Strengthened Default Mode Network Activation During Delay Discounting in Adolescents with Anorexia Nervosa After Partial Weight Restoration: A Longitudinal fMRI Study
Abstract
1. Introduction
2. Methods
2.1. Participants and Procedures
2.2. HC Participants
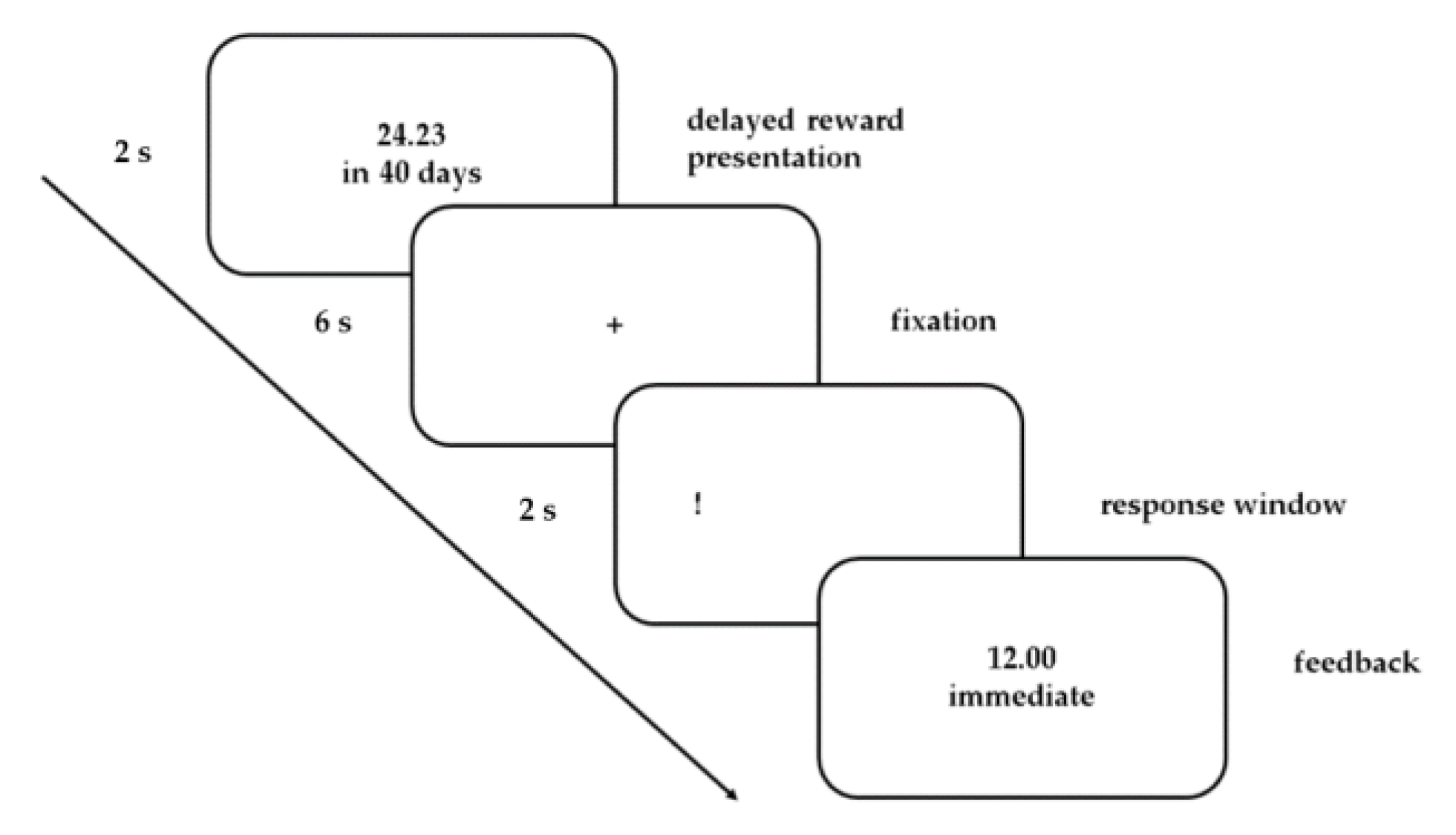
2.3. Task
2.4. Behavioral Data Analysis
2.5. FMRI Acquisition, Processing, and Analysis
3. Results
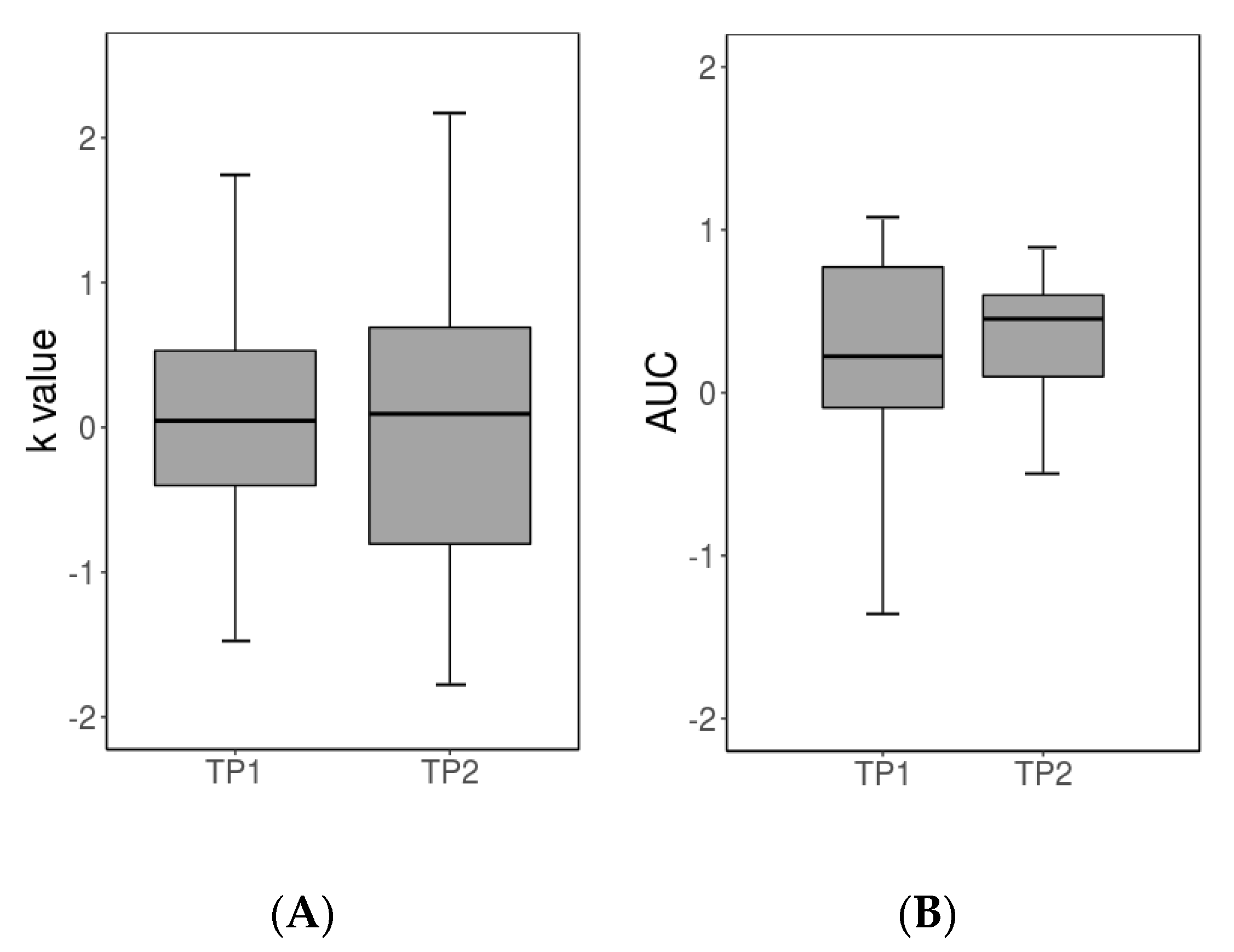
3.1. Behavioral Results
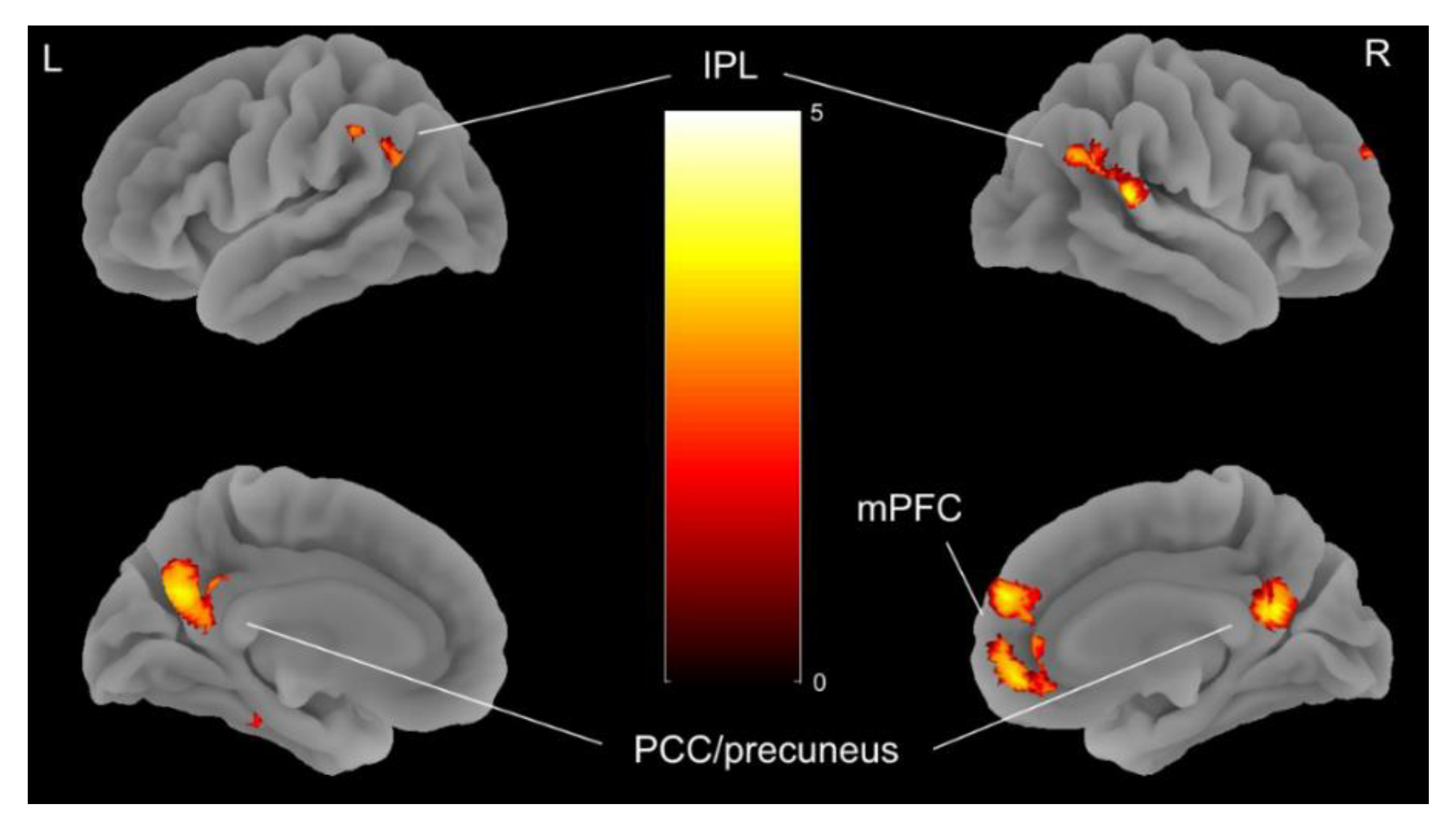
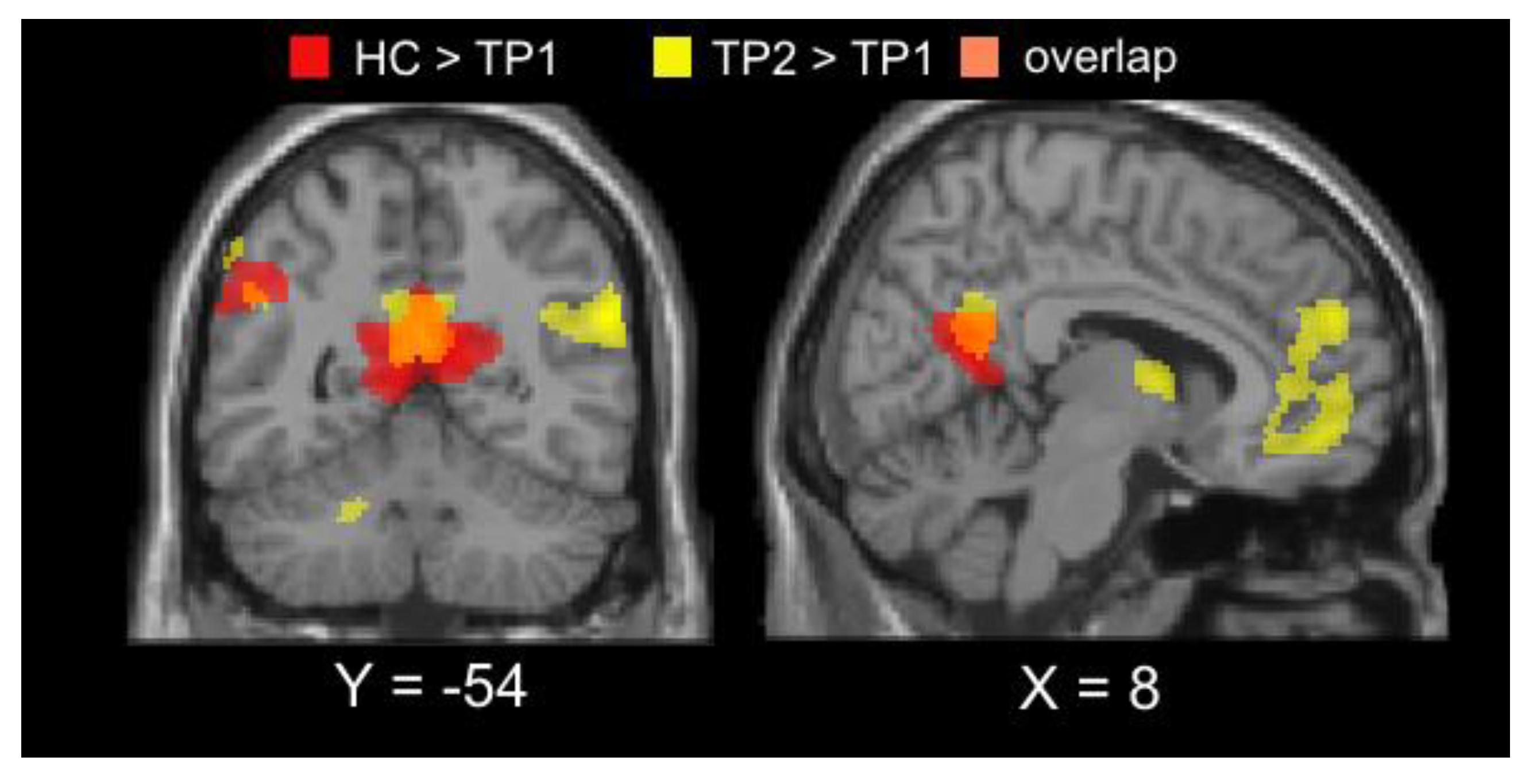
3.2. Imaging Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Treasure, J.; Zipfel, S.; Micali, N.; Wade, T.; Stice, E.; Claudino, A.; Schmidt, U.; Frank, G.K.; Bulik, C.M.; Wentz, E. Anorexia nervosa. Nat. Rev. Dis. Prim. 2015, 1, 15075. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Fudge, J.L.; Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009, 10, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Wierenga, C.E.; Ely, A.; Bischoff-Grethe, A.; Bailer, U.F.; Simmons, A.N.; Kaye, W.H. Are Extremes of Consumption in Eating Disorders Related to an Altered Balance between Reward and Inhibition? Front. Behav. Neurosci. 2014, 8, 410. [Google Scholar] [CrossRef]
- Bruch, H. The Golden Cage: The Enigma of Anorexia Nervosa; Harvard University Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Wu, M.; Brockmeyer, T.; Hartmann, M.; Skunde, M.; Herzog, W.; Friederich, H.-C. Reward-related decision making in eating and weight disorders: A systematic review and meta-analysis of the evidence from neuropsychological studies. Neurosci. Biobehav. Rev. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- O’Hara, C.B.; Campbell, I.C.; Schmidt, U. A reward-centred model of anorexia nervosa: A focussed narrative review of the neurological and psychophysiological literature. Neurosci. Biobehav. Rev. 2015, 52, 131–152. [Google Scholar] [CrossRef]
- Kroemer, N.B. Exaggerated Control Demands Over Reward-Related Behavior in Anorexia Nervosa. Biol. Psychiatry 2018, 83, 194–196. [Google Scholar] [CrossRef]
- Steinglass, J.E.; Berner, L.A.; Attia, E. Cognitive Neuroscience of Eating Disorders. Psychiatr. Clin. North Am. 2019, 42, 75–91. [Google Scholar] [CrossRef]
- Steinglass, J.E.; Walsh, B.T. Neurobiological model of the persistence of anorexia nervosa. J. Eat. Disord. 2016, 4, 19. [Google Scholar] [CrossRef]
