Association of Body Mass Index and Waist Circumference with All-Cause Mortality in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. NHIS Data Source
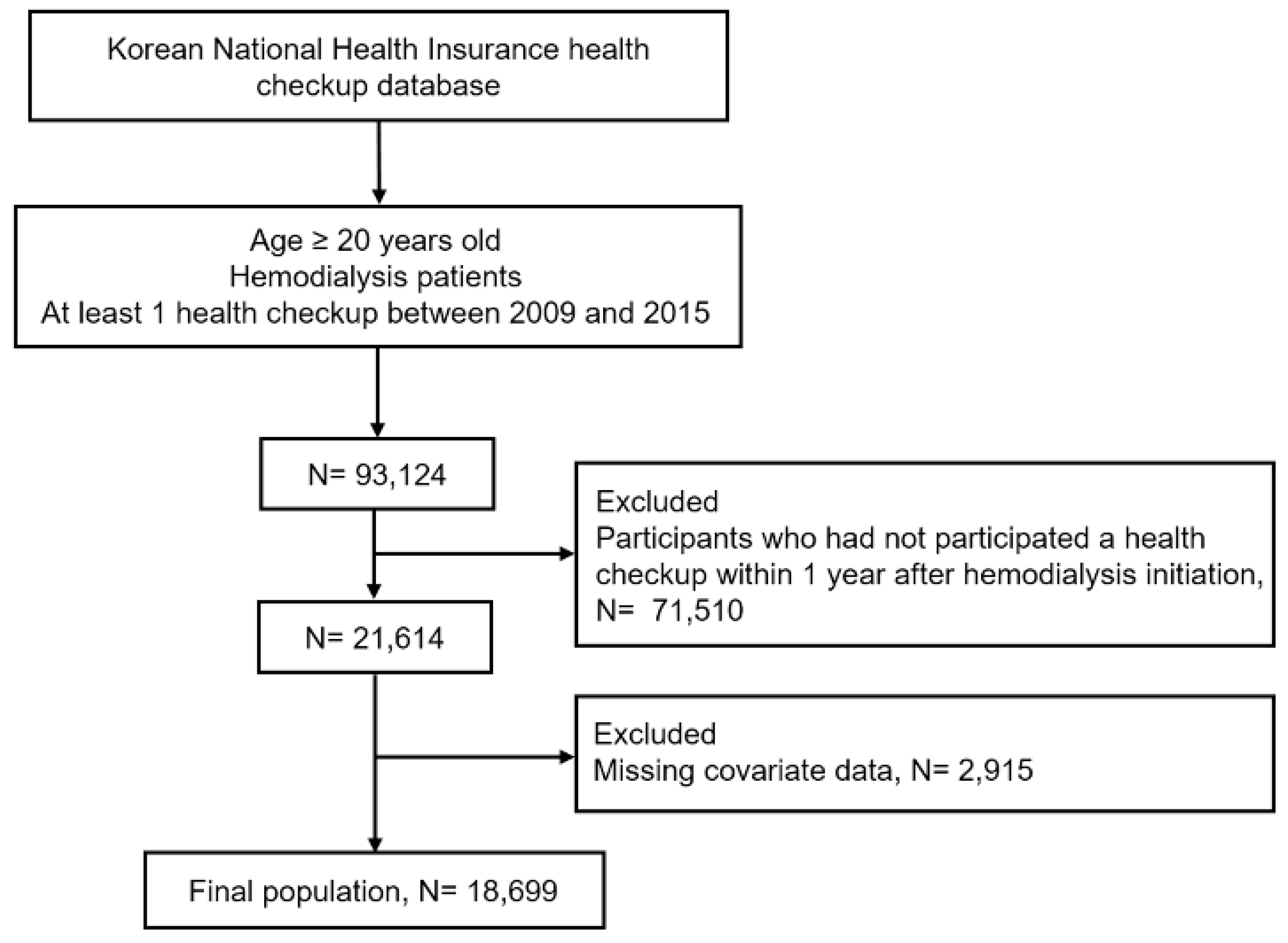
2.2. Study Population
2.3. Definitions of BMI and WC
2.4. Definitions of Health-Behavior Factors and Laboratory Measurements
2.5. Study Outcomes and Follow-Up
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Associations of BMI and WC with the Risk of Mortality
3.3. Subgroup Analyses of the Association of Obesity and Abdominal Obesity with All-Cause Mortality According to the BMI or WC Level
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chang, A.R.; Grams, M.E.; Ballew, S.H.; Bilo, H.; Correa, A.; Evans, M.; Gutierrez, O.M.; Hosseinpanah, F.; Iseki, K.; Kenealy, T.; et al. Adiposity and risk of decline in glomerular filtration rate: Meta-analysis of individual participant data in a global consortium. BMJ 2019, 364, k5301. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.F.; Zahmatkesh, G.; Ahmadi, E.; Streja, E.; Rhee, C.M.; Gillen, D.L.; De Nicola, L.; Minutolo, R.; Ricardo, A.C.; Kovesdy, C.P.; et al. Association of Body Mass Index with Clinical Outcomes in Non-Dialysis-Dependent Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Cardiorenal. Med. 2015, 6, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.L.; Kalantar-Zadeh, K.; Ma, J.Z.; Quarles, L.D.; Kovesdy, C.P. Association of body mass index with outcomes in patients with CKD. J. Am. Soc. Nephrol. 2014, 25, 2088–2096. [Google Scholar] [CrossRef]
- Polinder-Bos, H.A.; Diepen, M.V.; Dekker, F.W.; Hoogeveen, E.K.; Franssen, C.F.M.; Gansevoort, R.T.; Gaillard, C. Lower body mass index and mortality in older adults starting dialysis. Sci. Rep. 2018, 8, 12858. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef]
- Herselman, M.; Esau, N.; Kruger, J.M.; Labadarios, D.; Moosa, M.R. Relationship between body mass index and mortality in adults on maintenance hemodialysis: A systematic review. J. Ren. Nutr. 2010, 20, 281–292. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.M.; Han, K.D.; Jung, J.H.; Lee, S.S.; Oh, S.W.; Park, H.S.; Rhee, E.J.; Lee, W.Y.; Yoo, S.J. Waist Circumference and All-Cause Mortality Independent of Body Mass Index in Korean Population from the National Health Insurance Health Checkup 2009(-)2015. J. Clin. Med. 2019, 8, 72. [Google Scholar] [CrossRef]
- Postorino, M.; Marino, C.; Tripepi, G.; Zoccali, C.; Group, C.W. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J. Am. Coll. Cardiol. 2009, 53, 1265–1272. [Google Scholar] [CrossRef]
