An In Vivo Study in Rat Femurs of Bioactive Silicate Coatings on Titanium Dental Implants
Abstract
1. Introduction
2. Experimental Section
2.1. Samples Preparation and Characterization
2.2. Toxicity
2.2.1. Cells
2.2.2. Lactate Dehydrogenase (LDH) Activity
2.2.3. Hemolysis Assay
2.3. Surgical Procedure
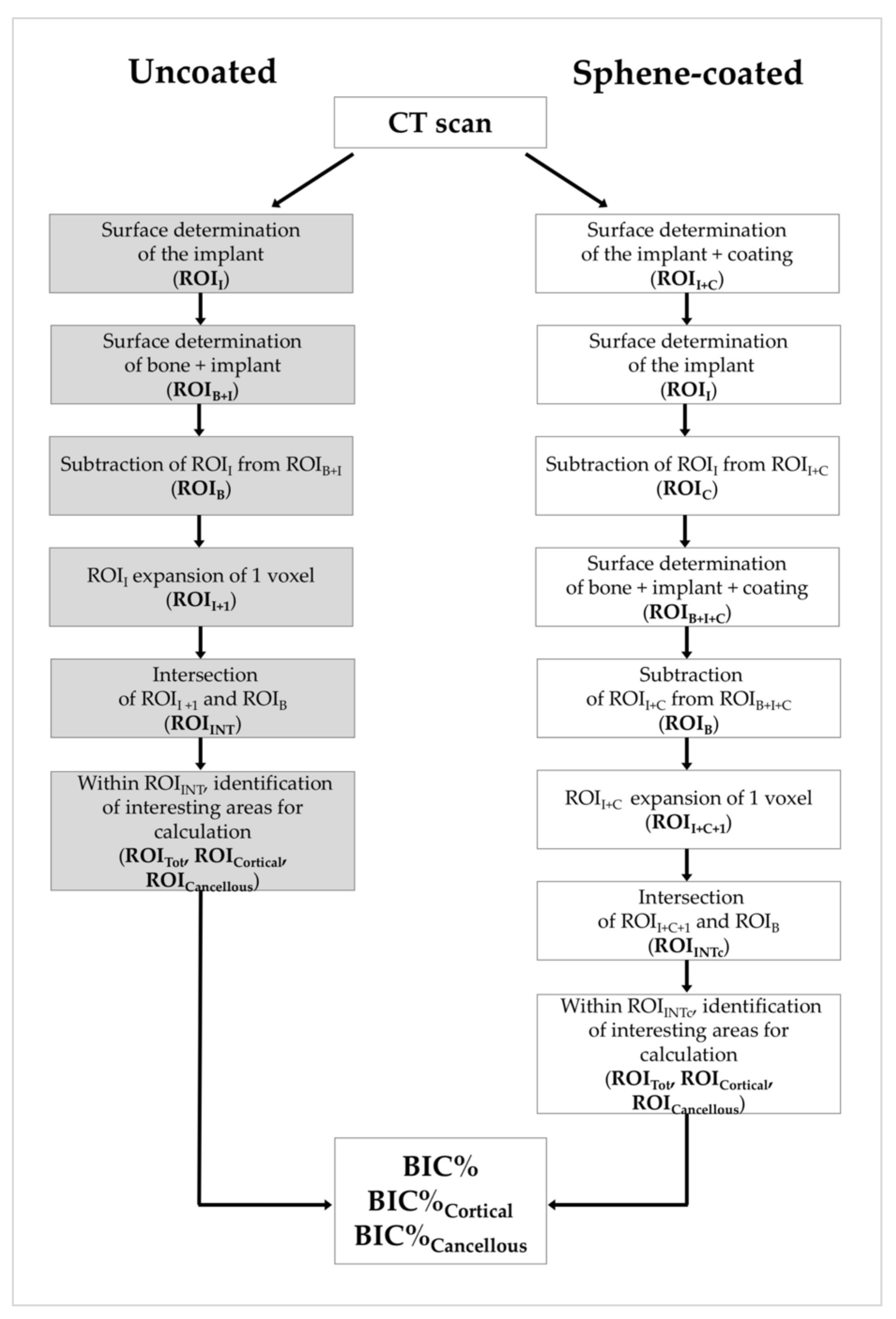
2.4. Micro-CT Analysis
2.5. Histological Processing
2.6. Histological Assessment
2.7. Synovial Fluid (SF) Analysis
2.8. Statistical Analysis
3. Results
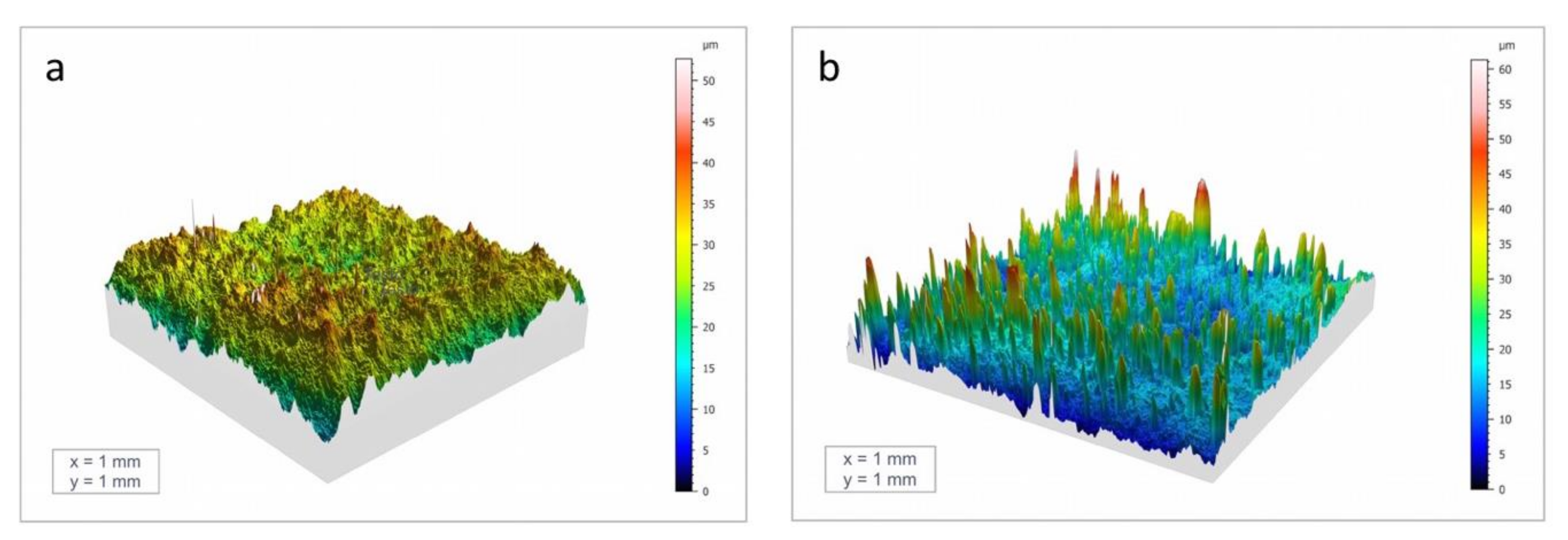
3.1. Surface and Coating Analysis
3.2. Toxicity
3.3. Clinical Observations in In Vivo Experiments
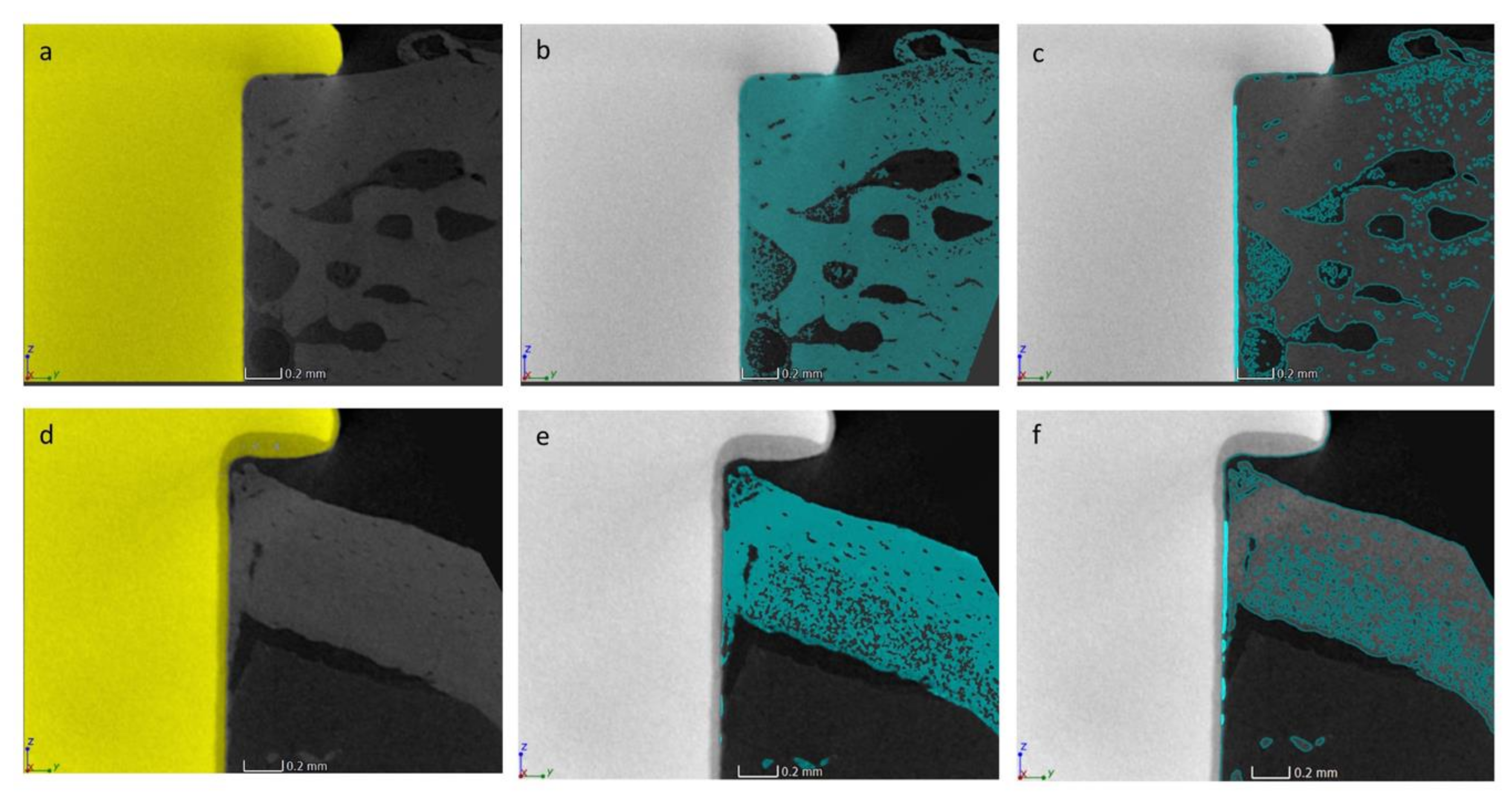
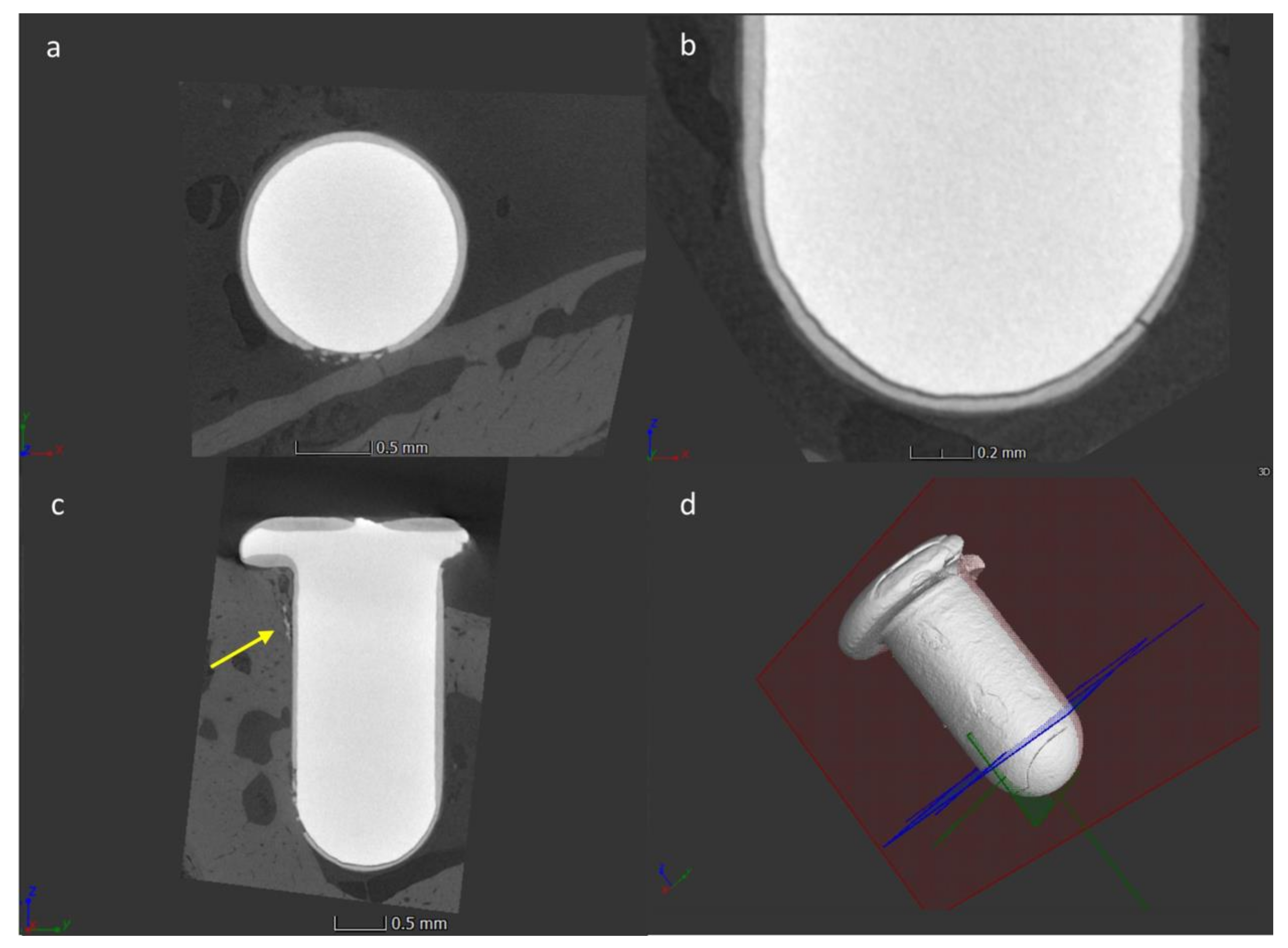
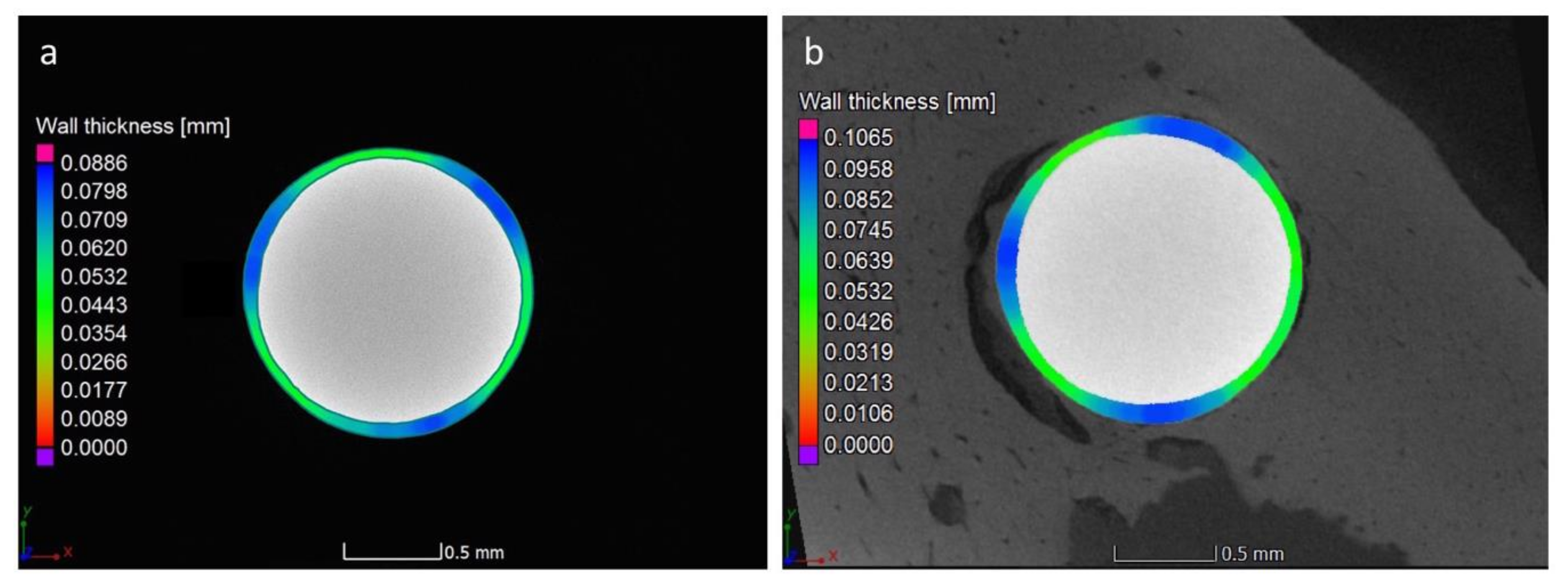
3.4. Micro-CT Analysis
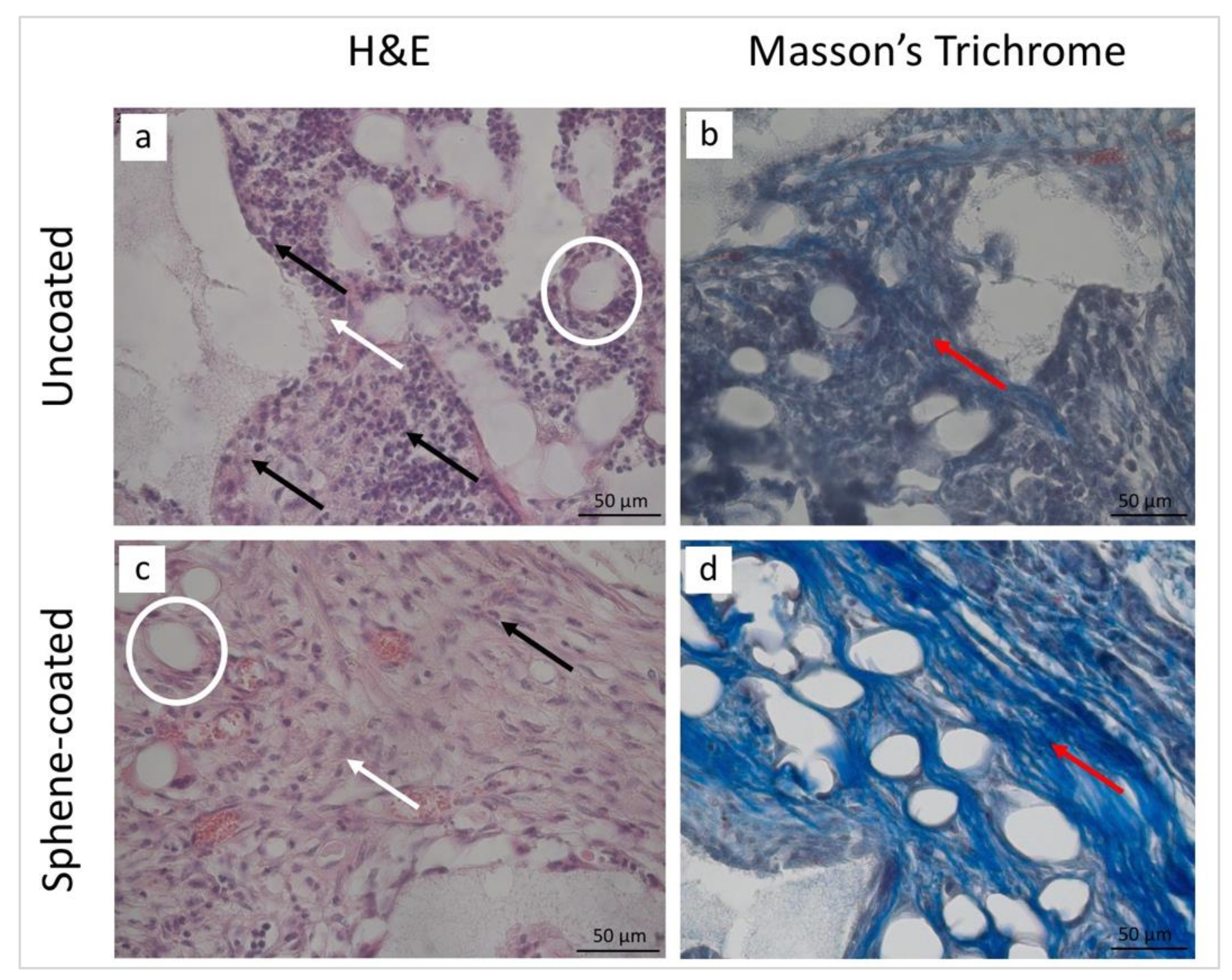
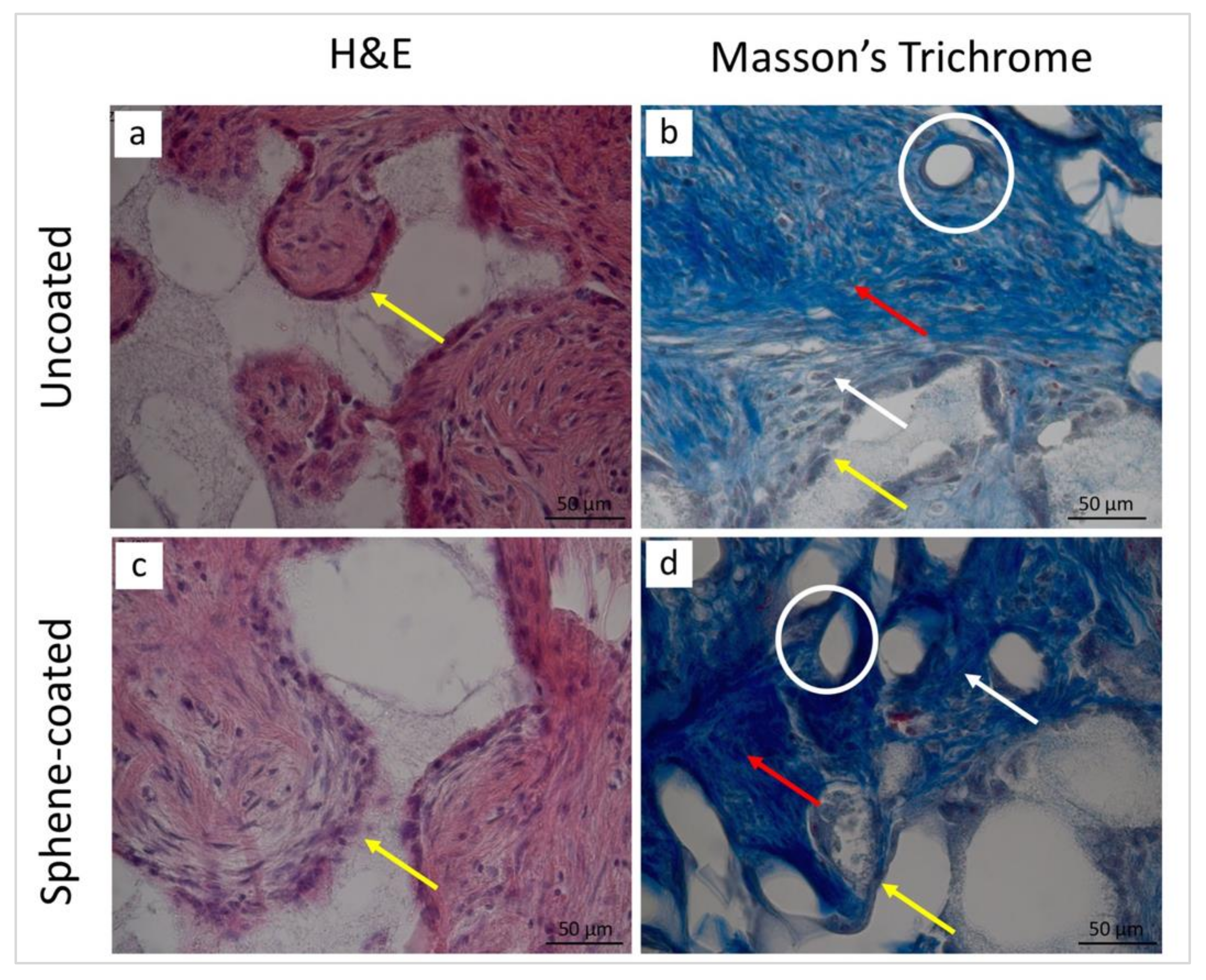
3.5. Histological and Histomorphometrical Analyses
3.6. Synovial Fluid (SF) Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Valenzuela, S.M.; Nissan, B.B.; Roest, R.; Knabe, C.; Radlanski, R.J.; Renz, H.; Evans, P.J. The effect of surface chemistry modification of titanium alloy on signalling pathways in human osteoblasts. Biomaterials 2005, 26, 7579–7586. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Barão, V.A. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of dental implant surface modifications on osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Brunello, G.; Elsayed, H.; Biasetto, L. Bioactive glass and silicate-based ceramic coatings on metallic implants: Open challenge or outdated topic? Materials 2019, 12, 2929. [Google Scholar] [CrossRef]
- Xuereb, M.; Camilleri, J.; Attard, N.J. Systematic review of current dental implant coating materials and novel coating techniques. Int. J. Prosthodont. 2015, 28, 51–59. [Google Scholar] [CrossRef]
- Aebli, N.; Krebs, J.; Stich, H.; Schawalder, P.; Walton, M.; Schwenke, D.; Gruner, H.; Gasser, B.; Theis, J.C. In vivo comparison of the osseointegration of vacuum plasma sprayed titanium- and hydroxyapatite-coated implants. J. Biomed. Mater. Res. A 2003, 66, 356–363. [Google Scholar] [CrossRef]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coat. Technol. 2011, 205, 2785–2792. [Google Scholar] [CrossRef]
- Wang, H.; Eliaz, N.; Xiang, Z.; Hsu, H.P.; Spector, M.; Hobbs, L.W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 2006, 27, 4192–4203. [Google Scholar] [CrossRef]