- McClelland, J.; Dalton, B.; Kekic, M.; Bartholdy, S.; Campbell, I.C.; Schmidt, U. A systematic review of temporal discounting in eating disorders and obesity: Behavioural and neuroimaging findings. Neurosci. Biobehav. Rev. 2016, 71, 506–528. [Google Scholar] [CrossRef]
- Lempert, K.M.; Steinglass, J.E.; Pinto, A.; Kable, J.W.; Simpson, H.B. Can delay discounting deliver on the promise of RDoC? Psychol. Med. 2019, 49, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Berns, G.S.; Laibson, D.; Loewenstein, G. Intertemporal choice–toward an integrative framework. Trends Cogn. Sci. 2007, 11, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Madden, G.J.; Bickel, W.K. Impulsivity: The Behavioral and Neurological Science of Discounting; American Psychological Association: Washington, DC, USA, 2010; ISBN 978-1-4338-0477-9. [Google Scholar]
- Decker, J.H.; Figner, B.; Steinglass, J.E. On Weight and Waiting: Delay Discounting in Anorexia Nervosa Pretreatment and Posttreatment. Biol. Psychiatry 2015, 78, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Steinglass, J.E.; Figner, B.; Berkowitz, S.; Simpson, H.B.; Weber, E.U.; Walsh, B.T. Increased Capacity to Delay Reward in Anorexia Nervosa. J. Int. Neuropsychol. Soc. 2012, 18, 773–780. [Google Scholar] [CrossRef]
- Steward, T.; Mestre-Bach, G.; Vintró-Alcaraz, C.; Agüera, Z.; Jiménez-Murcia, S.; Granero, R.; Fernández-Aranda, F. Delay discounting of reward and impulsivity in eating disorders: From anorexia nervosa to binge eating disorder. Eur. Eat. Disord. Rev. 2017, 25, 601–606. [Google Scholar] [CrossRef]
- Amlung, M.; Marsden, E.; Holshausen, K.; Morris, V.; Patel, H.; Vedelago, L.; Naish, K.R.; Reed, D.D.; McCabe, R.E. Delay Discounting as a Transdiagnostic Process in Psychiatric Disorders: A Meta-analysis. JAMA Psychiatry 2019, 76, 1176–1186. [Google Scholar] [CrossRef]
- Ritschel, F.; King, J.A.; Geisler, D.; Flohr, L.; Neidel, F.; Boehm, I.; Seidel, M.; Zwipp, J.; Ripke, S.; Smolka, M.N.; et al. Temporal delay discounting in acutely ill and weight-recovered patients with anorexia nervosa. Psychol. Med. 2015, 45, 1229–1239. [Google Scholar] [CrossRef]
- Bartholdy, S.; Rennalls, S.; Danby, H.; Jacques, C.; Campbell, I.C.; Schmidt, U.; O’daly, O.G. Temporal discounting and the tendency to delay gratification across the eating disorder spectrum. Eur. Eat. Disord. Rev. 2017, 25, 344–350. [Google Scholar] [CrossRef]
- Wierenga, C.E.; Bischoff-Grethe, A.; Melrose, A.J.; Irvine, Z.; Torres, L.; Bailer, U.F.; Simmons, A.; Fudge, J.L.; McClure, S.M.; Ely, A.; et al. Hunger does not motivate reward in women remitted from anorexia nervosa. Biol. Psychiatry 2015, 77, 642–652. [Google Scholar] [CrossRef]