- Postorino, M.; Marino, C.; Tripepi, G.; Zoccali, C.; Group, C.W. Abdominal obesity modifies the risk of hypertriglyceridemia for all-cause and cardiovascular mortality in hemodialysis patients. Kidney Int. 2011, 79, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.F.; Sarnak, M.J.; Tighiouart, H.; Griffith, J.L.; Kurth, T.; Salem, D.N.; Levey, A.S.; Weiner, D.E. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am. J. Kidney Dis. 2008, 52, 29–38. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; International Association for the Study of Obesity; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Lee, S.Y.; Park, H.S.; Kim, D.J.; Han, J.H.; Kim, S.M.; Cho, G.J.; Kim, D.Y.; Kwon, H.S.; Kim, S.R.; Lee, C.B.; et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 2007, 75, 72–80. [Google Scholar] [CrossRef]
- Mariat, C.; Alamartine, E.; Berthoux, F. Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 355, 1068–1069. [Google Scholar] [PubMed]
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235. [Google Scholar] [CrossRef]
- Kramer, H.; Gutierrez, O.M.; Judd, S.E.; Muntner, P.; Warnock, D.G.; Tanner, R.M.; Panwar, B.; Shoham, D.A.; McClellan, W. Waist Circumference, Body Mass Index, and ESRD in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am. J. Kidney Dis. 2016, 67, 62–69. [Google Scholar] [CrossRef]
- Stefansson, V.T.N.; Schei, J.; Solbu, M.D.; Jenssen, T.G.; Melsom, T.; Eriksen, B.O. Metabolic syndrome but not obesity measures are risk factors for accelerated age-related glomerular filtration rate decline in the general population. Kidney Int. 2018, 93, 1183–1190. [Google Scholar] [CrossRef]
- Hamer, M.; O’Donovan, G.; Stensel, D.; Stamatakis, E. Normal-Weight Central Obesity and Risk for Mortality. Ann. Intern. Med. 2017, 166, 917–918. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Anderson, J.E.; Kalantar-Zadeh, K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am. J. Kidney Dis. 2007, 49, 581–591. [Google Scholar] [CrossRef]
- Ladhani, M.; Craig, J.C.; Irving, M.; Clayton, P.A.; Wong, G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: A systematic review and meta-analysis. Nephrol. Dial. Transpl. 2017, 32, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.; Shoham, D.; McClure, L.A.; Durazo-Arvizu, R.; Howard, G.; Judd, S.; Muntner, P.; Safford, M.; Warnock, D.G.; McClellan, W. Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am. J. Kidney Dis. 2011, 58, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Meyre, D.; Froguel, P.; Horber, F.F.; Kral, J.G. Comment on: Valette; et al. Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLOS ONE 2012; 7(11):E48221. PLoS ONE 2014, 9, e93324. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Marchlewska, A.; Pecoits-Filho, R.; Heimburger, O.; Zhang, Z.; Hoff, C.; Holmes, C.; Axelsson, J.; Arvidsson, S.; Schalling, M.; et al. Adiponectin in renal disease: Relationship to phenotype and genetic variation in the gene encoding adiponectin. Kidney Int. 2004, 65, 274–281. [Google Scholar] [CrossRef]