- Artzi, Z.; Carmeli, G.; Kozlovsky, A. A distinguishable observation between survival and success rate outcome of hydroxyapatite-coated implants in 5-10 years in function. Clin. Oral Implant. Res. 2006, 17, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.; Harun, W.S.; Hassan, M.A.; Ghani, S.A.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. A review of bioactive silicate ceramics. Biomed. Mater. 2013, 8, 032001. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Miranzo, P.; Sainz, M.A. Thermally sprayed wollastonite and wollastonite-diopside compositions as new modulated bioactive coatings for metal implants. Ceram. Int. 2018, 44, 12896–12904. [Google Scholar] [CrossRef]
- Biasetto, L.; Elsayed, H. Sphene silicate ceramic coatings on cpTi substrates: Process upgrade. Surf. Coat. Technol. 2017, 312, 416–424. [Google Scholar] [CrossRef]
- Yi, D.; Wu, C.; Ma, X.; Ji, H.; Zheng, X.; Chang, J. Preparation and in vitro evaluation of plasma-sprayed bioactive akermanite coatings. Biomed. Mater. 2012, 7, 065004. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, Y.; Ji, H.; Huang, L.; Zheng, X. Excellent stability of plasma-sprayed bioactive Ca3ZrSi2O9 ceramic coating on Ti–6Al–4V. Appl. Surf. Sci. 2010, 256, 4677–4681. [Google Scholar] [CrossRef]
- Yi, D.; Wu, C.; Ma, B.; Ji, H.; Zheng, X.; Chang, J. Bioactive bredigite coating with improved bonding strength, rapid apatite mineralization and excellent cytocompatibility. J. Biomater. Appl. 2014, 28, 1343–1353. [Google Scholar] [CrossRef]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of silicon compounds on biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef]
- Elsayed, H.; Colombo, P. Crack-free silicate bioceramics from preceramic polymers. Adv. Appl. Ceram. 2016, 115, 193–199. [Google Scholar] [CrossRef]
- Elsayed, H.; Brunello, G.; Gardin, C.; Ferroni, L.; Badocco, D.; Pastore, P.; Sivolella, S.; Zavan, B.; Biasetto, L. Bioactive sphene-based ceramic coatings on cpTi substrates for dental implants: An in vitro study. Materials 2018, 11, 2234. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ramaswamy, Y.; Liu, X.; Wang, G.; Zreiqat, H. Plasma-sprayed CaTiSiO5 ceramic coating on Ti-6Al-4V with excellent bonding strength, stability and cellular bioactivity. J. R. Soc. Interface 2009, 6, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, Z.; Liu, X.; Zhou, X.; Ding, C.; Zreiqat, H. Nanostructured glass-ceramic coatings for orthopaedic applications. J. R. Soc. Interface 2011, 8, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yu, J.; Xie, Y.; Huang, L.; Ye, X.; Zheng, X. Chemical stability and antimicrobial activity of plasma sprayed bioactive Ca2ZnSi2O7 coating. J. Mater. Sci. Mater. Med. 2011, 22, 2781–2789. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Liu, Y.; Zhao, X.; Zou, D.; Zhu, C.; Jin, Y.; Huang, Q.; Sun, J.; Liu, X.; et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials 2013, 34, 3184–3195. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Soeparto, A.; Zreiqat, H. Incorporation of titanium into calcium silicate improved their chemical stability and biological properties. J. Biomed. Mater. Res. A. 2008, 86, 402–410. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Gale, D.; Yang, W.; Xiao, K.; Zhang, L.; Yin, Y.; Zreiqat, H. Novel sphene coatings on Ti-6Al-4V for orthopedic implants using sol-gel method. Acta Biomater. 2008, 4, 569–576. [Google Scholar] [CrossRef]
- Biasetto, L.; Bertolini, R.; Elsayed, H.; Ghiotti, A.; Bruschi, S. Use of cryogenic machining to improve the adhesion of sphene bioceramic coatings on titanium substrates for dental and orthopaedic applications. Ceram. Int. 2019, 45, 5941–5951. [Google Scholar] [CrossRef]
- Wancket, L.M. Animal models for evaluation of bone implants and devices: Comparative bone structure and common model uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef]
- Vandeweghe, S.; Coelho, P.G.; Vanhove, C.; Wennerberg, A.; Jimbo, R. Utilizing micro-computed tomography to evaluate bone structure surrounding dental implants: A comparison with histomorphometry. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, V.A.; Potapova, I.; Camenisch, K.; Nehrbass, D.; Richards, R.G.; Moriarty, T.F. In vivo MicroCT monitoring of osteomyelitis in a rat model. Biomed. Res. Int. 2015, 2015, 587857. [Google Scholar] [CrossRef] [PubMed]
- Meagher, M.J.; Parwani, R.N.; Virdi, A.S.; Sumner, D.R. Optimizing a micro-computed tomography-based surrogate measurement of bone-implant contact. J. Orthop. Res. 2018, 36, 979–986. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Cao, C.; Xu, Z.; Li, G.; Cao, H.; Liu, X.; Zhang, C.; Dong, Y. A comparison of micro-CT and histomorphometry for evaluation of osseointegration of PEO-coated titanium implants in a rat model. Sci. Rep. 2017, 7, 16270. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhu, W.Q.; Xu, L.N.; Ming, P.P.; Shao, S.Y.; Qiu, J. Osseointegration of titanium dental implant under fluoride exposure in rabbits: Micro-CT and histomorphometry study. Clin. Oral Implant. Res. 2019, 30, 1038–1048. [Google Scholar] [CrossRef]
- Geng, H.; Todd, N.M.; Devlin-Mullin, A.; Poologasundarampillai, G.; Kim, T.B.; Madi, K.; Cartmell, S.; Mitchell, C.A.; Jones, J.R.; Lee, P.D. A correlative imaging based methodology for accurate quantitative assessment of bone formation in additive manufactured implants. J. Mater. Sci. Mater. Med. 2016, 27, 112. [Google Scholar] [CrossRef]
