang, C.K.; Hirschhorn, R.; et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J. Exp. Med. 1997, 186, 1615–1620. [Google Scholar] [CrossRef]
- Dubey, R.K.; Baruscotti, I.; Stiller, R.; Fingerle, J.; Gillespie, D.G.; Mi, Z.; Leeners, B.; Imthurn, B.; Rosselli, M.; Jackson, E.K. Adenosine, Via A2B Receptors, Inhibits Human (P-SMC) Progenitor Smooth Muscle Cell Growth. Hypertension 2019, 75, 109–118. [Google Scholar] [CrossRef]
- Yang, D.; Koupenova, M.; McCrann, N.J.; Kopeikina, K.J.; Kagan, H.M.; Schreiber, B.; Ravid, K. The A2b adenosine receptor protects against vascular injury. Proc. Natl. Acad. Sci. USA 2008, 105, 792–796. [Google Scholar] [CrossRef]
- Fromonot, J.; Dignat-Georges, F.; Rossi, P.; Mottola, G.; Kipson, N.; Ruf, J.; Bonello, L.; Guieu, R.; Paganelli, F. Ticagrelor Improves Peripheral Arterial Function in Acute Coronary Syndrome Patients. J. Am. Coll. Cardiol. 2016, 67, 1967–1968. [Google Scholar] [CrossRef]
- Chen, J.-F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets--what are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef]
- Effendi, W.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Kitakaze, M.; Hori, M.; Kamada, T. Role of adenosine and its interaction with adrenoceptor activity in ischaemic and reperfusion injury of the myocardium. Cardiovasc. Res. 1993, 27, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Kocić, I.; Korolkiewicz, K. Negative inotropic action of alpha-1a adrenoceptor blocking agents: Role of adenosine and ATP-sensitive K+ channels. Gen. Pharmacol. Vasc. Syst. 1998, 30, 351–356. [Google Scholar] [CrossRef]
- Jackson, E.K.; Gillespie, D.G.; Mi, Z.; Cheng, N. Adenosine Receptors Influence Hypertension in Dahl Salt-Sensitive Rats. Hypertension 2018, 72, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lai, E.Y.; Huang, Y.; Eisner, C.; Mizel, D.; Wilcox, C.S.; Schnermann, J. Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors. Am. J. Physiol. Physiol. 2012, 303, F1166–F1175. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.Y.; Martinka, P.; Fähling, M.; Mrowka, R.; Steege, A.; Gericke, A.; Sendeski, M.; Persson, P.; Persson, A.E.G.; Patzak, A. Adenosine Restores Angiotensin II–Induced Contractions by Receptor-Independent Enhancement of Calcium Sensitivity in Renal Arterioles. Circ. Res. 2006, 99, 1117–1124. [Google Scholar] [CrossRef]
- Lai, E.; Patzak, A.; Steege, A.; Mrowka, R.; Brown, R.; Spielmann, N.; Persson, P.; Fredholm, B.; Persson, A. Contribution of adenosine receptors in the control of arteriolar tone and adenosine–angiotensin II interaction. Kidney Int. 2006, 70, 690–698. [Google Scholar] [CrossRef]
- Kuan, C.-J.; Herzer, W.A.; Jackson, E.K. Cardiovascular and Renal Effects of Blocking A1 Adenosine Receptors. J. Cardiovasc. Pharmacol. 1993, 21, 822–828. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.N.; Arner, A.; Boels, P.J.M.; Fredholm, B.B. Adenosine A1 receptors and vascular reactivity. Acta Physiol. 2010, 199, 211–220. [Google Scholar] [CrossRef]
- Yadav, V.R.; Teng, B.; Mustafa, S.J. Enhanced A(1) adenosine receptor-induced vascular contractions in mesenteric artery and aorta of in L-NAME mouse model of hypertension. Eur. J. Pharmacol. 2019, 842, 111–117. [Google Scholar] [CrossRef]
- Maimon, N.; Titus, P.A.; Sarelius, I.H. Pre-exposure to adenosine, acting via A2Areceptors on endothelial cells, alters the protein kinase A dependence of adenosine-induced dilation in skeletal muscle resistance arterioles. J. Physiol. 2014, 592, 2575–2590. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.-P.; Wu, F.; Li, P.-L.; Cowley, A.W. Effect of chronic salt loading on adenosine metabolism and receptor expression in renal cortex and medulla in rats. Hypertension 1999, 33, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.R.; Nadeem, A.; Talukder, M.A.H.; Sakhalkar, S.; Mustafa, S.J. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am. J. Physiol. Circ. Physiol. 2007, 292, H719–H725. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Fil, D.; Tilley, S.L.; Ledent, C.; Krahn, T.; Mustafa, S.J. Functional and RNA expression profile of adenosine receptor subtypes in mouse mesenteric arteries. J. Cardiovasc. Pharmacol. 2013, 61, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zou, A.-P.; Nithipatikom, K.; Li, P.-L.; Cowley, A.W. Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am. J. Physiol. Content 1999, 276, R790–R798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Wang, W.; Dai, Y.; Ning, C.; Luo, R.; Sun, K.; Glover, L.; Grenz, A.; Sun, H.; et al. Elevated ecto-5’-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ. Res. 2013, 112, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Manfredini, R.; Iannotta, V.; Pancaldi, C.; Cattabriga, E.; Uluoglu, C.; Borea, P.A.; Portaluppi, F. Effects of Doxazosin and Propranolol on A2AAdenosine Receptors in Essential Hypertension. Hypertension 2002, 40, 909–913. [Google Scholar] [CrossRef]
- Silhol, F.; Marlinge, M.; Guiol, C.; Chefrour, M.; Mace, P.; Criado, C.; Kipson, N.; Vaisse, B.; Vairo, D.; Sarlon, G.; et al. Characterization of adenosine A2 receptors in peripheral blood mononuclear cells of patients with fibromuscular dysplasia. Hypertens. Res. 2019, 43, 466–469. [Google Scholar] [CrossRef]
- Galié, N.; Torbicki, A.; Barst, R.; Dartevelle, P.; Haworth, S.; Higenbottam, T.; Olschewski, H.; Peacock, A.; Pietra, G.; Rubin, L.J.; et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur. Heart J. 2004, 25, 2243–2278. [Google Scholar] [CrossRef][Green Version]
- Xu, X.-Q.; Jing, Z.-C. High-altitude pulmonary hypertension. Eur. Respir. Rev. 2009, 18, 13–17. [Google Scholar] [CrossRef]
- Humbert, M.; Morrell, N.W.; Archer, S.L.; Stenmark, K.R.; MacLean, M.; Lang, I.M.; Christman, B.W.; Weir, E.; Eickelberg, O.; Voelkel, N.F.; et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43, S13–S24. [Google Scholar] [CrossRef] [PubMed]
- Alencar, A.K.N.; Montes, G.C.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Adenosine Receptors As Drug Targets for Treatment of Pulmonary Arterial Hypertension. Front. Pharmacol. 2017, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Saadjian, A.Y.; Paganelli, F.; Gaubert, M.; Levy, S.; Guieu, R.P. Adenosine plasma concentration in pulmonary hypertension. Cardiovasc. Res. 1999, 43, 228–236. [Google Scholar] [CrossRef]
- Saadjian, A.Y.; Paganelli, F.; Juin, M.A.; Devaux, C.; Lévy, S.; Guieu, R.P. Plasmabeta-endorphin and adenosine concentration in pulmonary hypertension. Am. J. Cardiol. 2000, 85, 858–863. [Google Scholar] [CrossRef]
- Mann, D.L. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ. Res. 2015, 116, 1254–1268. [Google Scholar] [CrossRef]
- Funaya, H.; Kitakaze, M.; Node, K.; Minamino, T.; Komamura, K.; Hori, M. Plasma Adenosine Levels Increase in Patients With Chronic Heart Failure. Circulation 1997, 95, 1363–1365. [Google Scholar] [CrossRef]
- Franceschi, F.; Deharo, J.-C.; Giorgi, R.; By, Y.; Monserrat, C.; Condo, J.; Ibrahim, Z.; Saadjian, A.; Guieu, R. Peripheral plasma adenosine release in patients with chronic heart failure. Heart 2008, 95, 651–655. [Google Scholar] [CrossRef]
- Gaubert, M.; Marlinge, M.; Kerbaul, F.; Resseguier, N.; Laine, M.; Cautella, J.; Cordier, C.; Colomb, B.; Kipson, N.; Thuny, F.; et al. Adenosine Plasma Level and A2A Receptor Expression in Patients With Cardiogenic Shock. Crit. Care Med. 2018, 46, e874–e880. [Google Scholar] [CrossRef]
- Asakura, M.; Asanuma, H.; Kim, J.; Liao, Y.; Nakamaru, K.; Fujita, M.; Komamura, K.; Isomura, T.; Furukawa, H.; Tomoike, H.; et al. Impact of Adenosine Receptor Signaling and Metabolism on Pathophysiology in Patients with Chronic Heart Failure. Hypertens. Res. 2007, 30, 781–787. [Google Scholar] [CrossRef]
- Matherne, G.P.; Linden, J.; Byford, A.M.; Gauthier, N.S.; Headrick, J.P. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc. Natl. Acad. Sci. USA 1997, 94, 6541–6546. [Google Scholar] [CrossRef]