- Horwich, T.B.; Fonarow, G.C.; Hamilton, M.A.; MacLellan, W.R.; Woo, M.A.; Tillisch, J.H. The relationship between obesity and mortality in patients with heart failure. J. Am. Coll. Cardiol. 2001, 38, 789–795. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Horwich, T.B.; Oreopoulos, A.; Kovesdy, C.P.; Younessi, H.; Anker, S.D.; Morley, J.E. Risk factor paradox in wasting diseases. Curr. Opin Clin. Nutr. Metab. Care 2007, 10, 433–442. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, J.C.; Ahn, S.Y.; Doh, K.; Jin, D.C.; Na, K.Y. Time-varying effects of body mass index on mortality among hemodialysis patients: Results from a nationwide Korean registry. Kidney Res. Clin. Pract. 2019, 38, 90–99. [Google Scholar] [CrossRef]
- Kittiskulnam, P.; Johansen, K.L. The obesity paradox: A further consideration in dialysis patients. Semin. Dial. 2019, 32, 485–489. [Google Scholar] [CrossRef]
- Delgado, C.; Chertow, G.M.; Kaysen, G.A.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Johansen, K.L. Associations of Body Mass Index and Body Fat with Markers of Inflammation and Nutrition Among Patients Receiving Hemodialysis. Am. J. Kidney Dis. 2017, 70, 817–825. [Google Scholar] [CrossRef]
- Hanks, L.J.; Tanner, R.M.; Muntner, P.; Kramer, H.; McClellan, W.M.; Warnock, D.G.; Judd, S.E.; Gutierrez, O.M.; Investigators, R. Metabolic subtypes and risk of mortality in normal weight, overweight, and obese individuals with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 2064–2071. [Google Scholar] [CrossRef][Green Version]
- Evans, P.D.; McIntyre, N.J.; Fluck, R.J.; McIntyre, C.W.; Taal, M.W. Anthropomorphic measurements that include central fat distribution are more closely related with key risk factors than BMI in CKD stage 3. PLoS ONE 2012, 7, e34699. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Sozio, S.M.; Jaar, B.G.; McAdams-DeMarco, M.A.; Estrella, M.M.; Tereshchenko, L.G.; Monroy-Trujillo, J.M.; Parekh, R.S. Association of Abdominal Adiposity with Cardiovascular Mortality in Incident Hemodialysis. Am. J. Nephrol. 2018, 48, 406–414. [Google Scholar] [CrossRef]
- Castro, A.C.M.; Bazanelli, A.P.; Nerbass, F.B.; Cuppari, L.; Kamimura, M.A. Waist circumference as a predictor of mortality in peritoneal dialysis patients: A follow-up study of 48 months. Br. J. Nutr. 2017, 117, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Postorino, M.; Marino, C.; Pizzini, P.; Cutrupi, S.; Tripepi, G.; Group, C.W. Waist circumference modifies the relationship between the adipose tissue cytokines leptin and adiponectin and all-cause and cardiovascular mortality in haemodialysis patients. J. Intern. Med. 2011, 269, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Liou, H.H.; Su, P.F.; Chang, M.Y.; Wang, H.H.; Chen, M.J.; Hung, S.Y. Abdominal obesity is the most significant metabolic syndrome component predictive of cardiovascular events in chronic hemodialysis patients. Nephrol. Dial. Transpl. 