- Chikazu, D.; Tomizuka, K.; Ogasawara, T.; Saijo, H.; Koizumi, T.; Mori, Y.; Yonehara, Y.; Susami, T.; Takato, T. Cyclooxygenase-2 activity is essential for the osseointegration of dental implants. Int. J. Oral Maxillofac. Surg. 2007, 36, 441–446. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Zhao, D.; Zou, H.; Sun, N.; Liang, X.; Dard, M.; Lanske, B.; Yuan, Q. Vitamin D supplementation enhances the fixation of titanium implants in chronic kidney disease mice. PLoS ONE 2014, 9, e95689, Erratum in: PLoS ONE 2014, 9, e99972. [Google Scholar] [CrossRef]
- Zheng, X.; Mo, A.; Wang, Y.; Guo, Y.; Wu, Y.; Yuan, Q. Effect of FK-506 (tacrolimus) therapy on bone healing of titanium implants: A histometric and biomechanical study in mice. Eur. J. Oral Sci. 2017, 125, 28–33. [Google Scholar] [CrossRef]
- Pelegrine, A.A.; Moy, P.K.; Moshaverinia, A.; Escada, A.L.D.A.; Calvo-Guirado, J.L.; Claro, A.P.R.A. Development of a novel nanotextured titanium implant. An experimental study in rats. J. Clin. Med. 2019, 8, 954. [Google Scholar] [CrossRef]
- Zhou, W.; Tangl, S.; Reich, K.M.; Kirchweger, F.; Liu, Z.; Zechner, W.; Ulm, C.; Rausch-Fan, X. The Influence of type 2 diabetes mellitus on the osseointegration of titanium implants with different surface modifications-A histomorphometric study in high-fat diet/low-dose streptozotocin-treated rats. Implant Dent. 2019, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Courtney, P.; Doherty, M. Joint aspiration and injection and synovial fluid analysis. Best Pr. Res. Clin. Rheumatol. 2013, 27, 137–169. [Google Scholar] [CrossRef] [PubMed]
- Punzi, L.; Oliviero, F. Arthrocentesis and synovial fluid analysis in clinical practice: Value of sonography in difficult cases. Ann. N. Y. Acad. Sci. 2009, 1154, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Strzelec-Nowak, D.; Kozioł-Montewka, M.; Niedźwiadek, J.; Bogut, A.; Blacha, J.; Mazurkiewicz, T. Investigation of the actual causes of hip joint implant loosening classified as aseptic--analysis of microbiological culture results and levels of inflammatory markers. Pol. J. Microbiol. 2015, 64, 129–135. [Google Scholar] [CrossRef]
- Delecrin, J.; Oka, M.; Takahashi, S.; Yamamuro, T.; Nakamura, T. Changes in joint fluid after total arthroplasty. A quantitative study on the rabbit knee joint. Clin. Orthop. Relat. Res. 1994, 240–249. [Google Scholar]
- Lin, T.; Tamaki, Y.; Pajarinen, J.; Waters, H.A.; Woo, D.K.; Yao, Z.; Goodman, S.B. Chronic inflammation in biomaterial induced periprosthetic osteolysis: NF-κB as a therapeutic target. Acta Biomater. 2014, 10. [Google Scholar] [CrossRef]