- King, J.A.; Bernardoni, F.; Geisler, D.; Ritschel, F.; Doose, A.; Pauligk, S.; Pásztor, K.; Weidner, K.; Roessner, V.; Smolka, M.N.; et al. Intact value-based decision-making during intertemporal choice in women with remitted anorexia nervosa? An fMRI study. J. Psychiatry Neurosci. 2019, 44, 180252. [Google Scholar] [CrossRef]
- King, J.A.; Ehrlich, S. The Elusive Nature of Delay Discounting as a Transdiagnostic Process in Psychiatric Disorders—The Devil Is in the Detail. JAMA Psychiatry 2020, 77, 325. [Google Scholar] [CrossRef] [PubMed]
- Scheres, A.; De Water, E.; Mies, G.W. The neural correlates of temporal reward discounting. Wiley Interdiscip. Rev. Cogn. Sci. 2013, 4, 523–545. [Google Scholar] [CrossRef] [PubMed]
- Kable, J.W.; Glimcher, P.W. The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 2007, 10, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.M.; Laibson, D.I.; Loewenstein, G.; Cohen, J.D. Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science 2004, 306, 503–507. [Google Scholar] [CrossRef]
- Amianto, F.; Northoff, G.; Abbate Daga, G.; Fassino, S.; Tasca, G.A. Is Anorexia Nervosa a Disorder of the Self? A Psychological Approach. Front. Psychol. 2016, 7, 520. [Google Scholar] [CrossRef]
- Stein, K.F.; Corte, C. Identity impairment and the eating disorders: Content and organization of the self-concept in women with anorexia nervosa and bulimia nervosa. Eur. Eat. Disord. Rev. 2007, 15, 58–69. [Google Scholar] [CrossRef]
- Oldershaw, A.; Startup, H.; Lavender, T. Anorexia Nervosa and a Lost Emotional Self: A Psychological Formulation of the Development, Maintenance, and Treatment of Anorexia Nervosa. Front. Psychol. 2019, 10, 219. [Google Scholar] [CrossRef]
- Bruch, H. Anorexia Nervosa: Therapy and theory. AJP 1982, 139, 1531–1538. [Google Scholar]
- Hershfield, H.E. Future self-continuity: How conceptions of the future self transform intertemporal choice: Hershfield. Ann. New York Acad. Sci. 2011, 1235, 30–43. [Google Scholar] [CrossRef]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Owens, M.M.; Gray, J.C.; Amlung, M.T.; Oshri, A.; Sweet, L.H.; MacKillop, J. Neuroanatomical foundations of delayed reward discounting decision making. NeuroImage 2017, 161, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.M.; Meyer, J.R.; Huettel, S.A. Functional neuroimaging of intertemporal choice models: A review. J. Neurosci. Psychol. Econ. 2010, 3, 27–45. [Google Scholar] [CrossRef]