2011, 26, 3689–3695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Roij van Zuijdewijn, C.L.; ter Wee, P.M.; Chapdelaine, I.; Bots, M.L.; Blankestijn, P.J.; van den Dorpel, M.A.; Nube, M.J.; Grooteman, M.P. A Comparison of 8 Nutrition-Related Tests to Predict Mortality in Hemodialysis Patients. J. Ren. Nutr. 2015, 25, 412–419. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Gillespie, I.A.; Tunks, J.; Addison, J.; Kronenberg, F.; Drueke, T.B.; Marcelli, D.; Schernthaner, G.; Eckardt, K.U.; Floege, J.; et al. Inflammation Modifies the Paradoxical Association between Body Mass Index and Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2016, 27, 1479–1486. [Google Scholar] [CrossRef]
- Ok, E.; Asci, G.; Toz, H.; Ok, E.S.; Kircelli, F.; Yilmaz, M.; Hur, E.; Demirci, M.S.; Demirci, C.; Duman, S.; et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: Results from the Turkish OL-HDF Study. Nephrol. Dial. Transpl. 2013, 28, 192–202. [Google Scholar] [CrossRef]
- Anderson, R.T.; Cleek, H.; Pajouhi, A.S.; Bellolio, M.F.; Mayukha, A.; Hart, A.; Hickson, L.J.; Feely, M.A.; Wilson, M.E.; Giddings Connolly, R.M.; et al. Prediction of Risk of Death for Patients Starting Dialysis: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1213–1227. [Google Scholar] [CrossRef]
- Lok, C.E.; Foley, R. Vascular access morbidity and mortality: Trends of the last decade. Clin. J. Am. Soc. Nephrol. 2013, 8, 1213–1219. [Google Scholar] [CrossRef]
- Doshi, M.; Streja, E.; Rhee, C.M.; Park, J.; Ravel, V.A.; Soohoo, M.; Moradi, H.; Lau, W.L.; Mehrotra, R.; Kuttykrishnan, S.; et al. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: A marginal structural model analysis. Nephrol. Dial. Transpl. 2016, 31, 1310–1319. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Total | BMI, kg/m2 | |||||
|---|---|---|---|---|---|---|---|
| <18.5 | 18.5–23 | 23–25 | 25–30 | ≥30 | P Value | ||
| Number | 18,699 | 1264 | 9534 | 3985 | 3439 | 477 | |
| Age, years, mean ± SD | 59.9 ± 10.9 | 57.0 ± 13.0 | 59.5 ± 11.0 | 61.2 ± 10.3 | 60.8 ± 10.3 | 56.8 ± 11.2 | <0.001 |
| Age group, years (%) | <0.001 | ||||||
| 20–40 | 565 (3.0) | 92 (7.3) | 303 (3.2) | 78 (2.0) | 65 (1.9) | 27 (5.7) | |
| 40–65 | 11,697 (62.6) | 807 (63.8) | 6066 (63.6) | 2396 (60.1) | 2107 (61.3) | 321 (67.3) | |
| ≥ 65 | 6437 (34.4) | 365 (28.9) | 3165 (33.2) | 1511 (37.9) | 1267 (36.8) | 129 (27.0) | |
| Female (%) | 8128 (43.5) | 760 (60.1) | 4077 (42.8) | 1516 (38.0) | 1498 (43.6) | 277 (58.1) | <0.001 |
| Place of residence (%) | 0.209 | ||||||