- Takei, I.; Takagi, M.; Ida, H.; Ogino, T.; Santavirta, S.; Konttinen, Y.T. High macrophage-colony stimulating factor levels in synovial fluid of loose artificial hip joints. J. Rheumatol. 2000, 27, 894–899. [Google Scholar]
- Fritz, J.; Lurie, B.; Potter, H.G. MR imaging of knee arthroplasty implants. Radiographics 2015, 35, 1483–1501. [Google Scholar] [CrossRef]
- Ferraris, S.; Bobbio, A.; Miola, M.; Spriano, S. Micro- and nano-textured, hydrophilic and bioactive titanium dental implants. Surf. Coat. Technol. 2015, 276, 374–383. [Google Scholar] [CrossRef]
- ISO 25178-2. GPS—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. National Centre for the Replacement, Refinement and Reduction of Amimals in Research. Animal research: Reporting in vivo experiments—The ARRIVE guidelines. J. Cereb. Blood Flow Metab. 2011, 31, 991–993. [Google Scholar] [CrossRef]
- Scanu, A.; Oliviero, F.; Ramonda, R.; Frallonardo, P.; Dayer, J.M.; Punzi, L. Cytokine levels in human synovial fluid during the different stages of acute gout: Role of transforming growth factor β1 in the resolution phase. Ann. Rheum. Dis. 2012, 71, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Al Farraj Aldosari, A.; Anil, S.; Alasqah, M.; Al Wazzan, K.A.; Al Jetaily, S.A.; Jansen, J.A. The influence of implant geometry and surface composition on bone response. Clin. Oral Implant. Res. 2014, 25, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.G.; Jeon, G.R.; Jeong, C.M.; Kim, Y.K.; Kim, S.G.; Cho, I.H.; Cho, Y.S.; Oh, J.S. Experimental study of bone response to hydroxyapatite coating implants: Bone-implant contact and removal torque test. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 411–418. [Google Scholar] [CrossRef]
- Faeda, R.S.; Spin-Neto, R.; Marcantonio, E.; Guastaldi, A.C.; Marcantonio, E., Jr. Laser ablation in titanium implants followed by biomimetic hydroxyapatite coating: Histomorphometric study in rabbits. Microsc. Res. Tech. 2012, 75, 940–948. [Google Scholar] [CrossRef]
- Todisco, M.; Trisi, P. Histomorphometric evaluation of six dental implant surfaces after early loading in augmented human sinuses. J. Oral Implant. 2006, 32, 153–166. [Google Scholar] [CrossRef]
- Weinlaender, M.; Beumer, J., 3rd; Kenney, E.B.; Lekovic, V.; Holmes, R.; Moy, P.K.; Plenk, H., Jr. Histomorphometric and fluorescence microscopic evaluation of interfacial bone healing around 3 different dental implants before and after radiation therapy. Int. J. Oral Maxillofac. Implant. 2006, 21, 212–224. [Google Scholar]