- Duriez, P.; Ramoz, N.; Gorwood, P.; Viltart, O.; Tolle, V. A Metabolic Perspective on Reward Abnormalities in Anorexia Nervosa. Trends Endocrinol. Metab. 2019, 30, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Schorr, M.; Miller, K.K. The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nat. Rev. Endocrinol. 2017, 13, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Sachs, K.V.; Harnke, B.; Mehler, P.S.; Krantz, M.J. Cardiovascular complications of anorexia nervosa: A systematic review. Int. J. Eat. Disord. 2016, 49, 238–248. [Google Scholar] [CrossRef]
- Frank, G.K.W. Altered Brain Reward Circuits in Eating Disorders: Chicken or Egg? Curr. Psychiatry Rep. 2013, 15, 396. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Geisler, D.; Bernardoni, F.; Ritschel, F.; Böhm, I.; Seidel, M.; Mennigen, E.; Ripke, S.; Smolka, M.N.; Roessner, V.; et al. Altered Neural Efficiency of Decision Making During Temporal Reward Discounting in Anorexia Nervosa. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 972–979. [Google Scholar] [CrossRef]
- Fichter, M.M.; Quadflieg, N. Strukturiertes Inventar für Anorektische und Bulimische Eßstörungen: (SIAB); Fragebogen (SIAB-S) und Interview (SIAB-EX) nach DSM-IV und ICD-10; Handanweisung; Hogrefe Verlag für Psychologie: Göttingen, Germany, 1999. [Google Scholar]
- Paul, T.; Thiel, A. Eating Disorder Inventory-2: EDI-2; deutsche Version; Manual: San Francisco, CA, USA; Hogrefe: Göttingen, Germany, 2005. [Google Scholar]
- Hautzinger, M.; Keller, F.; Kühner, C. BDI-II. Beck Depressions-Inventar Revision; Pearson Assessment and Information GmbH: Frankfurt, Germany, 2009. [Google Scholar]
- Petermann, F.; Petermann, M. HAWIK-IV; Hogrefe Verlag: Göttingen, Germany, 2010. [Google Scholar]
- Von Aster, M.; Neubauer, A.; Horn, R. WIE. Wechsler Intelligenztest fuer Erwachsene; Harcourt: Frankfurt, Germany, 2006. [Google Scholar]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Mon. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Hemmelmann, C.; Brose, S.; Vens, M.; Hebebrand, J.; Ziegler, A. [Percentiles of body mass index of 18-80-year-old German adults based on data from the Second National Nutrition Survey]. Dtsch. Med. Wochenschr. 2010, 135, 848–852. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Ripke, S.; Hübner, T.; Mennigen, E.; Müller, K.U.; Rodehacke, S.; Schmidt, D.; Jacob, M.J.; Smolka, M.N. Reward processing and intertemporal decision making in adults and adolescents: The role of impulsivity and decision consistency. Brain Res. 2012, 1478, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Vetter, N.C.; Steding, J.; Jurk, S.; Ripke, S.; Mennigen, E.; Smolka, M.N. Reliability in adolescent fMRI within two years—A comparison of three tasks. Sci. Rep. 2017, 7, 2287. [Google Scholar] [CrossRef] [PubMed]