| Urban living | 8360 (44.7) | 551 (43.6) | 4331 (45.4) | 1783 (44.7) | 1490 (43.3) | 205 (43.0) | |
| Rural living | 10,339 (55.3) | 713 (56.4) | 5203 (54.6) | 2202 (55.3) | 1949 (56.7) | 272 (57.0) | |
| Smoking (%) | <0.001 | ||||||
| Non | 12,410 (66.4) | 970 (76.7) | 6296 (66.0) | 2559 (64.2) | 2249 (65.4) | 336 (70.4) | |
| Former | 4273 (22.9) | 184 (14.6) | 2161 (22.7) | 1006 (25.2) | 834 (24.3) | 88 (18.5) | |
| Current | 2016 (10.8) | 110 (8.7) | 1077 (11.3) | 420 (10.5) | 356 (10.4) | 53 (11.1) | |
| Alcohol consumption | 0.018 | ||||||
| None (%) | 17,046 (91.2) | 1189 (94.1) | 8705 (91.3) | 3608 (90.5) | 3114 (90.6) | 430 (90.2) | |
| Moderate (%) | 1535 (8.2) | 71 (5.6) | 767 (8.0) | 353 (8.9) | 301 (8.8) | 43 (9.0) | |
| Heavy (%) | 118 (0.6) | 4 (0.3) | 62 (0.7) | 24 (0.6) | 24 (0.7) | 4 (0.8) | |
| Regular exercise (%) | <0.001 | ||||||
| No | 16,050 (85.8) | 1125 (89.0) | 8086 (84.8) | 3401 (85.3) | 3009 (87.5) | 429 (89.9) | |
| Yes | 2649 (14.2) | 139 (11.0) | 1448 (15.2) | 584 (14.7) | 430 (12.5) | 48 (10.1) | |
| Diabetes mellitus (%) | 8659 (46.3) | 450 (35.6) | 3999 (41.9) | 1975 (49.6) | 1927 (56.0) | 308 (64.6) | <0.001 |
| Hypertension (%) | 14,820 (79.3) | 982 (77.7) | 7589 (79.6) | 3142 (78.9) | 2730 (79.4) | 377 (79.0) | 0.554 |
| Dyslipidemia (%) | 6677 (35.7) | 322 (25.5) | 2972 (31.2) | 1546 (38.8) | 1560 (45.4) | 277 (58.1) | <0.001 |
| CKD (%) | 17,989 (96.2) | 1216 (96.2) | 9178 (96.3) | 3827 (96.0) | 3312 (96.3) | 456 (95.6) | 0.911 |
| Cardiovascular disease (%) | 4325 (23.1) | 268 (21.2) | 2096 (22.0) | 952 (23.9) | 898 (26.11) | 111 (23.3) | <0.001 |
| Cancer (%) | 555 (3.0) | 42 (3.3) | 298 (3.1) | 103 (2.6) | 96 (2.8) | 16 (3.4) | <0.001 |
| Low income (%) | <0.001 | ||||||
| No | 11710 (62.6) | 735 (58.2) | 5934 (62.2) | 2593 (65.1) | 2175 (63.3) | 273 (57.2) | |
| Yes | 6989 (37.4) | 529 (41.8) | 3600 (37.8) | 1392 (34.9) | 1264 (36.7) | 204 (42.8) | |
| BMI, kg/m2, mean ± SD | 22.7 ± 3.2 | 17.5 ± 0.9 | 21.0 ± 1.2 | 23.9 ± 0.6 | 26.7 ± 1.3 | 32.2 ± 2.1 | <0.001 |
| WC, cm, mean ± SD | 80.7 ± 9.6 | 68.2 ± 6.4 | 76.6 ± 6.7 | 84.0 ± 6.0 | 90.3 ± 6.8 | 100.2 ± 8.2 | <0.001 |
| SBP, mmHg, mean ± SD | 134.3 ± 20.1 | 132.7 ± 20.6 | 134.3 ± 20.2 | 134.4 ± 19.8 | 134.2 ± 19.9 | 136.7 ± 19.9 | 0.005 |
| DBP, mmHg, mean ± SD | 77.9 ± 11.8 | 78.1 ± 12.3 | 77.9 ± 11.9 | 77.8 ± 11.5 | 77.8 ± 11.7 | 79.8 ± 12.1 | 0.008 |
| Fasting glucose, mg/dL, mean ± SD | 114.5 ± 47.6 | 109.2 ± 45.9 | 111.6 ± 46.0 | 115.5 ± 47.4 | 120.8 ± 50.7 | 129.6 ± 53.0 | <0.001 |
| Total cholesterol, mg/dL, mean ± SD | 166.8 ± 39.1 | 165.9 ± 37.9 | 165.8 ± 38.2 | 167.7 ± 39.7 | 167.6 ± 40.2 | 174.4 ± 45.1 | <0.001 |
| High-density lipoprotein, mg/dL, mean ± SD | 49.0 ± 15.3 | 55.7 ± 17.1 | 51.1 ± 15.3 | 46.8 ± 14.9 | 44.2 ± 13.3 | 43.4 ± 11.9 | <0.001 |