- Ramaswamy, Y.; Wu, C.; Dunstan, C.R.; Hewson, B.; Eindorf, T.; Anderson, G.I.; Zreiqat, H. Sphene ceramics for orthopedic coating applications: An in vitro and in vivo study. Acta Biomater. 2009, 5, 3192–3204. [Google Scholar] [CrossRef]
- Butz, F.; Ogawa, T.; Chang, T.L.; Nishimura, I. Three-dimensional bone-implant integration profiling using micro-computed tomography. Int. J. Oral Maxillofac. Implant. 2006, 21, 687–695. [Google Scholar]
- Liu, S.; Broucek, J.; Virdi, A.S.; Sumner, D.R. Limitations of using micro-computed tomography to predict bone-implant contact and mechanical fixation. J. Microsc. 2012, 245, 34–42. [Google Scholar] [CrossRef]
- Bernhardt, R.; Kuhlisch, E.; Schulz, M.C.; Eckelt, U.; Stadlinger, B. Comparison of bone-implant contact and bone-implant volume between 2D-histological sections and 3D-SRµCT slices. Eur. Cell. Mater. 2012, 23, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Coelho, P.G.; Vandeweghe, S.; Schwartz-Filho, H.O.; Hayashi, M.; Ono, D.; Andersson, M.; Wennerberg, A. Histological and three-dimensional evaluation of osseointegration to nanostructured calcium phosphate-coated implants. Acta Biomater. 2011, 7, 4229–4234. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, A.; Shah, F.A.; Emanuelsson, L.; Omar, O.; Suska, F. A technique for evaluating bone ingrowth into 3D printed, porous Ti6Al4V implants accurately using X-ray micro-computed tomography and histomorphometry. Micron 2017, 94, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, O.; Probst, F.A.; Wolff, K.D.; Jeschke, A.; Weitz, J.; Deppe, H.; Kolk, A. Comparative 3D micro-CT and 2D histomorphometry analysis of dental implant osseointegration in the maxilla of minipigs. J. Clin. Periodontol. 2017, 44, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Park, J.I.; Chae, J.S.; Yeo, I.L. Comparison of micro-computed tomography and histomorphometry in the measurement of bone-implant contact ratios. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 87–95. [Google Scholar] [CrossRef]
- Sivolella, S.; Bressan, E.; Salata, L.A.; Urrutia, Z.A.; Lang, N.P.; Botticelli, D. Osteogenesis at implants without primary bone contact—An experimental study in dogs. Clin. Oral Implant. Res. 2012, 23, 542–549. [Google Scholar] [CrossRef]
- Abron, A.; Hopfensperger, M.; Thompson, J.; Cooper, L.F. Evaluation of a predictive model for implant surface topography effects on early osseointegration in the rat tibia model. J. Prosthet. Dent. 2001, 85, 40–46. [Google Scholar] [CrossRef]