- Gorgolewski, K.J.; Burns, C.D.; Madison, C.; Clark, D.; Halchenko, Y.; Waskom, M.L.; Ghosh, S.S. Nipype: A Flexible, Lightweight and Extensible Neuroimaging Data Processing Framework in Python. Front. Aging Neurosci. 2011, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Ripke, S.; Hübner, T.; Mennigen, E.; Müller, K.U.; Li, S.-C.; Smolka, M.N. Common Neural Correlates of Intertemporal Choices and Intelligence in Adolescents. J. Cogn. Neurosci. 2015, 27, 387–399. [Google Scholar] [CrossRef]
- Matthew, B.; Jean-Luc, A.; Romain, V.; Jean-Baptiste, P. Region of interest analysis using an SPM toolbox. In Proceedings of the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, 2–6 June 2002. [Google Scholar]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- McKiernan, K.A.; Kaufman, J.N.; Kucera-Thompson, J.; Binder, J.R. A Parametric Manipulation of Factors Affecting Task-induced Deactivation in Functional Neuroimaging. J. Cogn. Neurosci. 2003, 15, 394–408. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; van den Wildenberg, W.P.M.; Segalowitz, S.J.; Carter, C.S. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004, 56, 129–140. [Google Scholar] [CrossRef]
- Koffarnus, M.N.; Deshpande, H.U.; Lisinski, J.M.; Eklund, A.; Bickel, W.K.; LaConte, S.M. An adaptive, individualized fMRI delay discounting procedure to increase flexibility and optimize scanner time. Neuroimage 2017, 161, 56–66. [Google Scholar] [CrossRef]
- Park, R.J.; Godier, L.R.; Cowdrey, F.A. Hungry for reward: How can neuroscience inform the development of treatment for Anorexia Nervosa? Behav. Res. Ther. 2014, 62, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Compan, V.; Walsh, B.T.; Kaye, W.; Geliebter, A. How does the brain implement adaptive decision making to eat? J. Neurosci. 2015, 35, 13868–13878. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.W. Neuroimaging and eating disorders. Curr. Opin. Psychiatry 2019, 32, 478–483. [Google Scholar] [CrossRef]
