Endothelin-1 Serum Concentration is Lower in Children and Adolescents with High Myopia, a Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Statistical Methods
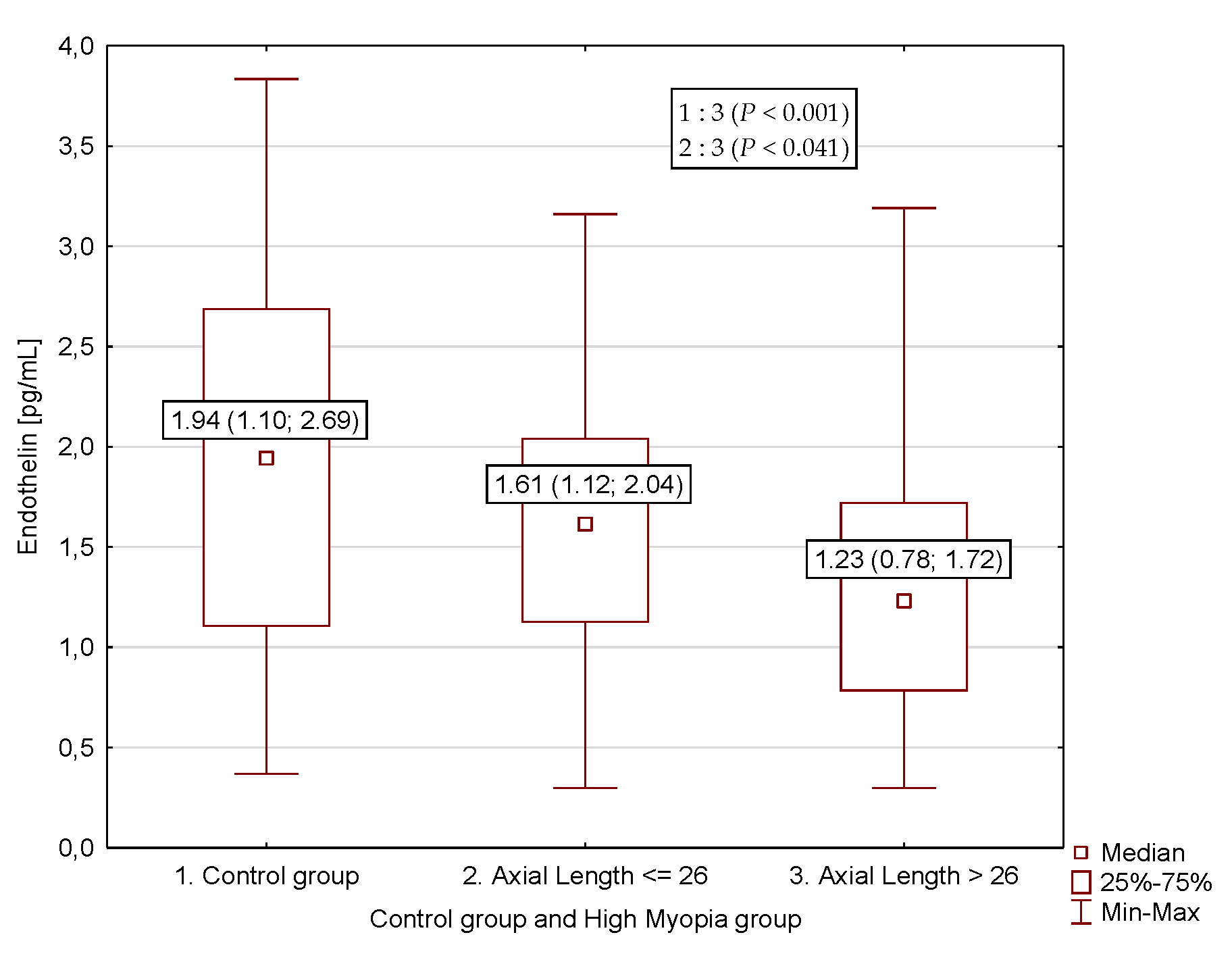
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grzybowski, A.; Kanclerz, P.; Tsubota, K.; Lanca, C.; Saw, S.M. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Harper, R.; Tromans, C.; Waterman, C.; Goldberg, D.; Haggerty, C.; Tullo, A. Quality of life in myopia. Br. J. Ophthalmol. 2000, 84, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.E.; Chung, I.; Krumholtz, I. An analysis of high myopia in a pediatric population less than 10 years of age. Optometry 2005, 76, 102–114. [Google Scholar] [CrossRef]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef]
- Mori, K.; Kurihara, T.; Uchino, M.; Torii, H.; Kawashima, M.; Sasaki, M.; Ozawa, Y.; Yamagishi, K.; Iso, H.; Sawada, N.; et al. High Myopia and Its Associated Factors in JPHC-NEXT Eye Study: A Cross-Sectional Observational Study. J. Clin. Med. 2019, 8, 1788. [Google Scholar] [CrossRef]
- Inoue, A.; Yanagisawa, M.; Kimura, S.; Kasuya, Y.; Miyauchi, T.; Goto, K.; Masaki, T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. USA 1989, 86, 2863–2867. [Google Scholar] [CrossRef] [PubMed]
- Jain, A. Endothelin-1-induced endoplasmic reticulum stress in disease. J. Pharmacol. Exp. Ther. 2013, 346, 163–172. [Google Scholar] [CrossRef]
- Lüscher, T.F. Endothelium-derived vasoactive factors and regulation of vascular tone in human blood vessels. Lung 1990, 168, 27–34. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Hynynen, M.M.; Khalil, R.A. The vascular endothelin system in hypertension–recent patents and discoveries. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 95–108. [Google Scholar] [CrossRef]
- Fedor, M.; Socha, K.; Urban, B.; Soroczyńska, J.; Matyskiela, M.; Borawska, M.H.; Bakunowicz-Łazarczyk, A. Serum Concentration of Zinc, Copper, Selenium, Manganese, and Cu/Zn Ratio in Children and Adolescents with Myopia. Biol. Trace Elem. Res. 2017, 176, 1–9. [Google Scholar] [CrossRef]
- Fedor, M.; Urban, B.; Socha, K.; Soroczyńska, J.; Krętowska, M.; Borawska, M.H.; Bakunowicz-Łazarczyk, A. Concentration of Zinc, Copper, Selenium, Manganese, and Cu/Zn Ratio in Hair of Children and Adolescents with Myopia. J. Ophthalmol. 2019, 2019, 5643848. [Google Scholar] [CrossRef]
- Dell Inc. Dell Statistica (Data Analysis Software System), Version 13. 2016. Available online: software.dell.com (accessed on 4 January 2020).
- Boesen, E.I.; Pollock, D.M. Cooperative role of ETA and ETB receptors in mediating the diuretic response to intramedullary hyperosmotic NaCl infusion. Am. J. Physiol.-Ren. Physiol. 2010, 299, F1424–F1432. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Kapetanakis, V.V.; Wathern, A.K.; Logan, N.S.; Gilmartin, B.; Whincup, P.H.; Cook, D.G.; Owen, C.G. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: Implications for aetiology and early prevention. Br. J. Ophthalmol. 2016, 100, 882–890. [Google Scholar] [CrossRef]