- Marinho, V.C.; Celletti, R.; Bracchetti, G.; Petrone, G.; Minkin, C.; Piattelli, A. Sandblasted and acid-etched dental implants: A histologic study in rats. Int. J. Oral Maxillofac. Implant. 2003, 18, 75–81. [Google Scholar]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Bressan, E.; Botticelli, D.; Sivolella, S.; Bengazi, F.; Guazzo, R.; Sbricoli, L.; Ricci, S.; Ferroni, L.; Gardin, C.; Velez, J.U.; et al. Adipose-derived stem cells as a tool for dental implant osseointegration: An experimental study in the dog. Int. J. Mol. Cell. Med. 2015, 4, 197–208. [Google Scholar] [PubMed]
- Soto-Peñaloza, D.; Caneva, M.; Viña-Almunia, J.; Martín-de-Llano, J.J.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M. Bone-healing pattern on the surface of titanium implants at cortical and marrow compartments in two topographic sites: An experimental study in rabbits. Materials 2018, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Wang, C.; Xue, Y.; Lin, K.; Chang, J.; Sun, J. Osteogenic differentiation of osteoblasts induced by calcium silicate and calcium silicate/β-tricalcium phosphate composite bioceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ramaswamy, Y.; Kwik, D.; Zreiqat, H. The effect of strontium incorporation into CaSiO3 ceramics on their physical and biological properties. Biomaterials 2007, 28, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Chang, J. In vitro degradation, bioactivity, and cytocompatibility of calcium silicate, dimagnesium silicate, and tricalcium phosphate bioceramics. J. Biomater. Appl. 2009, 24, 139–158. [Google Scholar] [CrossRef]
- Ogiso, M.; Yamashita, Y.; Matsumoto, T. The process of physical weakening and dissolution of the HA-coated implant in bone and soft tissue. J. Dent. Res. 1998, 77, 1426–1434. [Google Scholar] [CrossRef]
- Albrektsson, T.; Albrektsson, B. Osseointegration of bone implants. A review of an alternative mode of fixation. Acta Orthop. Scand. 1987, 58, 567–577. [Google Scholar] [CrossRef]
- Frisardi, G.; Barone, S.; Razionale, A.V.; Paoli, A.; Frisardi, F.; Tullio, A.; Lumbau, A.; Chessa, G. Biomechanics of the press-fit phenomenon in dental implantology: An image-based finite element analysis. Head Face Med. 2012, 8, 18. [Google Scholar] [CrossRef]
- Ostman, P.O.; Hellman, M.; Sennerby, L. Direct implant loading in the edentulous maxilla using a bone density-adapted surgical protocol and primary implant stability criteria for inclusion. Clin. Implant Dent. Relat. Res. 2005, 7, S60–S69. [Google Scholar] [CrossRef]
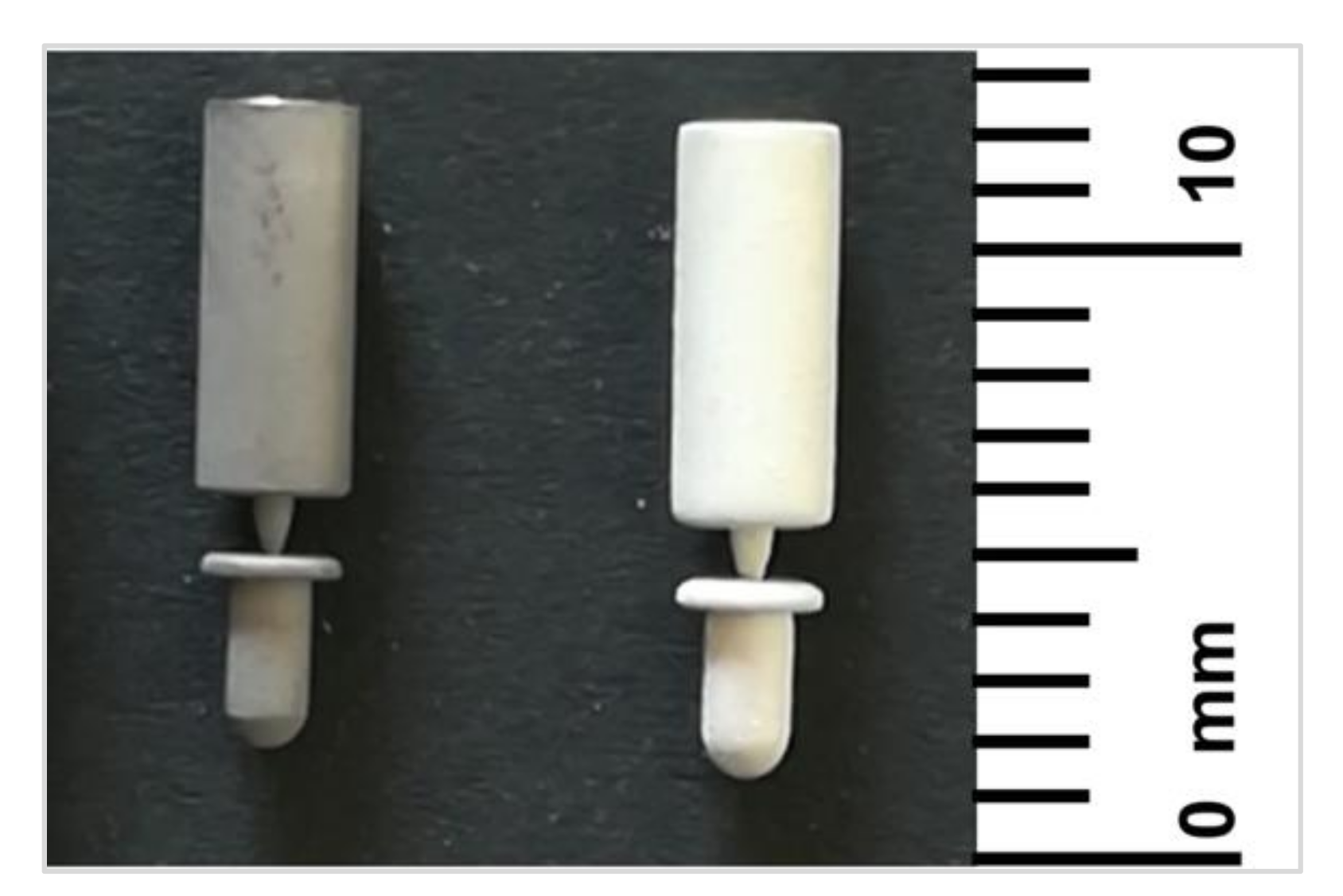
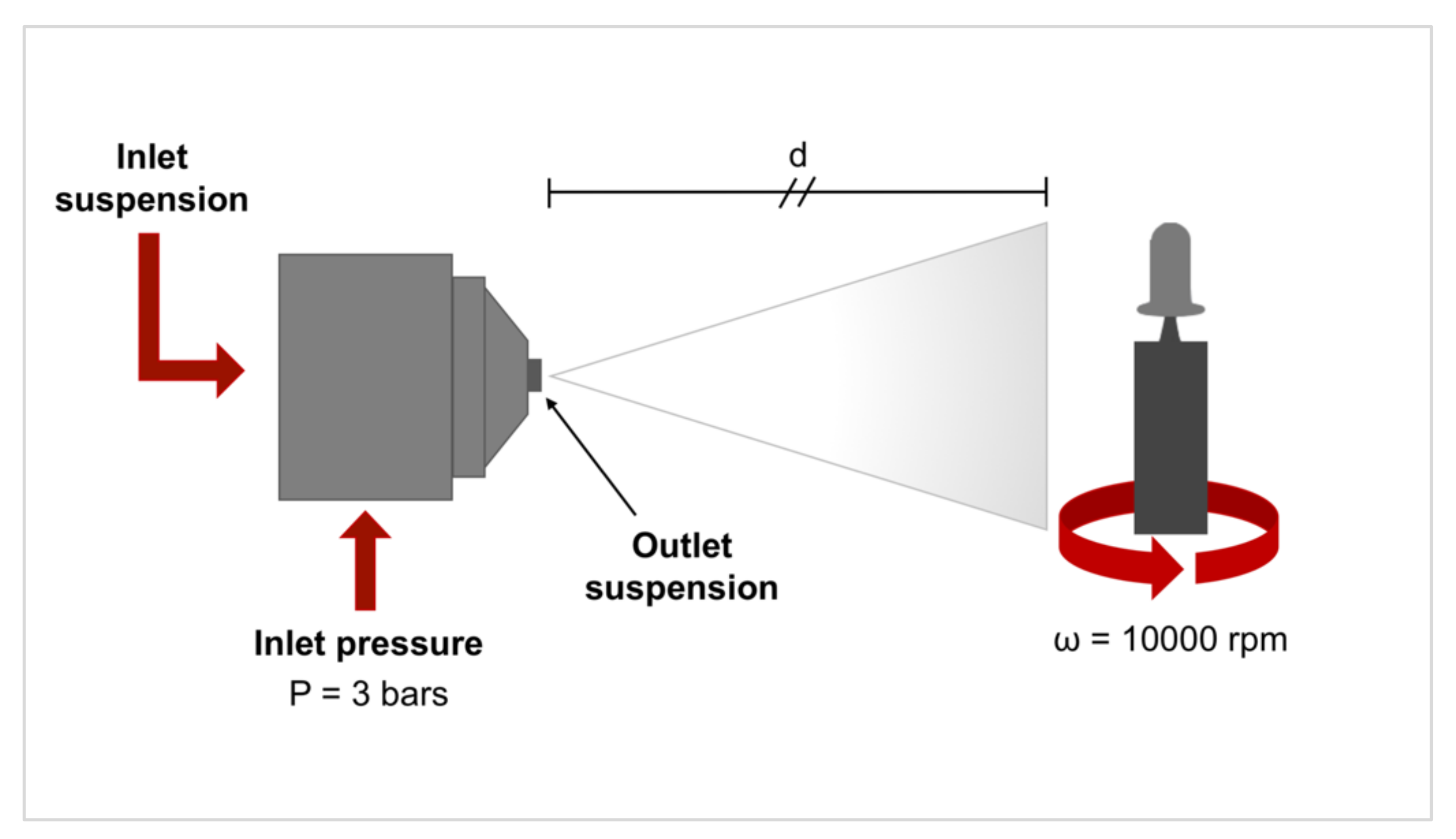
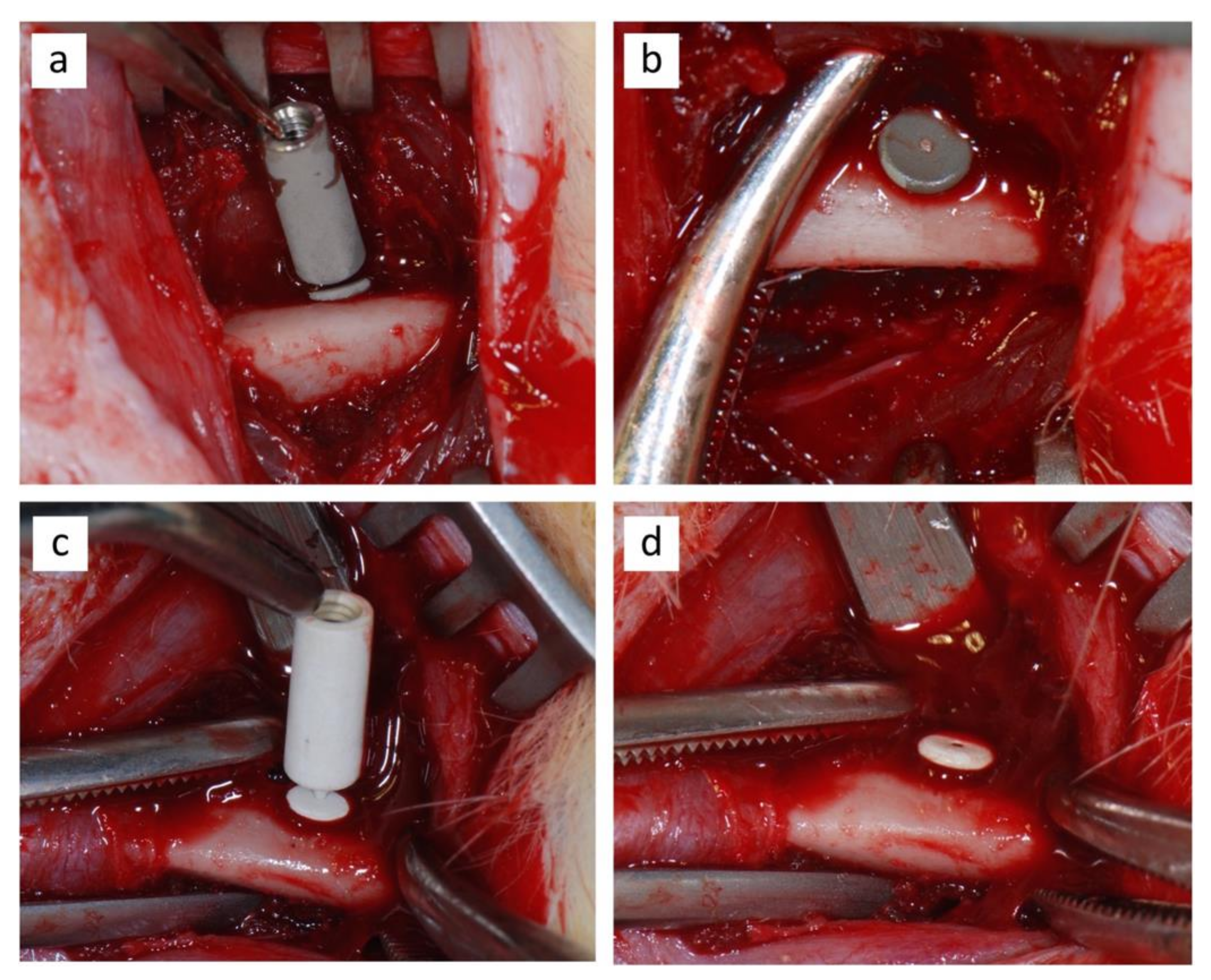

| Component Compositions * | Amount (g) |
|---|---|
| SiO2 precursor “MK polymer” | 3.65 |
| CaCO3 | 5.12 |