- Gaudio, S.; Wiemerslage, L.; Brooks, S.J.; Schiöth, H.B. A systematic review of resting-state functional-MRI studies in anorexia nervosa: Evidence for functional connectivity impairment in cognitive control and visuospatial and body-signal integration. Neurosci. Biobehav. Rev. 2016, 71, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Stopyra, M.A.; Friederich, H.-C. Neural Processing of Disorder-Related Stimuli in Patients with Anorexia Nervosa: A Narrative Review of Brain Imaging Studies. J. Clin. Med. 2019, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Essen, D.C.V.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [PubMed]
- Cowdrey, F.A.; Filippini, N.; Park, R.J.; Smith, S.M.; McCabe, C. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum. Brain Mapp. 2014, 35, 483–491. [Google Scholar] [CrossRef]
- Boehm, I.; Geisler, D.; King, J.A.; Ritschel, F.; Seidel, M.; Deza Araujo, Y.; Petermann, J.; Lohmeier, H.; Weiss, J.; Walter, M.; et al. Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. Front. Behav. Neurosci. 2014, 8, 346. [Google Scholar] [CrossRef]
- McFadden, K.L.; Tregellas, J.R.; Shott, M.E.; Frank, G.K.W. Reduced salience and default mode network activity in women with anorexia nervosa. J. Psychiatry Neurosci. 2014, 39, 178–188. [Google Scholar] [CrossRef]
- Via, E.; Goldberg, X.; Sánchez, I.; Forcano, L.; Harrison, B.J.; Davey, C.G.; Pujol, J.; Martínez-Zalacaín, I.; Fernández-Aranda, F.; Soriano-Mas, C.; et al. Self and other body perception in anorexia nervosa: The role of posterior DMN nodes. World J. Biol. Psychiatry 2018, 19, 210–224. [Google Scholar] [CrossRef]
- McAdams, C.J.; Krawczyk, D.C. Who am I? How do I look? Neural differences in self-identity in anorexia nervosa. Soc. Cogn. Affect. Neurosci. 2014, 9, 12–21. [Google Scholar] [CrossRef] [PubMed]
- McAdams, C.J.; Lohrenz, T.; Montague, P.R. Neural responses to kindness and malevolence differ in illness and recovery in women with anorexia nervosa. Hum. Brain Mapp. 2015, 36, 5207–5219. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Harper, J.A.; Van Enkevort, E.A.; Latimer, K.; Kelley, U.; McAdams, C.J. Neural activations are related to body-shape, anxiety, and outcomes in adolescent anorexia nervosa. J. Psychiatr. Res. 2017, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Berkman, E.T.; Livingston, J.L.; Kahn, L.E. Finding The “Self” in Self-Regulation: The Identity-Value Model. Psychol. Inq. 2017, 28, 77–98. [Google Scholar] [CrossRef]
- Qin, P.; Northoff, G. How is our self related to midline regions and the default-mode network? NeuroImage 2011, 57, 1221–1233. [Google Scholar] [CrossRef]