- Tedja, M.S.; Haarman, A.E.G.; Meester-Smoor, M.A.; Kaprio, J.; Mackey, D.A.; Guggenheim, J.A.; Hammond, C.J.; Verhoeven, V.J.M.; Klaver, C.C.W.; CREAM Consortium. IMI—Myopia Genetics Report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M89–M105. [Google Scholar] [CrossRef]
- Flammer, J.; Konieczka, K.; Flammer, A.J. The primary vascular dysregulation syndrome: Implications for eye diseases. EPMA J. 2013, 4, 14. [Google Scholar] [CrossRef]
- Salvatore, S.; Vingolo, E.M. Endothelin-1 role in human eye: A review. J. Ophthalmol. 2010, 2010, 354645. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, H.; Oku, H.; Li, Q.; Sakagami, K.; Puro, D.G. Endothelin-induced changes in the physiology of retinal pericytes. Investig. Ophthalmol. Vis. Sci. 2002, 43, 882–888. [Google Scholar]
- Taniguchi, T.; Okada, K.; Haque, M.S.; Sugiyama, K.; Kitazawa, Y. Effects of endothelin-1 on intraocular pressure and aqueous humor dynamics in the rabbit eye. Curr. Eye Res. 1994, 13, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.A.; Webb, D.J. The endothelin system and its potential as a therapeutic target in cardiovascular disease. Pharmacol. Ther. 1996, 72, 109–148. [Google Scholar] [CrossRef]
- Kohno, M.; Murakawa, K.; Yasunari, K.; Yokokawa, K.; Horio, T.; Kurihara, N.; Takeda, T. Prolonged blood pressure elevation after endothelin administration in bilaterally nephrectomized rats. Metabolism 1989, 38, 712–713. [Google Scholar] [CrossRef]
- Ikuno, Y.; Tano, Y. Retinal and choroidal biometry in highly myopic eyes with spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3876–3880. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Imamura, Y.; Margolis, R.; Slakter, J.S.; Spaide, R.F. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am. J. Ophthalmol. 2009, 148, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Shiragami, C.; Shirakata, Y.; Manabe, S.; Izumibata, S.; Shiraga, F. Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn. J. Ophthalmol. 2012, 56, 230–235. [Google Scholar] [CrossRef]
- Chen, F.K.; Yeoh, J.; Rahman, W.; Patel, P.J.; Tufail, A.; Da Cruz, L. Topographic variation and interocular symmetry of macular choroidal thickness using enhanced depth imaging optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 975–985. [Google Scholar] [CrossRef]
- Zheng, Q.; Zong, Y.; Li, L.; Huang, X.; Lin, L.; Yang, W.; Yuan, Y.; Li, Y.; He, H.; Gao, Q. Retinal vessel oxygen saturation and vessel diameter in high myopia. Ophthalmic Physiol. Opt. 2015, 35, 562–569. [Google Scholar] [CrossRef]
- Grudzińska, E.; Modrzejewska, M. Modern Diagnostic Techniques for the Assessment of Ocular Blood Flow in Myopia: Current State of Knowledge. J. Ophthalmol. 2018, 2018, 4694789. [Google Scholar] [CrossRef]
- Fleming, I.; Busse, R. Signal transduction of eNOS activation. Cardiovasc. Res. 1999, 43, 532–541. [Google Scholar] [CrossRef]