| TiO2 | 4.07 |
| Isopropyl alcohol | 14 |
| Parameter | Value |
|---|---|
| X-ray source voltage | 110 kV |
| X-ray source current | 62 μA |
| Exposure time | 1415 ms |
| Nr. of projections | 3142 |
| Voxel size | 3 μm |
| Temperature | 20 °C |
| Parameter | Value |
|---|---|
| X-ray source voltage | 170 kV |
| X-ray source current | 40 μA |
| Exposure time | 1415 ms |
| Nr. of projections | 2000 |
| Voxel size | 6.5 μm |
| Temperature | 20 °C |
| Sample | OD | HI | Results |
|---|---|---|---|
| Positive Control | 0.8700 ± 0.012 | 100% | Hemolitic |
| Negative control | 0.0134 ± 0.007 | 0% | Non-hemolitic |
| Sphene samples | 0.0123 ± 0.002 | 0% | Non-hemolitic |
| Sacrifice (days) | Arm (n implants) | BIC% | BIC%Cortical | BIC%Cancellous |
|---|---|---|---|---|
| 14 | Uncoated (5) | 48.1 ± 19.6 | 44.6 ± 18.6 | 42.2 ± 18.4 |
| Sphene-coated (5) | 50.5 ± 17.4 | 55.5 ± 16.1 | 41.5 ± 18.2 | |
| 28 | Uncoated (5) | 57.7 ± 15.4 | 62.2 ± 9.4 | 56.2 ± 14.1 |
| Sphene-coated (5) | 60.4 ± 17.7 | 53.9 ± 17.7 | 56.3 ± 14.8 |
| Uncoated | Sphene-coated | |||
|---|---|---|---|---|
| 14 days | 28 days | 14 days | 28 days | |
| Polymorphonuclear cells | 3 | 0 | 3 | 0 |
| Phagocytic cells | 3 | 0 | 3 | 0 |
| Fibroblasts | 1 | 2 | 1 | 2 |
| Endothelial cells | 2 | 2 | 2 | 2 |
| Collagen fibers | 2 | 3 | 2 | 3 |
| New bone | 0 | 2 | 0 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunello, G.; Biasetto, L.; Elsayed, H.; Sbettega, E.; Gardin, C.; Scanu, A.; Carmignato, S.; Zavan, B.; Sivolella, S. An In Vivo Study in Rat Femurs of Bioactive Silicate Coatings on Titanium Dental Implants. J. Clin. Med. 2020, 9, 1290. https://doi.org/10.3390/jcm9051290
Brunello G, Biasetto L, Elsayed H, Sbettega E, Gardin C, Scanu A, Carmignato S, Zavan B, Sivolella S. An In Vivo Study in Rat Femurs of Bioactive Silicate Coatings on Titanium Dental Implants. Journal of Clinical Medicine. 2020; 9(5):1290. https://doi.org/10.3390/jcm9051290
Chicago/Turabian StyleBrunello, Giulia, Lisa Biasetto, Hamada Elsayed, Elia Sbettega, Chiara Gardin, Anna Scanu, Simone Carmignato, Barbara Zavan, and Stefano Sivolella. 2020. "An In Vivo Study in Rat Femurs of Bioactive Silicate Coatings on Titanium Dental Implants" Journal of Clinical Medicine 9, no. 5: 1290. https://doi.org/10.3390/jcm9051290
APA StyleBrunello, G., Biasetto, L., Elsayed, H., Sbettega, E., Gardin, C., Scanu, A., Carmignato, S., Zavan, B., & Sivolella, S. (2020). An In Vivo Study in Rat Femurs of Bioactive Silicate Coatings on Titanium Dental Implants. Journal of Clinical Medicine, 9(5), 1290. https://doi.org/10.3390/jcm9051290