- Davey, C.G.; Pujol, J.; Harrison, B.J. Mapping the self in the brain’s default mode network. NeuroImage 2016, 132, 390–397. [Google Scholar] [CrossRef]
- Kirby, K.N. One-year temporal stability of delay-discount rates. Psychon. Bull. Rev. 2009, 16, 457–462. [Google Scholar] [CrossRef]
- Odum, A.L. Delay discounting: Trait variable? Behav. Process. 2011, 87, 1–9. [Google Scholar] [CrossRef]
- Lempert, K.M.; Phelps, E.A. The Malleability of Intertemporal Choice. Trends Cogn. Sci. (Regul. Ed.) 2016, 20, 64–74. [Google Scholar] [CrossRef]
- Steinberg, L.; Graham, S.; O’Brien, L.; Woolard, J.; Cauffman, E.; Banich, M. Age Differences in Future Orientation and Delay Discounting. Child Dev. 2009, 80, 28–44. [Google Scholar] [CrossRef]
- Albrecht, K.; Volz, K.G.; Sutter, M.; Laibson, D.I.; von Cramon, D.Y. What is for me is not for you: Brain correlates of intertemporal choice for self and other. Soc. Cogn. Affect. Neurosci. 2011, 6, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Fuglset, T.S. Set-shifting, central coherence and decision-making in individuals recovered from anorexia nervosa: A systematic review. J. Eat. Disord. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Steinglass, J.E.; Dalack, M.; Foerde, K. The promise of neurobiological research in anorexia nervosa. Curr. Opin. Psychiatry 2019, 32, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Broyd, S.J.; Demanuele, C.; Debener, S.; Helps, S.K.; James, C.J.; Sonuga-Barke, E.J.S. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Biobehav. Rev. 2009, 33, 279–296. [Google Scholar] [CrossRef]
- Domakonda, M.J.; He, X.; Lee, S.; Cyr, M.; Marsh, R. Increased Functional Connectivity between Ventral Attention and Default Mode Networks in Adolescents with Bulimia Nervosa. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 232–241. [Google Scholar] [CrossRef]
- Uniacke, B.; Wang, Y.; Biezonski, D.; Sussman, T.; Lee, S.; Posner, J.; Steinglass, J. Resting-state connectivity within and across neural circuits in anorexia nervosa. Brain Behav. 2019, 9, e01205. [Google Scholar] [CrossRef]
- Ehrlich, S.; King, J.A.; Boehm, I. Editorial: Connecting the Nodes of Altered Brain Network Organization in Eating Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 156–158. [Google Scholar] [CrossRef]
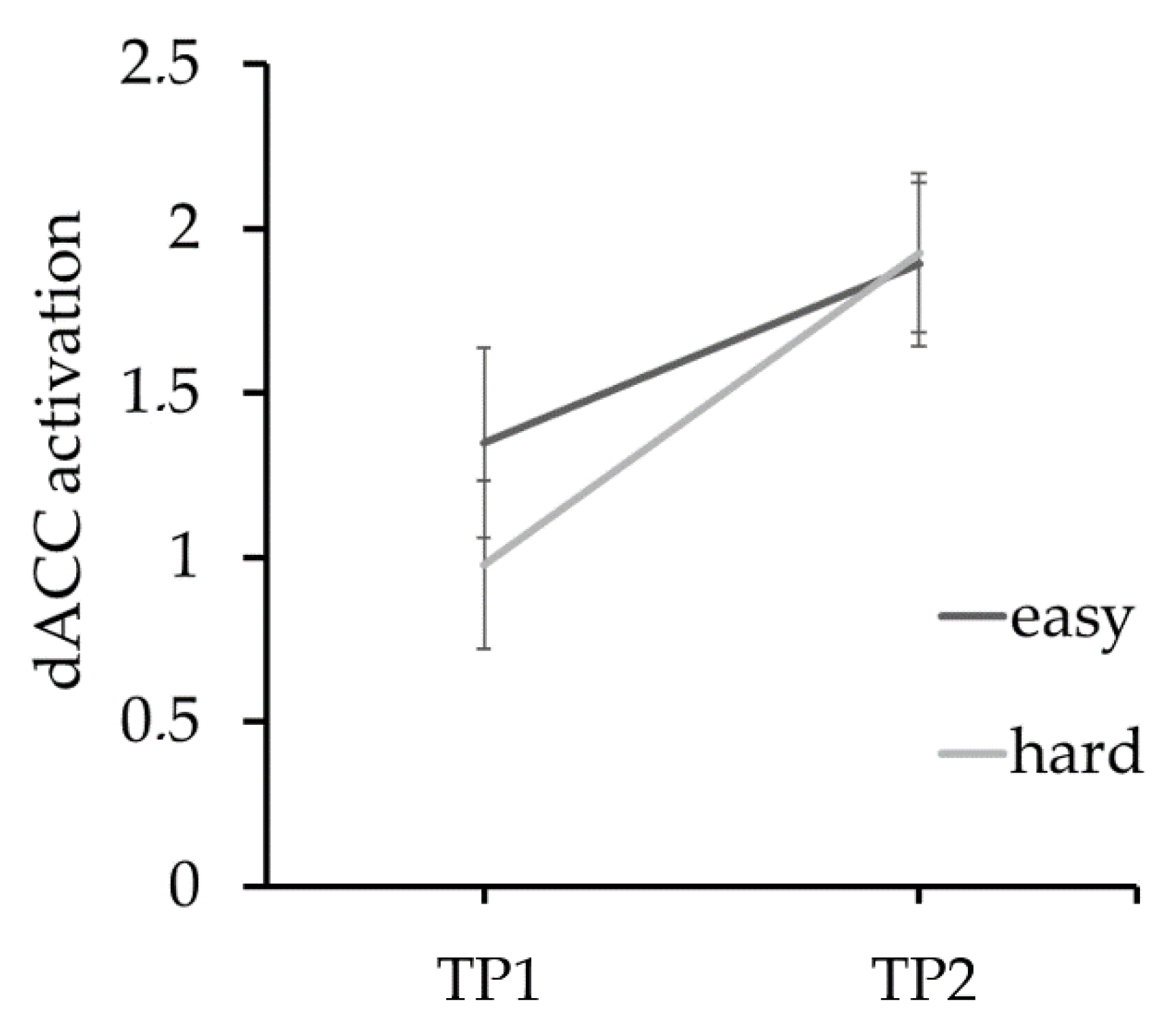
| TP1 | TP2 | t | p | |
|---|---|---|---|---|
| Age (years) | 15.5 ± 2.2 | 15.8 ± 2.2 | −11.7 | <0.001 |
| IQ | 114 ± 11.1 | - | - | - |
| BMI-SDS | −2.9 ± 1.0 | −0.7 ± 0.6 | −13.7 | <0.001 |