- Zucchi, R.; Cerniway, R.J.; Ronca-Testoni, S.; Morrison, R.R.; Ronca, G.; Matherne, G.P. Effect of cardiac A(1) adenosine receptor overexpression on sarcoplasmic reticulum function. Cardiovasc. Res. 2002, 53, 326–333. [Google Scholar] [CrossRef][Green Version]
- Varani, K.; Laghi-Pasini, F.; Camurri, A.; Capecchi, P.L.; Maccherini, M.; Diciolla, F.; Ceccatelli, L.; Lazzerini, P.E.; Ulouglu, C.; Cattabeni, F.; et al. Changes of peripheral A 2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB J. 2002, 17, 280–282. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory Cytokines in Heart Failure: Mediators and Markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef]
- Zhai, Y.-J.; Liu, P.; He, H.-R.; Zheng, X.-W.; Wang, Y.; Yang, Q.-T.; Lu, T.; Lyu, J. The Association of ADORA2A and ADORA2B Polymorphisms with the Risk and Severity of Chronic Heart Failure: A Case-Control Study of a Northern Chinese Population. Int. J. Mol. Sci. 2015, 16, 2732–2746. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Everett, T.H.; Wilson, E.; Chang, R.; Zeng, D.; Belardinelli, L.; Olgin, J.E. Blockade of A2B adenosine receptor reduces left ventricular dysfunction and ventricular arrhythmias 1 week after myocardial infarction in the rat model. Heart Rhythm. 2014, 11, 101–109. [Google Scholar] [CrossRef]
- Lu, Z.; Fassett, J.; Xu, X.; Hu, X.; Zhu, G.; French, J.; Zhang, P.; Schnermann, J.; Bache, R.J.; Chen, Y. Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle. Circulation 2008, 118, 1713–1721. [Google Scholar] [CrossRef]
| Disease | Purinergic Abnormalities |
|---|---|
| Atrial fibrillation | High APL in the left atria [64]. |
| High A2AR expression in left atria [46,68]. Heterogenous expression of A1R in right atria [69] | |
| Reflex (neurohumoral) syncope | |
| Vasovagal syncope | |
| High APL [70] | |
| High A2AR expression [71,72] | |
| CC variant in the second exon of the gene [72] | |
| Syncope without prodrome, normal heart and normal electrocardiogram | |
| Low APL [73,74] | |
| Low A2AR expression [73] | |
| Hypoxic conditions | |
| TLOC during dive | High APL [75,76] |
| Sleep apnea | High hypoxanthine and xanthine serum levels [77] |
| High APL and serum uric acid [78,79] | |
| Altitude hypoxia | High APL, high A2BR expression and low ENT1 [80] |
| Hypertension conditions | |
| Systemic hypertension | High APL [81] |
| High A2AR expression [82] | |
| Pulmonary Hypertension | Low APL in pulmonary arteries [83,84] |
| Heart failure | CHF: High APL [85,86] |
| Increase in A2AR expression [87] | |
| AHF: High APL and high A2AR expression [88] |
| Supra Ventricular Level | Interruption of tachycardia [89] |
| AVB [92] | |
| AVB in low adenosine syncope patients [73,103] | |
| Asystole, myoclonic jerk [93] | |
| Fetal bradycardia [95] | |
| Atrial fibrillation [60] | |
| Atrial premature complex [62] | |
| Atrial ectopic beats [63] | |
| Flutter [62] | |
| Ventricular Level | Increased QT interval in LQTS [99] |
| Torsade de pointes in LQTS [100] | |
| Ventricular tachycardia [96,97] | |
| Torsade de pointes [98,102] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guieu, R.; Deharo, J.-C.; Maille, B.; Crotti, L.; Torresani, E.; Brignole, M.; Parati, G. Adenosine and the Cardiovascular System: The Good and the Bad. J. Clin. Med. 2020, 9, 1366. https://doi.org/10.3390/jcm9051366
Guieu R, Deharo J-C, Maille B, Crotti L, Torresani E, Brignole M, Parati G. Adenosine and the Cardiovascular System: The Good and the Bad. Journal of Clinical Medicine. 2020; 9(5):1366. https://doi.org/10.3390/jcm9051366
Chicago/Turabian StyleGuieu, Régis, Jean-Claude Deharo, Baptiste Maille, Lia Crotti, Ermino Torresani, Michele Brignole, and Gianfranco Parati. 2020. "Adenosine and the Cardiovascular System: The Good and the Bad" Journal of Clinical Medicine 9, no. 5: 1366. https://doi.org/10.3390/jcm9051366
APA StyleGuieu, R., Deharo, J.-C., Maille, B., Crotti, L., Torresani, E., Brignole, M., & Parati, G. (2020). Adenosine and the Cardiovascular System: The Good and the Bad. Journal of Clinical Medicine, 9(5), 1366. https://doi.org/10.3390/jcm9051366