| Low-density lipoprotein, mg/dL, mean ± SD | 93.3 ± 32.8 | 90.0 ± 30.7 | 92.9 ± 32.0 | 94.7 ± 33.7 | 93.9 ± 33.9 | 95.0 ± 37.3 | <0.001 |
| Triglyceride, mg/dL, (25th 75th) | 108.0 (107.2–108.8) | 91.2 (89.0–93.4) | 97.8 (96.9–98.7) | 116.3 (114.5–118.1) | 131.6 (129.4–133.7) | 160.2 (152.6–168.1) | <0.001 |
| Group | Number | Death | Follow-Up Duration, Person-Year | Mortality Rate, Per 100 Person-Year | Model 1, HR (95% CI) a | Model 2, HR (95% CI) b | Model 3, HR (95% CI) c |
|---|---|---|---|---|---|---|---|
| BMI group, kg/m2 | |||||||
| <18.5 | 1264 | 380 | 5146.1 | 7.38 | 1.46 (1.31–1.62) | 1.48 (1.33–1.65) | 1.67 (1.49–1.87) |
| 18.5–23 | 9534 | 2586 | 41213.6 | 6.27 | reference | reference | reference |
| 23–25 | 3985 | 1059 | 17444.3 | 6.07 | 0.87 (0.81–0.93) | 0.84 (0.78–0.90) | 0.76 (0.70–0.82) |
| 25–30 | 3439 | 846 | 14609.4 | 5.79 | 0.86 (0.80–0.93) | 0.80 (0.74–0.86) | 0.65 (0.59–0.72) |
| ≥30 | 477 | 104 | 1987.5 | 5.23 | 1.03 (0.85–1.25) | 0.86 (0.70–1.04) | 0.60 (0.48–0.75) |
| WC group, cm, (male/female) | |||||||
| <80/<75 | 7006 | 1640 | 31042.7 | 5.28 | 1.00 (0.92,1.08) | 1.08 (1.00–1.18) | 0.80 (0.73–0.88) |
| 80–85/75–80 | 4125 | 1100 | 17857.8 | 6.16 | 0.97 (0.89,1.06) | 0.99 (0.91–1.08) | 0.88 (0.80–0.96) |
| 85–90/80–85 | 3368 | 968 | 14165.0 | 6.83 | reference | reference | reference |
| 90–95/85–90 | 2167 | 657 | 8990.5 | 7.31 | 1.02 (0.93–1.13) | 1.00 (0.90–1.10) | 1.12 (1.01–1.24) |
| 95–100/90–95 | 1140 | 338 | 4789.0 | 7.06 | 1.06 (0.94–1.20) | 0.97 (0.86–1.10) | 1.25 (1.10–1.42) |
| ≥100/≥95 | 893 | 272 | 3555.8 | 7.65 | 1.19 (1.04–1.36) | 1.07 (0.93–1.22) | 1.68 (1.45–1.96) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.S.; Han, K.-D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Body Mass Index and Waist Circumference with All-Cause Mortality in Hemodialysis Patients. J. Clin. Med. 2020, 9, 1289. https://doi.org/10.3390/jcm9051289
Kim CS, Han K-D, Choi HS, Bae EH, Ma SK, Kim SW. Association of Body Mass Index and Waist Circumference with All-Cause Mortality in Hemodialysis Patients. Journal of Clinical Medicine. 2020; 9(5):1289. https://doi.org/10.3390/jcm9051289
Chicago/Turabian StyleKim, Chang Seong, Kyung-Do Han, Hong Sang Choi, Eun Hui Bae, Seong Kwon Ma, and Soo Wan Kim. 2020. "Association of Body Mass Index and Waist Circumference with All-Cause Mortality in Hemodialysis Patients" Journal of Clinical Medicine 9, no. 5: 1289. https://doi.org/10.3390/jcm9051289
APA StyleKim, C. S., Han, K.-D., Choi, H. S., Bae, E. H., Ma, S. K., & Kim, S. W. (2020). Association of Body Mass Index and Waist Circumference with All-Cause Mortality in Hemodialysis Patients. Journal of Clinical Medicine, 9(5), 1289. https://doi.org/10.3390/jcm9051289