| BMI (kg /m²) | 14.9 ± 1.2 | 18.8 ± 1.2 | −15.3 | <0.001 |
| Duration of current episode (months) | 11.6 ± 16.8 | - | - | - |
| BDI-II | 20.0 ± 9.5 | 12.1 ± 6.4 | 4.4 | <0.001 |
| SCL-90-R (Global Severity Index) | 0.9 ± 0.5 | 0.6 ± 0.4 | 4.3 | <0.001 |
| EDI-2 total | 203.6 ± 38.6 | 188.3 ± 41.8 | 2.4 | 0.03 |
| EDI-2 Drive for thinness | 30.1 ± 8.5 | 25.5 ± 10.5 | 2.2 | 0.04 |
| EDI-2 Body dissatisfaction | 36.5 ± 10.3 | 37.5 ± 13.3 | −0.3 | 0.75 |
| EDI-2 Bulimia | 10.1 ± 2.9 | 8.5 ± 1.9 | 3.01 | 0.01 |
| Hemisphere | Voxels | x | y | z | tmax | |
|---|---|---|---|---|---|---|
| TP1<TP2 | ||||||
| Middle occipital gyrus | L/R | 495 | 22 | −98 | 14 | 6.05 |
| TP1>TP2 | ||||||
| Angular gyrus | L | 368 | −54 | −68 | 32 | −4.35 |
| Angular gyrus | R | 582 | 56 | −56 | 25 | −5.60 |
| Posterior cingulate/precuneus | L/R | 754 | 6 | −54 | 20 | −4.67 |
| Medial Frontal Gyrus | L/R | 600 | 8 | 54 | 26 | −4.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doose, A.; King, J.A.; Bernardoni, F.; Geisler, D.; Hellerhoff, I.; Weinert, T.; Roessner, V.; Smolka, M.N.; Ehrlich, S. Strengthened Default Mode Network Activation During Delay Discounting in Adolescents with Anorexia Nervosa After Partial Weight Restoration: A Longitudinal fMRI Study. J. Clin. Med. 2020, 9, 900. https://doi.org/10.3390/jcm9040900
Doose A, King JA, Bernardoni F, Geisler D, Hellerhoff I, Weinert T, Roessner V, Smolka MN, Ehrlich S. Strengthened Default Mode Network Activation During Delay Discounting in Adolescents with Anorexia Nervosa After Partial Weight Restoration: A Longitudinal fMRI Study. Journal of Clinical Medicine. 2020; 9(4):900. https://doi.org/10.3390/jcm9040900
Chicago/Turabian StyleDoose, Arne, Joseph A. King, Fabio Bernardoni, Daniel Geisler, Inger Hellerhoff, Tomas Weinert, Veit Roessner, Michael N. Smolka, and Stefan Ehrlich. 2020. "Strengthened Default Mode Network Activation During Delay Discounting in Adolescents with Anorexia Nervosa After Partial Weight Restoration: A Longitudinal fMRI Study" Journal of Clinical Medicine 9, no. 4: 900. https://doi.org/10.3390/jcm9040900
APA StyleDoose, A., King, J. A., Bernardoni, F., Geisler, D., Hellerhoff, I., Weinert, T., Roessner, V., Smolka, M. N., & Ehrlich, S. (2020). Strengthened Default Mode Network Activation During Delay Discounting in Adolescents with Anorexia Nervosa After Partial Weight Restoration: A Longitudinal fMRI Study. Journal of Clinical Medicine, 9(4), 900. https://doi.org/10.3390/jcm9040900