- Tsou, J.K.; Gower, R.M.; Ting, H.J.; Schaff, U.Y.; Insana, M.F.; Passerini, A.G.; Simon, S.I. Spatial regulation of inflammation by human aortic endothelial cells in a linear gradient of shear stress. Microcirculation 2008, 15, 311–323. [Google Scholar] [CrossRef]
| Parameter | High Myopia Patients | Control Group | p Values |
|---|---|---|---|
| Number, n | 57 | 29 | |
| Age, years | 14 (13; 16) | 14 (11; 15) | 0.181 |
| Gender (female/male), n (%) | 31 (54)/26 (46) | 17 (59)/12 (41) | 0.709 |
| Age group (≤13/>13 year), n (%) | 23 (40)/34 (60) | 13 (45)/16 (55) | 0.691 |
| Endothelin-1, pg/mL | 1.47 (0.91; 1.87) | 1.94 (1.1; 2.69) | 0.005 |
| Features | High Myopia Patients, n = 57 |
|---|---|
| Visual acuity of right eye | 1.0 (0.7; 1.0) |
| Visual acuity of left eye | 0.8 (0.6; 1.0) |
| Refractive error of right eye, D | −6.8 (−8.6; −6) |
| Refractive error of left eye, D | −6.8 (−9.5; −6) |
| Axial length of right eye, mm | 25.9 (25.3; 26.6) |
| Axial length of left eye, mm | 25.8 (25.3; 26.6) |
| Peripheral chorioretinal atrophy, yes/no, n (%) | 11 (19)/46 (81) |
| Parameter | High Myopia Patients | Control Group | |
|---|---|---|---|
| Axial Length of Eye | |||
| >26 mm | ≤26 mm | ||
| Number, n | 29 | 28 | 29 |
| Gender (female/male), n (%) | 13 (45)/16 (55) | 18 (64)/10 (36) | 17 (59)/12 (41) |
| Age group (≤13/>13 year), n (%) | 12 (41)/17 (59) | 11 (39)/17 (61) | 13 (45)/16 (55) |
| Endothelin-1, pg/mL | 1.23 (0.78; 1.72) a,b | 1.61 (1.12; 2.04) | 1.94 (1.1; 2.69) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powierza, K.; Żelazowska-Rutkowska, B.; Sawicka-Powierza, J.; Mikołuć, B.; Urban, B.; Zaremba, W.; Cylwik, B.; Bakunowicz-Łazarczyk, A. Endothelin-1 Serum Concentration is Lower in Children and Adolescents with High Myopia, a Preliminary Study. J. Clin. Med. 2020, 9, 1327. https://doi.org/10.3390/jcm9051327
Powierza K, Żelazowska-Rutkowska B, Sawicka-Powierza J, Mikołuć B, Urban B, Zaremba W, Cylwik B, Bakunowicz-Łazarczyk A. Endothelin-1 Serum Concentration is Lower in Children and Adolescents with High Myopia, a Preliminary Study. Journal of Clinical Medicine. 2020; 9(5):1327. https://doi.org/10.3390/jcm9051327
Chicago/Turabian StylePowierza, Katarzyna, Beata Żelazowska-Rutkowska, Jolanta Sawicka-Powierza, Bożena Mikołuć, Beata Urban, Wojciech Zaremba, Bogdan Cylwik, and Alina Bakunowicz-Łazarczyk. 2020. "Endothelin-1 Serum Concentration is Lower in Children and Adolescents with High Myopia, a Preliminary Study" Journal of Clinical Medicine 9, no. 5: 1327. https://doi.org/10.3390/jcm9051327
APA StylePowierza, K., Żelazowska-Rutkowska, B., Sawicka-Powierza, J., Mikołuć, B., Urban, B., Zaremba, W., Cylwik, B., & Bakunowicz-Łazarczyk, A. (2020). Endothelin-1 Serum Concentration is Lower in Children and Adolescents with High Myopia, a Preliminary Study. Journal of Clinical Medicine, 9(5), 1327. https://doi.org/10.3390/jcm9051327





